Abstract
The present study is the first account of in vitro carotenoid pigment induction in Cleome rosea, a Brazilian herbaceous species frequently found near the coast in ecosystems submitted to intense anthropic degradation. Micropropagated plants obtained from in vitro roots were used as source of internodal explants for callus cultures. The callus cultures were induced on MS medium supplemented with different concentrations of 2,4-dichlorophenoxyacetic acid (2,4-D) and 4-amino-3,5,6-trichloropicolinic acid (picloram) in the light or in the dark. The use of different culture media (B5, Nitsch and White) supplemented with 2,4-D was also evaluated. Although callogenesis was obtained in all treatments, a high production of biomass was achieved from the cultures that were maintained in light. The highest biomass accumulation was reached by cultures established on MS medium supplemented with 0.2 mg L−1 2,4-D. The exposure of cultures to light was an essential factor for carotenoid production. Pigment induction was observed on calli maintained in all tested media, and the highest pigment yield was obtained by cultures established on MS medium with 0.2 mg L−1 2,4-D. Callus cultures were subjected to treatments with elicitors (chitosan, methyl jasmonate, and yeast extract) at different concentrations and exposure times. The highest pigment production was achieved on cultures treated with 60 mg L−1 methyl jasmonate (MeJa), which resulted in a six-fold increase in the carotenoid yield when compared to non-elicited cultures. A chromatographic analysis showed that the addition of MeJa induced β-carotene production. Elicited and non-elicited calli were used to establish cell suspension cultures. These cultures were evaluated during three subsequent subcultures, showing an increased production of carotenoids during the first subculture. This study showed that in vitro cultures of C. rosea, especially after elicitation, may become an efficient alternative source of carotenoids indicating the success of plant tissue culture techniques for secondary metabolite production.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carotenoids are yellow to orange-red tetraterpenoid pigments that are distributed in some non-photosynthetic bacteria and fungi and in all photosynthetic organisms (Tanaka et al. 2008). Carotenoids are valuable compounds with many functions in the nutraceutical, cosmetic and food industries (Jaswir et al. 2011). These pigments have long been used as food colorants and, due to their strong antioxidant properties, they have a broad range of functions regarding human health, including the prevention of chronic diseases, such as cardiovascular diseases and cancer (Fraser and Bramley 2004; Kopsell and Kopsell 2006; Bélanger and Johns 2008). The economic demand for these pigments is growing, and it is estimated that the global market of carotenoids has reached US$ 1.2 billion in 2010, with a chance to grow to US$ 1.4 billion in 2018 (BCC Research 2011). The most useful carotenoids are obtained by chemical synthesis or extracted from plants, and most of the biotechnological processes used to obtain these pigments are based on the cultivation of microorganisms, such as yeast and microalgae (Dufossé 2009). However, the increasing demand for natural pigments and the challenge of producing these compounds on an industrial scale have led to an intense search for new production strategies. In this context, production by plant cell culture techniques becomes a viable alternative system. In fact, many in vitro protocols have been successfully established from a great variety of plant species, especially considering the production of pharmaceutically important compounds (Collin 2001; Rao and Ravishankar 2002; Simões et al. 2012; Wilson and Roberts 2012). Moreover, many studies have been undertaken to find practical approaches to increase the yield of secondary metabolites under in vitro conditions. Some of these approaches include the use of elicitors, which involves exposing cell cultures to biotic stress factors or to compounds that are regarded as signaling molecules in plant stress responses (Karuppusamy 2009; Aijaz et al. 2011).
The genus Cleome comprises herbaceous annual or perennial plants and shrubs that are widely distributed in tropical and subtropical regions. Many of these species are used in traditional medicine and have been investigated with respect to their pharmacological and phytochemical aspects (Aparadh et al. 2012). Cleome rosea is a Brazilian herbaceous species frequently found near the coast in ecosystems submitted to intense anthropic degradation. The medicinal potential of C. rosea has been investigated (Simões et al. 2006, 2010a; Simões-Gurgel et al. 2012), and biotechnological studies performed using this species have been efficient in developing in vitro propagation protocols (Simões et al. 2004, 2009a, b) and also in establishing callus and cell suspension culture producers of anthocyanic pigments (Simões et al. 2009b; Simões-Gurgel et al. 2011). Due to the promising results already achieved with C. rosea, the present work aimed to develop in vitro carotenoid-producing cell lines and to optimize the pigment production by applying elicitation methods.
Materials and methods
Plant material
In vitro propagated plants of C. rosea previously established from root segments were used as an explant source (Simões et al. 2009a). These plants were kept on MS medium (Murashige and Skoog 1962) with 30 g L−1 sucrose devoid of growth regulators (MS0). The medium was adjusted to a pH of 5.8 before adding agar (8 g L−1), autoclaved at 121 °C for 15 min and dispensed (30 mL) into flasks (11 × 5 cm) closed with polypropylene caps. For subcultures, the apical portion (2 cm) was excised and inoculated in fresh MS0 medium. The flasks were maintained in a growth chamber at 26 ± 2 °C under a 16 h photoperiod provided by cool white fluorescent tubes (45 μmol m−2 s−1). After approximately 30 days in culture, these plants were used to initiate the callus cultures.
Callus cultures
The cultures were initiated from internodal explants (0.5 cm) inoculated on MS medium supplemented with 30 g L−1 sucrose and with the auxins 2,4-dichlorophenoxyacetic acid (2,4-D) or 4-amino-3,5,6-trichloro-2-pyridinecarboxylic acid (picloram) at concentrations of 0.2, 0.5, 1.0, 5.0 and 10.0 mg L−1. The culture flasks were incubated in a growth chamber at 26 ± 2 °C in the dark or under a 16 h photoperiod (45 μmol m−2 s−1). In a second set of experiments, the callogenesis process was evaluated using the B5 (Gamborg et al. 1968), Nitsch (Nitsch and Nitsch 1969) and White (White 1934) basal culture media. These media were supplemented with 20 g L−1 sucrose and 2,4-D at the same concentrations previously used. These cultures were incubated in a growth chamber at 26 ± 2 °C under light (16 h photoperiod/45 μmol m−2 s−1). All experiments were performed using 15 flasks containing four explants per treatment and were repeated twice. The callus biomass accumulation was estimated after 60 days of culture based on fresh (FW) and dry (DW) weight. The dry mass was obtained after drying at 45 °C to a constant weight.
Carotenoid extraction, identification and quantification
Extraction of carotenoids was conducted according to Jacques et al. (2009) with small modifications. Briefly, 2 g callus samples were ground with cold acetone (25 mL). The mixture was agitated for 10 min, followed by filtration using Whatman No. 1 filter paper. The filtrate was transferred into a separation funnel and partitioned with petroleum ether (20 mL). To remove the acetone, the filtrate was washed with distilled water (100 mL) and the lower phase was discarded. The procedure was repeated twice. The petroleum ether layer was filtrated using Whatman No. 1 filter paper covered with 5 g of anhydrous sodium sulfate to remove residual water. The petroleum ether extracts were pooled, and the volume was adjusted to 25 mL with petroleum ether.
The extracts were submitted to spectrophotometric analysis (300–600 nm) using a UV–vis spectrophotometer (Shimadzu UV – 160, Japan). After this first analysis, the absorbance was measured at 450 nm to determine the total carotenoid content using the following formula:
where A Absorbance at 450 nm, V Total extract volume, P Sample weight and E1%1cm Extinction coefficient of β-carotene in petroleum ether = 2592.
For the HPLC analysis, the extracts were evaporated until dryness under reduced pressure using a rotary vacuum evaporator (Markoni, Brazil) at 40 °C, lyophilized and then resuspended in acetone PA (1 mg mL−1). The analysis was performed using a Dionex HPLC system (Dionex Ultimate 3000, Sunnyvale, California USA) coupled with a photodiode array detection (DAD) system, a Rheodyne auto-sampler and the Chromeleon software package. The samples were analyzed using an Acclaim C18 column (250 × 4.6 mm i.d., 5 μm particle size) with an injection volume of 20 μL and column temperature of 29 °C. The flow rate was 2 mL min−1, and the detection was at 450 nm. The HPLC analysis utilized a mobile phase of methanol:acetonitrile:ethyl acetate (80:10:10 v/v/v) (Campos et al. 2003). The chromatograms were compared with an authentic β-carotene standard.
Elicitation
To determine the effect of the elicitation treatments on the biomass accumulation and production of carotenoids, three different elicitors were tested at different concentrations. Yeast extract (YE) was dissolved in deionized water and used at concentrations of 200 and 400 mg L−1. Methyl jasmonate (MeJa) was first dissolved in a few drops of dimethylsulfoxide, diluted with deionized water and then applied at concentrations of 20, 40 and 60 mg L−1. Chitosan (CS) was dissolved in 2 % (v/v) acetic acid, diluted with deionized water and then used at 1.0, 2.5 and 5.0 mg L−1. Sixty-day-old-calli with the highest biomass accumulation and production of carotenoids according to the first series of experiments were used in this assay. The elicitor solutions were added to the culture media and calli were maintained in contact with the elicitors for 7 or 14 days, and then transferred to fresh medium without these substances. Biomass accumulation (FW and DW) and total carotenoid (TC) production, expressed as μg β-carotene g−1, were evaluated 60 and 90 days after the elicitation treatment. The experiments were performed using 15 flasks containing four calli per treatment and was repeated twice.
Establishment of cell suspension cultures
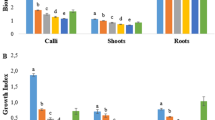
Cell suspension cultures (CSC) were initiated from non-elicited and elicited callus cultures established in the cultures conditions that resulted in the highest biomass accumulation and production of carotenoids. The CSC were obtained by transferring 1.5 g of friable calli (1.50 ± 0.10 g FW/0.12 ± 0.06 g DW) to 50 mL Erlenmeyer flasks containing 25 mL of liquid medium with the same composition used for the callus culture. The flasks were closed with double-aluminum caps and maintained on a shaker (New Brunswick Scientific, Enfield, Connecticut, USA) (110 rpm) at 26 ± 2 °C with a 16 h photoperiod (45 μmol m−2 s−1). To determine the growth pattern, the cells were harvested by filtering through a nylon mesh (45 μm) using a filter unit (Nalgene™ Cat. No. 320-2533, Kansas City, Missouri, USA) connected to a vacuum pump (GAST Manufacturing, Rochester, Indiana, USA). The biomass accumulation was estimated by measuring the FW and DW. Cells were collected every 4–5 days during a 43 day period, and each point of the growth curve represents the mean of three independent determinations (flasks). After establishing the growth curve, the CSC were evaluated at the exponential growth phase for three subcultures. During each subculture, the biomass accumulation (FW and DW) and total carotenoid content (TC) were determined as previously described. All experiments were performed using ten flasks per treatment and was repeated twice.
Statistical analysis
The experiments were organized in a completely randomized design and repeated twice. Data were analyzed using a one-way analysis of variance (ANOVA), and the differences among the means were tested using the Tukey test at a 5 % level of significance. The analyses were performed using the statistical software GraphPad Prism 5.
Results and discussion
Callus cultures
The callogenic response in cultures maintained on MS medium supplemented with 2,4-D or picloram was observed in the first week through the swelling of the explants with subsequent production of friable and non-organogenic calli. This response was initially observed at the cut end that was in direct contact with the culture medium, and the response was subsequently observed throughout the whole explant. The callogenic process was positively affected by light (Fig. 1a).
Callus cultures of C. rosea. a Cultures established on MS medium supplemented with 0.2 mg L−1 2,4-D in the light or dark after 60 days in culture; b Cultures established on MS medium supplemented with 10.0 mg L−1 2,4-D in the light or dark after 60 days in culture; c Callus produced on MS + 0.2 mg L−1 2,4-D showing the production of pigment in its surface after 3 weeks in culture; d Rhizogenic callus produced on B5 medium supplemented with 0.2 mg L−1 2,4-D; e Organogenic callus showing the development of buds (arrows) produced on Nitsch medium supplemented with 0.5 mg L−1 2,4-D. Bars a = 1.4 cm; b = 1.4 cm; c = 0.7 cm; d = 0.8 cm; e = 0.94 cm
The use of 2,4-D at lower concentrations (0.2, 0.5, 1.0 mg L−1) resulted in the highest production of callus biomass, mainly on medium supplemented with 0.2 mg L−1 2,4-D (Fig. 2). On the other hand, cultures established on media supplemented with 5.0 and 10.0 mg L−1 2,4-D exhibited a significant reduction in callus growth (Fig. 1b). This inhibitory effect can be related to a herbicide-like activity of 2,4-D, as reported by Wernicke and Milkovits (1984). The presence of high concentrations of 2,4-D also inhibited callus growth on cultures of Litchi chinensis (Mesquita et al. 2003) and Salyx humboldtiana (Santos et al. 2005). The inhibitory action caused by the high concentrations of 2,4-D was not observed on cultures established on media with picloram when this phytoregulator was used at high concentrations (Fig. 2).
Biomass accumulation (fresh and dry weight) in callus cultures of C. rosea maintained on MS medium supplemented with different concentrations of 2,4-D or picloram after 60 days of culture in the presence of light. Asterisks over the bars of the same color in each graph indicate that the means are not significantly different by the Tukey test at 5 %
The calli maintained under light produced an orange pigment on their surface after the third week in culture (Fig. 1c). The spectrophotometric analysis of the extracts prepared with these calli presented maximum absorption bands at approximately 451 nm and 478 nm, which is a characteristic for the absorbance spectra of carotenoids. The HPLC-DAD analysis of calli obtained on MS medium supplemented with 0.2 mg L−1 2,4-D showed the presence of three peaks (Fig. 3a) that were characteristic for the absorption spectra of carotenoids, with maximum absorption bands between 452 nm and 475 nm (Peak 1), 451 nm and 478 nm (Peak 2) and between 452 nm and 477 nm (Peak 3). Peak 3 was identified as β-carotene after the co-injection of callus samples with the β-carotene commercial standard, resulting in an increase in the peak area intensity (Fig. 3b).
HPLC-DAD profiles of callus samples of C. rosea. a Callus maintained on MS medium supplemented with 0.2 mg L−1 2,4-D; b Co-injection of callus samples with the β-carotene commercial standard, showing an increase in the intensity of peak 3; c Callus maintained on MS medium supplemented with 0.2 mg L−1 2,4-D after 60 days of elicitation with methyl jasmonate (60 mg L−1/7 days). Peak numbers refers to carotenoids as reported in the text
Based on the results obtained on MS medium, new assays were performed to evaluate the influence of different basal culture media on callus growth associated with carotenoid induction. Considering the previous results, the cultures were maintained under light and the media were supplemented with different concentrations of 2,4-D. Although the callogenic process was achieved in all treatments, organogenic calli were obtained in cultures maintained on B5 and Nitsch media. The calli cultured on B5 medium showed the production of roots (Fig. 1d), whereas they formed buds on the Nitsch medium (Fig. 1e). The cultures maintained on White medium resulted in non-organogenic calli. However, compared to the cultures established on B5 and Nitsch media, calli produced on White medium achieved the lowest biomass accumulation (Fig. 4). As verified on MS medium, the callus cultures maintained on B5, Nitsch and White media achieved the highest biomass contents at the lowest concentrations of 2,4-D (Fig. 4) and showed the induction of carotenoids on their surface.
Biomass accumulation (fresh and dry weight) in callus cultures of C. rosea maintained on B5 (a), Nitsch (b) and White (c) culture media supplemented with different concentrations of 2,4-D after 60 days of culture in the presence of light. Asterisks over the bars in each graph indicate that the means are not significantly different by the Tukey test at 5 %
The callus cultures of C. rosea established on MS medium supplemented with 2,4-D exhibited the greatest biomass accumulation associated with the carotenoid production. Although only a few studies have demonstrated the induction of carotenoids by plant cell tissue culture strategies, most of them used the 2,4-D, such as in cell suspension cultures of Vaccinium ashei (Nawa et al. 1993), callus and cell suspension cultures of Barringtonia racemosa (Behbahani et al. 2011) and callus cultures of Calendula officinalis (Legha et al. 2012). The in vitro induction of carotenoids has also been achieved on culture media supplemented with 2-(4-chlorophenyl-thio) triethylamine hydrohloride (CPTA), such as in cell suspension cultures of tomatoes (Fosket and Radin 1983; Rhodes et al. 1991; Engelmann et al. 2010).
The light, which was an important factor for callus growth, was essential for carotenoid induction on callus cultures of C. rosea because the production of pigments on the callus surface was only observed under this condition. It is well-known that light participates in the regulation of carotenoid biosynthesis (Bramley 2002; Simkin et al. 2003; Pizarro and Stange 2009). The presence of light was also important for lycopene synthesis induction in callus and cell suspension cultures of Barringtonia racemosa (Behbahani et al. 2011). In Citrus callus, effect of light on carotenoid biosynthesis varied with different genotypes and, although light regulates the expression of several carotenogenesis genes, its presence may not necessarily result in significant changes in carotenoid production (Gao et al. 2011).
Elicitation
To optimize the production of carotenoids, calli developed under the culture conditions selected as the most efficient for the high biomass accumulation of friable and non-organogenic calli, i.e., MS medium supplemented with 0.2 mg L−1 2,4-D and maintained in light, were exposed to different elicitors.
The elicitation process was an efficient strategy for optimizing the carotenoid production in callus cultures of C. rosea, regardless of the elicitor used. Table 1 presents the effect of chitosan (CS) on the total carotenoid (TC) accumulation. The highest TC accumulation 60 days after the elicitation process was reached by calli treated with 5 mg L−1 CS for 7 days (7.91 ± 2.07 μg g−1). The carotenoid content was three-times greater than the pigment production obtained with non-elicited control cultures (1.37 ± 0.06 μg g−1). The TC content 90 days after the elicitation continued to increase, mainly on cultures treated with 1 mg L−1 and 5 mg L−1 CS. In addition, to effectively optimize the production of carotenoids, the elicitation process did not result in significant changes in the biomass accumulation.
When yeast extract (YE) was used as an elicitor, an increase in the carotenoid content was achieved 60 days after elicitation (Table 1). The maximum TC accumulation was observed in cultures exposed to 200 mg L−1 YE over 14 days (8.66 ± 0.81 μg g−1 vs. 3.56 ± 1.16 μg g−1 in the control cultures). Nevertheless, the production was reduced after 90 days in culture. Unlike CS, a longer exposure time (14 days) to YE resulted in an increased production of pigments. However, as previously observed with the CS treatment, the elicitation process did not significantly influence the biomass accumulation.
Elicitation with methyl jasmonate (MeJa) was the most effective treatment in optimizing the carotenoid production (Table 1). A maximum TC accumulation after 60 days of elicitation was obtained when 60 mg L−1 MeJa was added to the cultures, independently of the exposure time (13.62 ± 1.87 μg g−1 and 13.59 ± 1.34 μg g−1, after 7 and 14 days, respectively). These values represent a six-fold increase in pigment production when compared to the non-elicited control cultures (1.89 ± 1.04 μg g−1). Although the cultures exhibited a reduction in carotenoid content after 90 days of elicitation with MeJa, the productivity levels remained higher, especially in cultures treated with 60 mg L−1 MeJa for 7 days (9.43 ± 3.54 μg g−1). As already observed with the other elicitors tested, the treatment with MeJa did not result in significant changes in the callus growth.
The kinetics of carotenoid accumulation varied with the different elicitors applied to callus cultures of C. rosea. The highest yield was achieved 60 days after treatment in cultures exposed to YE and MeJa. The use of CS resulted in a higher production 90 days after treatment. As reported by Vasconsuelo and Boland (2007), although the classes of metabolites induced by the elicitation process depend on the plant species, the kinetics of induction and/or accumulation levels vary with the different elicitors.
In the present work, the elicitor exposure time was not a relevant factor in inducing the carotenoid accumulation. Vasconsuelo and Boland (2007) reported that the time required for maximum secondary metabolite accumulation is characteristic of each plant species and is normally preceded by an increase in activity of the involved metabolic enzymes. The exposure time, like the elicitor concentration, must be empirically determined for each culture.
The biomass accumulation in callus cultures of C. rosea was not influenced by the elicitors used. However, elicitation can lead to significant changes in growth rates. A significant reduction in the biomass accumulation was observed after elicitation with MeJa on callus cultures of Taxus x media var. Hatfieldii (Furmanowa et al. 1997) and hairy roots of cotton (Frankfater et al. 2009). In contrast, an increase in biomass was achieved in cell suspension cultures of Hypericum perforatum after the addition of jasmonic acid (Walker et al. 2002).
The chromatographic analysis of extracts obtained from the elicited calli treated with MeJa (60 mg L−1 for 7 days) revealed a significant increase in the β-carotene content (Fig. 3c) when compared to the non-elicited calli (Fig. 3a, peak 3). The identification was confirmed after the co-injection of callus samples with a β-carotene commercial standard. However, the peaks related to the other two carotenoids, previously observed on the non-elicited calli (Fig. 3a, peaks 1 and 2), were not detected in the elicited cultures. The use of MeJa also led to qualitative changes in the alkaloid pattern produced by somatic hybrid cell suspension cultures of Rauwolfia serpentina x Rhazya stricta (Sheludko et al. 1999).
Cell suspension cultures
The CSC were established from non-elicited and elicited (60 mg L−1 MeJa for 7 days) calli. Regardless of the callus source, the cell suspensions were characterized by a 4-day lag phase followed by an exponential phase lasting until day 24 of culture, with the most significant growth occurring between days 14 and 18. The stationary phase began after day 24 (Fig. 5) and was followed by a gradual reduction in cell density. Despite the lower yield achieved when compared to the callus cultures, the CSC presented an increase in the biomass accumulation during the subcultures, achieving a two-fold increase at the third subculture (Table 2). The CSC initiated from elicited calli exhibited the greatest production of carotenoids at the third subculture, although the TC accumulation did not show significant differences when compared to cultures initiated from non-elicited calli.
The results from the present work show that in vitro cultures of C. rosea, especially after elicitation, may become an efficient alternative source of carotenoids. Therefore, the developed protocol could be used as a suitable system for the in vitro production of such pigments.
Abbreviations
- B5:
-
Gamborg medium
- CSC:
-
Cell suspension culture
- CS:
-
Chitosan
- 2,4-D:
-
2,4-dichlorophenoxy acetic acid
- MeJa:
-
Methyl jasmonate
- MS:
-
Murashige and Skoog medium
- Picloram:
-
4-amino-3,5,6-trichloropicolinic acid
- TC:
-
Total carotenoid
- YE:
-
Yeast extract
References
Aijaz A, Jain S, Hariharan AG (2011) Effect of elicitation on the production of phyto-constituents through plant tissue culture technique—a review. Int J Drug Discov Herb Res 1:84–90
Aparadh VT, Mahamuni RJ, Karadge BA (2012) Taxonomy and physiological studies in spider flower (Cleome species): a critical review. Plant Sci Feed 2:25–46
BCC Research (2011) The global market of carotenoids. BCC Research, Wellesley
Behbahani M, Shanehsazzadeh M, Hessami MJ (2011) Optimization of callus and cell suspension cultures of Barringtonia racemosa (Lecythidaceae family) for lycopene production. Sci Agric 68:69–76
Bélanger J, Johns T (2008) Biological diversity, dietary diversity, and eye health in developing country populations: establishing the evidence-base. EcoHealth 5:244–256
Bramley PM (2002) Regulation of carotenoid formation during tomato fruit ripening and development. J Exp Bot 53:2107–2113
Campos FM, Pinheiro-Sant’ana MH, Stringheta PC, Chaves JBP (2003) Levels of beta carotene in leafy vegetables prepared in commercial restaurants in Viçosa, Brazil. Braz J Food Technol 6:163–169
Collin HA (2001) Secondary product formation in plant tissue cultures. Plant Growth Regul 34:119–134
Dufossé L (2009) Microbial and microalgal carotenoids as colourants and supplements. In: Britton G, Liaanen-Jensen S, Pfander H (eds) Carotenoids. Volume 5. Nutrition and health. Birkhauser Verlang, Basel, pp 83–98
Engelmann NJ, Campbell JK, Rogers RB, Rupassara SI, Garlick PJ, Lila MA, Erdman JW (2010) Screening and selection of high carotenoid producing in vitro tomato cell culture lines for [13C]-carotenoid production. J Agric Food Chem 58:9979–9987
Fosket DE, Radin DN (1983) Induction of carotenogenesis in cultured cells of Lycopersicon esculentum. Plant Sci Lett 30:165–175
Frankfater CR, Dowd MK, Triplett BA (2009) Effect of elicitors on the production of gossypol and methylated gossypol in cotton hairy roots. Plant Cell Tissue Organ Cult 98:341–349
Fraser P, Bramley P (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Furmanowa M, Gowniak K, Sykowska-Baranek K, Zgórka G, Józefczyk A (1997) Effect of picloram and methyl jasmonate on growth and taxane accumulation in callus culture of Taxus x media var. Hatfieldii. Plant Cell Tissue Organ Cult 49:75–79
Gamborg OL, Miller RA, Ojima K (1968) Nutrient requirements of suspension cultures of soybean root cells. Exp Cell Res 50:151–158
Gao H, Xu J, Liu X, Liu B, Deng X (2011) Light effect on carotenoids production and expression of carotenogenesis genes in citrus callus of four genotypes. Acta Physiol Plant 33:2485–2492
Jacques AC, Pertuzatti PB, Barcia MT, Zambiazi RC (2009) Bioactive compounds in small fruits cultivated in the southern region of Brazil. Braz J Food Technol 12:123–127
Jaswir I, Noviendri D, Hasrini RF, Octavianti F (2011) Carotenoids: sources, medicinal properties and their application in food and nutraceutical industry. J Med Plants Res 5:7119–7131
Karuppusamy S (2009) A review on trends in production of secondary metabolites from higher plants by in vitro tissue, organ and cell cultures. J Med Plants Res 3:1222–1239
Kopsell DA, Kopsell DE (2006) Accumulation and bioavailability of dietary carotenoids in vegetable crops. Trends Plant Sci 11:499–507
Legha MR, Prasad KV, Singh SK, Kaur C, Arora A, Kumar S (2012) Induction of carotenoid pigments in callus cultures of Calendula officinalis L. in response to nitrogen and sucrose levels. In Vitro Cell Dev Biol Plant 48:99–106
Mesquita AC, Paiva R, Gomes AC, Paiva PDO, Artiaga EJ, Santos CG, Paiva LV (2003) Efeito do 2.4-D e ANA na formação de calos em explantes foliares de lichieira. Rev Ceres 50:595–601
Murashige T, Skoog F (1962) A revised medium for rapid growth and biossays with tobacco tissue cultures. Physiol Plant 15:473–497
Nawa Y, Asano S, Motoori S, Ohtani T (1993) Production of anthocyanins, carotenoids, and proanthocianins by cultured cells of rabbiteye blueberry (Vaccinium ashei Reade). Biosci Biotechol Biochem 5:770–774
Nitsch JP, Nitsch C (1969) Haploid plants from pollen grains. Science 163:85–87
Pizarro L, Stange C (2009) Light-dependent regulation of carotenoid biosynthesis in plants. Cienc Inv Agr 36:143–162
Rao SR, Ravishankar GA (2002) Plant cell cultures: chemical factories of secondary metabolites. Biotechnol Adv 20:101–153
Rhodes MJ, Spencer A, Hamill JD (1991) Plant cell culture in the production of flavour compounds. Biochem Soc Trans 19:702–706
Santos BR, Paiva R, Martinotto C, Nogueira RC, Paiva PDO (2005) Induction of friable callus in leaf explants of Salix (Salyx humboldtiana Willd). Ciênc Rural 35:510–514
Simkin AJ, Zhu C, Kuntz M, Sandmann G (2003) Light–dark regulation of carotenoid biosynthesis in pepper (Capsicum annuum) leaves. J Plant Physiol 160:439–443
Simões C, Albarello N, Callado CH, Castro TC, Mansur E (2010a) Somatic embryogenesis and plant regeneration from callus cultures of Cleome rosea Vahl. Braz Arch Biol Technol 53:679–686
Simões C, Albarello N, Callado CH, Castro TC, Mansur E (2009a) New approaches for shoot production and in vitro root cultures of Cleome rosea Vahl. Plant Cell Tissue Organ Cult 98:79–86
Simões C, Albarello N, Castro TC, Mansur E (2012) Production of anthocyanins by plant cell and tissue culture strategies. In: Orhan IE (ed) Biotechnological production of plant secondary metabolites. Bentham Science Publishers, pp 67–86
Simões C, Bizarri CHB, Castro TC, Coutada LCM, Silva AJR, Albarello N, Mansur E (2009b) Anthocyanin production in callus cultures of Cleome rosea: modulation by culture conditions and characterization of pigments by means of HPLC-DAD/ESIMS. Plant Physiol Biochem 47:895–903
Simões C, Castro TC, Cordeiro LS, Albarello N, Mansur E, Romanos MTV (2010b) Antiviral activity of Cleome rosea extracts from field-grown plants and tissue culture-derived materials against acyclovir-resistant Herpes simplex viruses type 1 (ACVr-HSV-1) and type 2 (ACVr-HSV-2). World J Microbiol Biotechnol 26:93–99
Simões C, Mattos JCP, Sabino KCC, Caldeira-de-Araújo A, Coelho MGP, Albarello N, Figueiredo SFL (2006) Medicinal potencial from in vivo and acclimatized plants of Cleome rosea Vahl ex DC. (Capparaceae). Fitoterapia 77:94–99
Simões C, Santos AS, Albarello N, Figueiredo SFL (2004) Shoot organogenesis and plantlet regeneration from stem explants of Cleome rosea Vahl (Capparaceae). J Plant Biotechnol 6:199–204
Simões-Gurgel C, Cordeiro LS, Castro TC, Callado CH, Albarello N, Mansur E (2011) Establishment of anthocyanin-producing cell suspension cultures of Cleome rosea Vahl ex DC. (Capparaceae). Plant Cell Tissue Organ Cult 106:537–545
Simões-Gurgel C, Rocha AS, Cordeiro LS, Gayer CRM, Castro TC, Coelho MGP, Albarello N, Mansur E, Rosa ACP (2012) Antibacterial activity of field-grown plants, in vitro propagated plants, callus and cell suspension cultures of Cleome rosea Vahl. J Pharm Res 5:3304–3308
Sheludko Y, Gerasimineko I, Unger M, Kostenyuk I, Stoeckigt J (1999) Induction of alkaloid diversity in hybrid plant cell cultures. Plant Cell Rep 18:911–918
Tanaka Y, Sasaki N, Ohmiya A (2008) Biosynthesis of plant pigments: anthocyanins, betalains and carotenoids. Plant J 54:733–749
Vasconsuelo A, Boland R (2007) Molecular aspects of the early stages of secondary metabolites in plants. Plant Sci 172:861–875
Walker TS, Bais HP, Vivanco JM (2002) Jasmonic acid-induced hypericin production in cell suspension cultures of Hypericum perforatum L. (St. John’s wort). Phytochemistry 60:289–293
Wernicke W, Milkovits L (1984) Developmental gradients in wheat leaves: response of leaf segments in different genotypes cultured in vitro. J Plant Physiol 115:49–58
White PR (1934) Potentially unlimited growth of excised tomato root tips in a liquid medium. Plant Physiol 9:585–600
Wilson SA, Roberts SC (2012) Recent advances towards development and commercialization of plant cell culture processes for the synthesis of biomolecules. Plant Biotechnol J 10:249–268
Acknowledgments
The authors are grateful to Dr. Ivan G. Ribeiro of Rio de Janeiro State University for his valuable comments that improved the text and to Maria Francisca Santoro Assunção for technical assistance. This research work was supported by Fundação Carlos Chagas Filho de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Silva da Rocha, A., Rocha, E.K., Alves, L.M. et al. Production and optimization through elicitation of carotenoid pigments in the in vitro cultures of Cleome rosea Vahl (Cleomaceae). J. Plant Biochem. Biotechnol. 24, 105–113 (2015). https://doi.org/10.1007/s13562-013-0241-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-013-0241-7