Abstract
The objective of the study was to explore the effect of zinc oxide nanoparticles (ZnO-NPs) through root treatment on growth biomarkers, photosynthetic traits, activities of carbonic anhydrase and various antioxidant enzymes as well as the yield of tomato plants. 20 days old seedlings of tomato (cv. PKM-1) were dipped in double distilled water (control), 10, 50, 100 or 200 ppm of ZnO-NPs for 15, 30 or 45 min, respectively. At the stage of 45 and 60 days after sowing (DAS), the plants developed from ZnO-NPs (10 ppm for 30 min) showed improved growth and photosynthetic attributes, enhanced activity of various antioxidant enzymes (e.g., catalase, peroxidase and superoxide dismutase) and higher accumulation of proline and protein content over the non-treated plant. The fruits number and yield in ZnO-NPs treated plants (10 ppm; 30 min) were higher whereas ascorbic acid was lower than the non-treated plant. These fruits possessed higher levels of β-carotene and lycopene content. The root dipping with 10 ppm for 30 min of ZnO-NPs was found to be best for most of the evaluated parameters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The “Nano-Era” that came in light in 1990s is now a progressive field of science and technology for the researchers. The nanoparticles (NPs) are the center of attraction that has the size ranges in between 1 and 100 nm. NPs have been used as building blocks (Madhumitha et al. 2015) in the field of chemistry, physics, medicine and biology (Biswas and Wu 2005). The size, shape and surface area of NPs play an important role in the performance (Edison and Sethuraman 2012). The application of NPs leads to their accumulation in subcellular regions which ultimately cause the change in various physiological parameters leads to better performance of the crop (Garcia-Sanchez et al. 2015; Schwab et al. 2016). Zinc being essential micronutrient plays an important role in many integral metabolic processes (Rout and Das 2003). Important optoelectrical, physical and antimicrobial activities of ZnO-NPs offers great potential to enhance the productivity of crop (Hussain et al. 2016). NPs enter into plants roots by apoplastic pathway and transported to shoots via the vascular system (Lin and Xing 2008; Lin et al. 2009). The uptake of different types of NPs in various crop plants has been reported previously (Lin and Xing 2008; Lin et al. 2009; Wild and Jones 2009; Whiteside et al. 2009). In Lolium plants, ZnO-NPs has been uptake and accumulate in various cell organelles and alter several physiological and biochemical processes (Lin and Xing 2007; Zhao et al. 2012; Mukherjee et al. 2014; Schwab et al. 2016). ZnO-NPs enhanced growth, photosynthesis and antioxidant enzymes in Brassica nigra (Zafar et al. 2016). There were several studies on different crop plants that show the significant enhancement in plant growth and development after the exposure of ZnO-NPs (Dimkpa et al. 2013; Liu et al. 2015; Zafar et al. 2016; Wang et al. 2018). Tomato was selected as the experimental species because it is one of the most important “protective foods” widely known for its outstanding antioxidant, antidiabetic and anticancerous properties. Therefore, keeping these points into consideration, the present research was conducted with an aim to explore the effect of ZnO-NPs through root dipping on the performance of the tomato plants.
Materials and methods
Plant materials
The seeds of tomato (Lycopersicon esculentum Mill. cv. PKM-1) were purchased from National Seed Corporation Ltd., New Delhi, India. The healthy seeds of uniform sizes were surface-sterilized with 1% sodium hypochlorite solution for 10 min, followed by repeated washing with double distilled water (DDW).
Source of nanoparticles
ZnO-NPs were obtained from Sigma Aldrich Chemicals Pvt. Ltd, India. Requisite concentration (10, 50, 100 or 200 ppm) of ZnO-NPs were dissolved in 10 mL of DDW in a 100 mL volumetric flask. Surfactant (Tween-20) was used prior to treatment. The roots were washed with 0.01% Tween 20 for 1 min before dipping in NPs.
Treatment pattern and experimental design
The experiment was conducted under a randomized block design in 75 pots (6-inch diameter). The sterilized seeds were sown to make the nursery. At 20 days after sowing (DAS), seedlings were transplanted into maintaining pots, filled with soil and farmyard manure (6:1). The roots were dipped in 0 (control), 10, 50, 100 or 200 ppm of ZnO-NPs for 15, 30 or 45 min. The pots were kept in the net house of the Department of Botany, Aligarh Muslim University, Aligarh India, and were allowed to grow under natural conditions. The plants of each treatment were uprooted at 45 and 60 DAS to assess the various parameters.
Plant growth analysis
The plants were uprooted from all the treatments and washed properly to remove the adhering soils. The length of root and shoot were measured through a meter scale. The fresh and dry masses were weighed under electronic balance. The leaf area was measured by using a portable leaf area meter (ADC Bioscientific, Hoddesdon, Herts, UK).
Chlorophyll content (SPAD values)
Chlorophyll was measured in intact leaves with the help of SPAD chlorophyll meter (SPAD-502; Konica, Minolta, Inc., Japan).
Photosynthetic attributes
Photosynthetic attributes [net photosynthetic rate (PN), stomatal conductance (gs), intercellular CO2 concentration (Ci), and transpiration rate (E)] were measured in fully expanded uppermost leaves of the plant in each treatment by the portable photosynthetic system (LI-COR 6400, LI-COR, Lincoln, NE, USA). The air temperature, relative humidity, CO2 concentration, and photosynthetic photon flux density were maintained at 25 °C, 85%, 600 ppm, and 800 μmol mol−2 s−1, respectively.
Determination of carbonic anhydrase (CA) and nitrate reductase (NR) activity
Carbonic anhydrase activity (CA, 4.2.1.1) was determined in the leaves by the method of Dwivedi and Randhawa (1974), whereas, Jaworski (1971) was applied to assess the nitrate reductase (NR, 1.6.6.1) activity.
Protein estimation
Protein in the leaf samples was analysed by the method of Bradford (1976).
Antioxidant assay
Antioxidant enzymes activities i.e. catalase (CAT, 1.11.1.6), peroxidase (POX, 1.11.1.7) and superoxide dismutase (SOD, 1.15.1.1) were analysed, as described by Khan et al. (2015). Enzyme activity was expressed on a fresh mass basis and in the form of nM (H2O2) decomposed g−1 (FM) for CAT, U g−1(FM) for POX and U g−1 (FM) for SOD.
Determination of proline content
Amount of proline in the newly formed leaf was determined through the method of Bates et al. (1973).
Scanning electron microscopy (SEM)
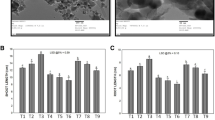
Attachment of ZnO-NPs with tomato plant root was examined by SEM (JEOL, JSM-6510LV, JAPAN) at the Ultra Sophisticated Instrumentation Facility Centre, AMU, Aligarh, India. Root samples were fixed in 2.5% glutaraldehyde in 0.05 M phosphate buffer (pH = 7.1) for 8 h, and dehydrated in an ethanol series. The surface characteristics of the samples (Fig. 9) were evaluated at an accelerating voltage 15 kV.
Analysis of lycopene, β-carotene, and ascorbic acid content
In ripe fruits, the amount of lycopene was measured by the method of Ranganna (1976). β-carotene in the fruit was analysed as described in Sadasivam and Manickam (1997). In the mature fruit, ascorbic acid content was determined following the method of Raghuramulu et al. (1983).
Yield parameters
At the stage of harvesting (180 DAS), 9 plants (3 plant from each pot) representing each treatment were randomly sampled and counted for the number of fruits per plant and weight to assess fruit yield per plant.
Statistical analysis
Data were statistically analyzed using SPSS, 17.0 for Windows (SPSS, Chicago, IL, USA). Standard error was calculated and analysis of variance (ANOVA) was performed to determine the least significance difference (LSD) between treatment means with the level of significance at p ≤ 0.05.
Results
Growth parameters
The data presented in Figs. 1, 2 and 3a, b revealed that the length, fresh mass as well as dry mass of root and shoot and leaf area increased as the growth progressed from 45 to 60 DAS. The values increased further when roots were dipped in a lower concentration of ZnO-NPs for various time. Out of the four concentrations and three different times the root dipping in 10 ppm and 30 min proved best and increased the values by 21% and 27% (shoot length), 18% and 30% (root length), 17% and 24% (shoot fresh mass), 18% and 27% (root fresh mass), 18% and 30% (shoot dry mass), 19% and 25% (root dry mass) and 20% and 24% (leaf area) respectively over control at 45 and 60 DAS. Duration of the root dipping is statistically non-significant to each other. The values for all growth parameters were decreased at 100 and 200 ppm of ZnO-NPs irrespective of dipping time.
Effect of different concentrations (0, 10, 50, 100 or 200 ppm) and durations (15, 30 or 45 min) of zinc oxide nanoparticles (ZnO-NPs) on shoot length (a, b), root length (c, d) and shoot fresh mass (e, f) in the leaves of Lycopersicon esculentum seedlings at 45 and 60 DAS respectively. All the data are the mean of five replicates (n = 5) and vertical bars shows standard errors (± SE)
Effect of different concentrations (0, 10, 50, 100 or 200 ppm) and durations (15, 30 or 45 min) of ZnO-NPs on root fresh mass (a, b), shoot dry mass (C,D) and root dry mass (e, f) in the leaves of Lycopersicon esculentum seedlings at 45 and 60 DAS respectively. All the data are the mean of five replicates (n = 5) and vertical bars shows standard errors (± SE)
Effect of different concentrations (0, 10, 50, 100 or 200 ppm) and durations (15, 30 or 45 min) of ZnO-NPs on leaf area (a, b), SPAD chlorophyll (c, d) and net photosynthetic rate (e, f) in the leaves of Lycopersicon esculentum seedlings at 45 and 60 DAS respectively. All the data are the mean of five replicates (n = 5) and vertical bars shows standard errors (± SE)
Chlorophyll content (SPAD values)
As evident from the Fig. 3c, d that SPAD values in the plant increased by dipping of roots in ZnO-NPs and further increased as growth progressed. Maximum SPAD value was recorded, when the roots were dipped in 10 ppm of ZnO-NPs for 30 min and were about 27% and 34% more as compared to control at 45 and 60 DAS.
Photosynthetic parameters
The data presented in the Figs. 3e, f and 4, indicate that the photosynthetic parameters were increased as the growth progressed from 45 to 60 DAS irrespective of the treatment. However, concentrations (10 and 50 ppm) increased it further at both the stages. Out of the various concentrations 10 ppm for 30 min was more effective and increased the PN by 32% and 41%, gs by 30% and 33%, Ci by 31% and 34% and E by 30% and 36% at 45 and 60 DAS respectively over their control. However, at higher concentrations, photosynthetic parameters were significantly decreased and the maximum decrease was recorded at 200 ppm of ZnO-NPs.
Effect of different concentrations (0, 10, 50, 100 or 200 ppm) and durations (15, 30 or 45 min) of ZnO-NPs on stomatal conductance (a, b), internal CO2 concentration (c, d) and transpiration rate (e, f) in the leaves of Lycopersicon esculentum seedlings at 45 and 60 DAS respectively. All the data are the mean of five replicates (n = 5) and vertical bars shows standard errors (± SE)
Carbonic anhydrase and nitrate reductase activity
It is evident from Fig. 5a–d that leaf CA and NR activity were increased with the advancement of the age of the plant. It further increased with the lower (10 and 50 ppm) concentrations of ZnO-NPs at both the stages of growth. Maximum CA and NR activity were noted in the plants at 10 ppm of ZnO-NPs dipped in for 30 min which was 20% and 30% and 23% and 32% higher as compared to control respectively at 45 and 60 DAS.
Effect of different concentrations (0, 10, 50, 100 or 200 ppm) and durations (15, 30 or 45 min) of ZnO-NPs on carbonic anhydrase activity (a, b), nitrate reductase activity (c, d) and proline content (e, f) in the leaves of Lycopersicon esculentum seedlings at 45 and 60 DAS respectively. All the data are the mean of five replicates (n = 5) and vertical bars shows standard errors (± SE)
Protein content
Leaf protein content was also increased with the advancement of the age of the plant (Fig. 7a, b). It was further increased by the lower concentrations of ZnO-NPs (10 and 50 ppm) whereas maximum protein was recorded in the leaf of the plant grown in the presence of 10 ppm of ZnO-NPs dipped for 30 min. The higher concentrations proved to be inhibitory and decreased the level of protein.
Antioxidant enzymes
The activity of antioxidant enzymes (CAT, POX and SOD) were increased as the growth of the plant progressed from 45 to 60 DAS (Fig. 6a–f). These enzymes were increased further with the application of lower concentrations (10 and 50 ppm) of ZnO-NP. Maximum activities for CAT, POX, and SOD were generated when plants were grown in the presence of 10 ppm of ZnO-NPs dipped in 30 min irrespective of the days of sampling. These activities were increased by 55% and 60% (CAT), 54% and 63% (POX) and 52% and 59% (SOD) at 45 and 60 DAS respectively over their controls. Duration of soaking was non-significant in CAT and SOD whereas found significant difference in POX.
Effect of different concentrations (0, 10, 50, 100 or 200 ppm) and durations (15, 30 or 45 min) of ZnO-NPs on catalase activity (a, b), peroxidase activity (c, d) and superoxide dismutase activity (e, f) in the leaves of Lycopersicon esculentum seedlings at 45 and 60 DAS respectively. All the data are the mean of five replicates (n = 5) and vertical bars shows standard errors (± SE)
Proline assay
Plant raised with the root exposed to ZnO-NPs (10, 50, 100 or 200 ppm) had significantly more proline content than the control irrespective of the duration of dipping (Fig. 5e, f). Out of all these concentrations, 10 ppm of ZnO-NPs (30 min) proved best and enhanced the proline content by 14% and 16% as compared to their respective control at 45 and 60 DAS. A significant maximum decrease was recorded at 200 ppm of ZnO-NPs.
Number of fruits and fruit yield
The number of fruits and fruit yield were significantly improved by the ZnO-NPs. Lower concentrations (10 and 50 ppm) were more effective. The maximum values for both the parameters were recorded in the plants grown from the roots dipped for 30 min at 10 ppm and was about 28% (Number of fruits) and 30% (fruit yield) higher over the control (Fig. 7c,d).
Effect of different concentrations (0, 10, 50, 100 or 200 ppm) and durations (15, 30 or 45 min) of ZnO-NPs on protein content (a, b) in the leaves at 45 and 60 DAS, number of fruits (c), fruits yield (d), lycopene content (e) and β-carotene content (f) in the fruit of Lycopersicon esculentum seedlings at 180 DAS. All the data are the mean of five replicates (n = 5) and vertical bars shows standard errors (± SE)
Lycopene, β-carotene and ascorbic acid content
The content of lycopene, β-carotene, and ascorbic acid were significantly improved by the application of lower concentrations of ZnO-NPs (10 and 50 ppm). The maximum values for lycopene and β-carotene were recorded when the plant received 10 ppm of ZnO-NPs dipped for 30 min and was about 23% (Lycopene) and 26% (β-carotene) (Fig. 7e, f) more as compared to control. Contrary to lycopene and β-carotene, ascorbic acid followed a completely different pattern. Highest ascorbic acid content was found in the plant treated with 200 ppm of ZnO-NPs (Fig. 8).
Scanning electron microscope (SEM)
SEM analysis was carried out to find the effect of ZnO-NPs on the root of tomato plants. In the root of control plants, no attachment of NPs has been seen (Fig. 9a), whereas, root dipped for 30 min in 10 ppm of ZnO-NPs shows the clear attachment of NPs on the root surface (Fig. 9b). It might be possible that attachment of ZnO-NPs with root surface enhanced the physiological and biochemical parameters of the plants.
Discussion
The main processes that determine the quality and quantity of plant growth are cell division, enlargement, and differentiation. Growth regulating substances of the plant get involved through the alteration of transcription, translation and/or sensitivity of the tissue. In the present study plant exposed to the different concentration of ZnO-NPs (10, 50, 100 or 200 ppm), enhanced the values for all the growth traits which were reflected in the form of an increase in length, fresh and dry mass of root and shoot and also leaf area (Figs. 1, 2, 3a, b). This could be attributed to the multifaceted action of ZnO-NPs on vital processes like plant growth and development (Mukherjee et al. 2016). Application of ZnO-NPs enhanced the amount of Zn in plants (Garcia-Gomez et al. 2018) since Zn included as a micronutrient for plants and required for the synthesis of tryptophan which is a precursor of IAA (Brennan 2005). Few other findings also display the same results with a marked increase in biomass of the plant (Prasad et al. 2012; Raliya and Tarafdar 2013). Although, plant growth (shoot and root length, fresh and dry mass as well as leaf area) were strongly inhibited under high concentrations of ZnO-NPs (100 and 200 ppm). These results are in accordance with those of Chen et al. (2018), who found that growth of the plant significantly decreased in 100 and 200 ppm of ZnO-NPs treated Oryza sativa and with Wang et al. (2018), who reported ZnO-NPs exposure caused a reduction in growth and photosynthetic parameters in the tomato plant. The scanning electron micrograph of control and treated root also reveals the attachment of ZnO-NPs on its surface (Fig. 9) which may help to increase the performance of the tomato plants.
Nitrate reductase (NR) is the enzymes which catalyzed the NAD (P) H mediated reduction of nitrate (NO3−) into nitrite (NO2−) (Campbell 1999), to ensure the sufficient supply of nitrogen in the plants for the complete growth and yield (Srivastava 1995). The process of nitrate reduction depends on mainly three factors (a) substrate (nitrate) levels in the cytoplasm (b) the level of functional NR and/or (c) the activity level of functional NR. More importantly, we observed that various concentrations of ZnO-NPs through the root dipping enhance NR activity (Fig. 5c, d). The probable reason for this increase is an expression of the interaction of the acid with NR specific inhibitors whose presence is claimed by Srivastava (1980).
Application of ZnO-NPs enhanced the SPAD values in a concentration-dependent manner and highest level was noted in the plant treated with 10 ppm and 50 ppm, whereas the plant samples treated with a higher concentration (100 or 200 ppm) showed a lower level of chlorophyll (Fig. 3c, d). This is in accordance with the findings of Wang et al. (2018). According to Gurmani et al. (2012), application of Zn enhanced the chlorophyll content in tomato plants. Similar observations were also found in hydroponic culture, where the application of Zn improved the chlorophyll, protein, and mineral contents in Vigna radiata plant (Samreen et al. 2017). The mechanism at the back of increased chlorophyll content is the role of Zn as the important nutrient for the plant. Zn plays important role on plant metabolism via changing the action of vital enzymes, such as carbonic anhydrase. In the present study, CA activity was also increased by root dipping treatment of the 10 and 50 ppm of ZnO-NPs (Fig. 5a, b). It is in agreement of the other studies where ZnO-NPs application enhanced chlorophyll content (Prasad et al. 2012; Raliya and Tarafdar 2013; Mukherjee et al. 2016) and CA activity in different crop plants (Siddiqui et al. 2014; Faizan et al. 2018).
In plants, various factors regulate the process of photosynthesis and related attributes. The increase in photosynthetic attributes (Figs. 3e, f, 4) after the exposure of ZnO-NPs may be due to the increase of light absorbance which further helps to protect the chloroplast from aging and extend the photosynthetic time of the chloroplast which ultimately leads to the increased photosynthesis (Yang et al. 2006). Therefore, the above factor might have contributed to the increase in the rate of photosynthesis (Fig. 3e, f) which is in conformity with Yang et al. (2006), where the same result was found after using TiO2-NPs.
In plants, ROS is formed as a natural by-product of the normal metabolism of O2 and have promising roles in cell signaling and homeostasis (Ray et al. 2012). Reduction of O2 gives rise to ROS that includes the superoxide (O2−), singlet oxygen (1O2), hydroxyl radical (HO.) and hydrogen peroxide (H2O2). Imbalance in ROS causes oxidative stress, the higher formation of ROS damage to DNA, proteins and lipid and finally cell death (Tripathy and Oelmuller 2012). To overcome the toxic effect of oxidative stress plant activates enzymatic (CAT, POX, and SOD) and non-enzymatic (proline) antioxidants (Tripathy and Oelmuller 2012; Sewelam et al. 2016). In the present investigation, the level of antioxidant enzymes was also increased in the presence of ZnO-NPs (Fig. 6a, f). Findings of the present study are in coherence with the observations of Hu et al. (2013), where ZnO-NPs application enhances the activity of antioxidant enzymes. Moreover, lower concentration of ZnO-NPs also enhanced the antioxidant capacity in Brassica napus (Kouhi et al. 2015). Proline content in tomato leaves was also increased in the presence of ZnO-NPs. Accumulation of proline is the main factor that supports plant growth under challenging environments (Torabian et al. 2016). The enhancement in proline content of tomato leaf may be due to the expression of gene encoding key enzymes of proline synthesis that is controlled by stress (Torabian et al. 2016). In agreement with our results, proline content increased by ZnO-NPs in banana (Helaly et al. 2014).
In the present study, protein content in tomato significantly enhanced in the plants treated with 10 and 50 ppm of ZnO-NPs (Fig. 7a, b). Similarly, Krishnaraj et al. (2012) reported the positive role of Ag-NPs on an enhanced level of protein in Bacopa monnieri. Raliya and Tarafdar (2013) also reported increased protein content in cluster bean. Zinc plays an important role as a structural and catalytic component of protein and enzymes for normal growth and development of plant (Broadley et al. 2007). These conclusions are supported in the literature (Zhao et al. 2012; Faizan et al. 2018).
The fruit bearing capacity of the plant is mainly determined by the density of the flowers retained in the progenitor body (Zhao et al. 1987). Application of ZnO-NPs increased the growth of the tomato plants (Figs. 1, 2, 3a, b) which also leads to increase in yield parameters. The number of fruits and fruit yield in plants elevated through the application of ZnO-NPs (Fig. 7c, d), that might be due to the slowdowns of the process of senescence. This view was also supported by the study of Karuppanapandian et al. (2011), where Ag-NPs applications inhibit/decreased the process of senescence.
In tomato fruit, lycopene and β-carotene content are very important nutritional parameters. Fruits developed from the treatment of ZnO-NPs had an increased amount of both lycopene and β-carotene (Fig. 7e, f). The possible reason for this increase may be the increased level of chlorophyll by reducing the activity of chlorophyll degrading enzyme, cholorophyyllase (Benedetti and Arruda 2002). These pigments also enhanced by the increased synthesis of chlorophyll precursor, 5-aminolaevulinic acid. The present study supports the results of Kole et al. (2013), where carbon NPs increased the concentration of lycopene. However, the content of ascorbic acid in ZnO-NPs treated fruits significantly reduced (Fig. 8). It is believed that ZnO-NPs decreased the level of ascorbic acid due to the inhibitory effect of metal ions on enzyme activity of ascorbic acid metabolism and reduction of its pool due to the ROS neutralization and its use for plant growth and development, which was further supported by the results of Zelenchukova et al. (2015).
Conclusions
It can be deduced from the present findings that the application of ZnO-NPs through the root is helpful to increase the growth and yield of tomato plants and can be employed under field condition to confer the above findings.
Abbreviations
- CA:
-
Carbonic anhydrase
- CAT:
-
Catalase
- Ci:
-
Intercellular CO2 concentration
- DAS:
-
Days after sowing
- DDW:
-
Double distilled water
- E:
-
Transpiration rate
- gs:
-
Stomatal conductance
- mL:
-
Millilitre
- NO2− :
-
Nitrite
- NO3− :
-
Nitrate
- NP:
-
Nanoparticles
- NR:
-
Nitrate reductase
- PN :
-
Net photosynthetic rate
- POX:
-
Peroxidase
- ppm:
-
Part per million
- ROS:
-
Reactive oxygen species
- SEM:
-
Scanning electron microscopy
- SOD:
-
Superoxide dismutase
- ZnO-NPs:
-
Zinc oxide nanoparticles
References
Bates L, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39:205–207
Benedetti CE, Arruda P (2002) Altering the expression of the chlorophyllase Gene ATHCOR1 in transgenic arabidopsis caused changes in the chlorophyll-to-chlorophyllide ratio. Biochem Proc Macro Stress 128:1255–1263
Biswas P, Wu CY (2005) Nanoparticles and the environment. J Air Waste Manag Assoc 55:708–746
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brennan RF (2005) Zinc application and its availability to plants. Ph. D. Dissertation, School of Environmental Science, Division of Science and Engineering, Murdoch University
Broadley MR, White PJ, Hammond JP, Zelko I, Lux A (2007) Zinc in plants. New Phytol 173:677–702
Campbell WH (1999) Nitrate reductase structure, function and regulation: bridging the gap between biochemistry and physiology. Annu Rev Plant Physiol Mol Biol 50:277–303
Chen J, Dou R, Yang Z, You T, Gao X, Wang L (2018) Phytotoxicity and bioaccumulation of zinc oxide nanoparticles in rice (Oryza sativa L.). Plant Physiol Biochem 130:604–612
Dimkpa CO, Latta DE, McLean JE, Britt DW, Boyanov MI, Anderson AJ (2013) Fate of CuO and ZnO nano- and microparticles in the plant environment. Environ Sci Technol 47:4734–4742
Dwivedi RS, Randhawa NS (1974) Evaluation of rapid test for hidden hunger of zinc in plants. Plant Soil 40:445–451
Edison TJI, Sethuraman MG (2012) Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem 47:1351–1357
Faizan M, Faraz A, Yusuf M, Khan ST, Hayat S (2018) Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 56:678–686
Garcia-Gomez C, Garcia S, Obrador AF, Gonzalez D, Babin M, Fernandez MD (2018) Effects of aged ZnO NPs and soil type on Zn availability, accumulation and toxicity to pea and beet in greenhouse experiment. Ecotoxicol Environ Safety 160:222–230
Garcia-Sanchez S, Bernales I, Cristobal S (2015) Early response to nanoparticles in the Arabidopsis transcriptome compromises plant defence and root-hair development through salicylic acid signalling. BMC Genom 16:341
Gurmani AR, Khan SU, Andaleep R, Waseem K, Khan A (2012) Soil application of zinc improves growth and yield of tomato. Int J Agric Biol 14:91–96
Helaly MN, El-Metwally MA, El-Hoseiny H, Omar SA, El-Sheery NI (2014) Effect of nanoparticles on biological contamination of in vitro cultures and organogenic regeneration of banana. Aust J Crop Sci 8:612–624
Hu C, Liu Y, Li X, Li M (2013) Biochemical responses of duckweed (Spirodela polyrhiza) to zinc oxide nanoparticles. Arch Environ Cont Toxicol 64:643–651
Hussain I, Singh NB, Singh A, Singh H, Singh SC (2016) Green synthesis of nanoparticles and its potential application. Biotechnol Lett 38:545–560
Jaworski EG (1971) Nitrate reductase assay in intact plant tissue. Biochem Biophys Res Commun 43:1274–1279
Karuppanapandian T, Wang HW, Prabakaren N, Jeyalakshmi K, Kwon M, Manoharan K, Kim W (2011) 2, 4-dichlorophenoxyacetic acid-induced leaf senescence in mung bean (Vigna radiata L. Wilczek) and senescence inhibition by co-treatment with silver nanoparticles. Plant Physiol Biochem 49:168–177
Khan TA, Fariduddin Q, Yusuf M (2015) Lycopersicon esculentum under low temperature stress: an approach toward enhanced antioxidants and yield. Environ Sci Pollut Res 22:14178–14188
Kole C, Kole P, Randunu KM, Choudhary P, Podila F, Ke PC, Rao AM, Marcus RK (2013) Nanobiotechnology can boost crop production and quality: first evidence from increased plant biomass, fruit yield and phytomedicine content in bitter melon (Momordica charantia). BMC Biotechnol 13:37
Kouhi SMM, Lahouti M, Ganjeali A, Entezari MH (2015) Comparative phytotoxicity of ZnO nanoparticles, ZnO microparticles, and Zn2+ on rapeseed (Brassica napus L.): investigating a wide range of concentrations. Toxicol Environm Chem 96:861–868
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. plant growth metabolism. Process Biochem 47:651–658
Lin D, Xing B (2007) Phytotoxicity of nanoparticles: inhibition of seed germination and root growth. Environ Pollut 150:243–250
Lin D, Xing B (2008) Root uptake and phytotoxicity of ZnO nanoparticles. Environ Sci Technol 42:5580–5585
Lin S, Reppert J, Hu Q, Hudson JS, Reid ML, Ratnikova TA, Rao AM, Luo H, Ke PC (2009) Uptake, translocation, and transmission of carbon nanomaterials in rice plants. Small 5:1128–1132
Liu X, Wang F, Shi Z, Tong R, Shi X (2015) Bioavailability of Zn in ZnO nanoparticle-spiked soil and the implications to maize plants. J Nanopart Res 17:175
Madhumitha G, Elango G, Roopan SM (2015) Bio-functionalized doped silver nanoparticles and its antimicrobial studies. J Sol Gel Sci Technol 73:476–483
Mukherjee A, Peralta-Videa JR, Bandyopadhyay S, Rico CM, Zhao L, Gardea-Torresdey JL (2014) Physiological effects of nanoparticulate ZnO in green peas (Pisum sativum L.) cultivated in soil. Metallomics 6:132–138
Mukherjee A, Sun Y, Morelius E, Tamez C, Bandyopadhyay S, Niu G, White JC, Peralta-Videa JR, Gardea-Torresdey JL (2016) Differential toxicity of bare and hybrid ZnO nanoparticles in Green Pea (Pisum sativum L.): a life cycle study. Front Plant Sci 12:1242
Prasad TNVKV, Sudhakar P, Sreenivasulu Y, Latha P, Munaswamy V, Raja Reddy K, Sreeprasad TS, Sajanlal PR, Pradeep T (2012) Effect of nanoscale zinc oxide particles on the germination, growth and yield of peanut. J Plant Nutr 35:905–927
Raghuramulu N, Nair MK, Kalyanasundarum S (1983) A manual of laboratory techniques. National Institute of Nutrition, Silver Prints, Hyderabad
Raliya R, Tarafdar JC (2013) ZnO nanoparticle biosynthesis and its effect on phosphorous-mobilizing enzyme secretion and gum contents in cluster bean (Cyamopsis tetragonoloba L.). Agric Res 2:48–57
Ranganna S (1976) Manual of analysis of fruit and vegetable products. McGraw Hill, New Delhi, p 77
Ray PD, Huang BW, Tsuji Y (2012) Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal 24:981–990
Rout GR, Das P (2003) Effect of metal toxicity on plant growth and metabolism. Agronomie 23:3–11
Sadasivam S, Manickam A (1997) Biochemical methods. New Age International Publishers, New Delhi, pp 187–188
Samreen T, Shah HU, Ullah S, Javid M (2017) Zinc effect on growth rate, chlorophyll, protein and mineral contents of hydroponically grown mung beans plant (Vigna radiata). Arab J Chem 10:1802–1807
Schwab F, Zhai G, Kern M, Turner A, Schnoor JL, Wiesner MR (2016) Barriers, pathways and processes for uptake, translocation, and accumulation of nanomaterials in plants. Critical review. Nanotoxicology 10:257–278
Sewelam N, Kazan K, Schenk PM (2016) Global plant stress signaling: reactive oxygen species at the cross-road. Front Plant Sci 7:187
Siddiqui MH, Al-Whaibi MH, Faisal M, Al Sahli AA (2014) Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ Toxicol Chem 33:2429–2437
Srivastava HS (1980) Regulation of nitrate reductase activity in higher plants. Phytochem 19:72533
Srivastava HS (1995) Nitrate reductase. In: Srivastava HS, Sing RP (eds) Nitrogen nutrition in higher plants. Associated Publishing Co., New Delhi, pp 145–164
Torabian S, Zahedi M, Khoshgoftar AH (2016) Effects of foliar spray of two kinds of zinc oxide on the growth and ion concentration of sunflower cultivars under salt stress. J Plant Nutr 39:172–180
Tripathy BC, Oelmuller R (2012) Reactive oxygen species generation and signaling in plants. Plant Signal Behav 7:1621–1633
Wang XP, Li QQ, Pei ZM, Wang SC (2018) Effects of zinc oxide nanoparticles on the growth, photosynthetic traits, and antioxidative enzymes in tomato plants. Biol Plant 62:801–808
Whiteside MD, Treseder KK, Atsatt PR (2009) The brighter side of soils: quantum dots track organic nitrogen through fungi and plants. Ecology 90:100–108
Wild E, Jones KC (2009) Novel method for the direct visualization of in vivo nanomaterials and chemical interactions in plants. Environ Sci Technol 43:5290–5294
Yang F, Hong F, You W, Liu C, Gao F, Wu C, Yang P (2006) Influence of nano-anatase TiO2 on the nitrogen metabolism of growing spinach. Biol Trace Elem Res 110:179–190
Zafar H, Ali A, Ali JS, Haq IU, Zia M (2016) Effect of ZnO nanoparticles on Brassica nigra seedlings and stem explants: growth dynamics and antioxidative response. Front Plant Sci 7:535
Zelenchukova NS, Ivanytskyy AE, Tayupova RR (2015) Features of growth and development of Eruca sativa under the polyethylene film low emission cover based on copper and silver compounds. Bull TSPU 2:122–127
Zhao YJ, Luo WH, Wang YQ, Xu RJ (1987) Retarding effects of brassinolide on maturation and senescence of hypocotyls segments of mung bean seedlings. Acta Physiol Sinica 13:129–135
Zhao L, Peng B, Hernandez-Viezcas JA, Rico C, Sun Y, Peralta-Videa JR, Tang X, Niu G, Jin L, Varela A, Zhang J, Gardea-Torresdey JL (2012) Stress response and tolerance of Zea mays to CeO2 nanoparticles: cross talk among H2O2, heat shock protein, and lipid peroxidation. ACS Nano 6:9615–9622
Acknowledgements
The authors are thankful to Chairman, Department of Botany, Aligarh Muslim University, India for providing the necessary facilities for conducting the experimental work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
No conflict of interest among the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faizan, M., Faraz, A. & Hayat, S. Effective use of zinc oxide nanoparticles through root dipping on the performance of growth, quality, photosynthesis and antioxidant system in tomato. J. Plant Biochem. Biotechnol. 29, 553–567 (2020). https://doi.org/10.1007/s13562-019-00525-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-019-00525-z