Abstract
Mungbean yellow mosaic India virus (MYMIV) causing yellow mosaic disease in grain legumes belongs to the genus Begomovirus of the family Geminiviridae. The virus has a bipartite genome comprising two circular single stranded DNA components (DNA A and DNA B) and is transmitted by the whitefly, Bemisia tabaci Genn. DNA A encodes for coat protein on the viral strand and for replication associated proteins and transcription activator proteins in the complementary strand. The coat protein plays key role in whitefly transmission. In the present communication, the regions in coat protein that govern whitefly transmission and systemic spread were mapped by constructing deletion mutations. Results showed that inoculation with viral constructs having N′ terminal deletion of 75 and 150 amino acids affected systemic spread and pathogenicity in cowpea, mungbean and blackgram plants contrasting to French bean which developed symptoms similar to wild type. Assembly of particles and whitefly transmission were not seen in all the mutations. However there was minimum, six folds increase in viral DNA levels in French bean plants inoculated with N′ terminal deletions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yellow mosaic disease caused by Mungbean yellow mosaic India virus (MYMIV) and Mungbean yellow mosaic virus (MYMV) is a major constraint in improving productivity of grain legumes. These two viruses belong to the Genus Begomovirus, family Geminiviridae which encompass members having characteristic twinned geminate particles encapsidating one circular single stranded DNA genome. Members of the genus Begomovirus infect dicotyledonous plants and are transmitted by the vector whitefly, Bemisia tabaci Genn. Majority of New World begomoviruses and some Old World begomoviruses like MYMIV have two genomic components, referred to as DNA A and DNA B. DNA A encodes for coat protein gene (ORF AV1) on the viral strand and replication associated protein (ORF AC1 and AC3) and transcription activator protein (ORF AC2) on the complementary strand. DNA B encodes for proteins facilitating export/import of viral genome into nucleus (ORF BV1) and cell to cell movement (ORF BC1) of viral genome.
In all the geminiviruses, there is only one type of coat protein that forms the twinned structure (Böttcher et al. 2004; Kittelmann and Jeske 2008). Investigation on the fine structure revealed (Zhang et al. 2001) that the CP subunits are arranged with quasi-icosahedral symmetry with 110 copies of CP fitted in pentamer capsomeres. The tertiary structure was modelled as an eight stranded β barrel motif with N′ terminal α helix. The authors suggested that the N′ terminal helix was involved in maintaining particle structure encapsidating genomic DNA.
The coat protein in geminiviruses is a multifunctional protein; besides encapsidation the three major functions of viral pathogenicity affected by coat protein are, viral DNA replication, intra, inter cellular movement and long distance transport of viral genome and the most important function being vector mediated transmission.
Begomoviruses are transmitted in a circulative and non propagative manner, by only one species of whitefly, Bemisia tabaci Genn. There is extreme conservation in the virion particles to bring about specific recognition and interaction between the virion particles and the vector. CP replacement studies (Briddon et al. 1989) between leaf hopper transmitted Beet curly top virus and the whitefly transmitted African cassava mosaic virus and mutation studies (Etessami et al. 1989; Klinkenberg et al. 1989) clearly established the importance of CP in the whitefly transmission.
However, exactly the domains in coat protein that would interact with the vector is not yet identified for many begomoviruses of economic importance. It is especially relevant in the context of current attempts to develop transgenic resistance against viruses: transgenic resistance through RNAi approach targeting domains in CP governing transmission is looked upon as an alternative strategy to manage the disease. The present study attempts to identify the region of coat protein involved in transmission, for the important yellow mosaic virus, MYMIV.
In order to decipher the domains of coat protein of MYMIV that play a role in whitefly transmission, we have constructed four deletion mutants (two N′ terminal and two C′ terminal) within the virion-sense ORF AVI and carried out infectivity test by agroinoculation (Mandal et al. 1997).
Results showed that in all the four deletions, particle formation and whitefly transmission were affected. However there was considerable viral DNA accumulation and symptom expression in French bean.
Materials and methods
Plasmid constructs
Full length clones of blackgram isolate Mungbean yellow mosaic India virus- [India:NewDelhi:Blackgram 3:1991] [MYMIV-[IN:ND:Bg3:91] (GenBank Acc No. DNA-A: AF126406 and DNA-B: AF142440), were used in this analysis.
Deletion mutagenesis
Coat protein gene in MYMIV is 692 nucleotides long and spans from nucleotide coordinate 398 to 1090. The strategy to generate deletion mutation was as follows. The primers were designed to generate two N′ terminal (AVI-nΔ75 and AVI-nΔ150) and two C′ terminal (AVI-cΔ57 and AVI-cΔ108) deletions. The details of the primers (Operon), number of nucleotides expected to be deleted and the respective co-ordinates in the viral genome are given in Table 1.
From the clone of DNA-A in pUC18 vector, a 1.8 kb fragment of DNA A representing origin of replication and replication associated ORF in the complementary strand were restricted as HindIII/KpnI fragment and ligated to pBin19 vector restricted with HindIII/KpnI to get pBinA0.6mer.
The nested deleted fragments of different sizes were generated as represented in Fig. 1 and the fragments were PCR amplified from the DNA-A clone of MYMIV (GenBank Acc. No. DNA-A: AF126406) using appropriate combination of specific primers and cloned in pGEMT-Easy vector. These CP gene fragments (580 bp, 358 bp of N′ terminal and 751 bp, 601 bp of C′ terminal) were released from pGEMT-Easy vector as BamHI/SacI fragment which was ligated in pUC18 having 1.8 kb partial fragment of the same DNA-A clone to generate ~2.7 kb genome. This ~2.7 kb genome with deletion mutations were released from pUC18 and ligated to HindIII restricted pBinA0.6mer, to make an infectious partial tandem repeat (PTR) constructs.
These clones were confirmed through colony PCR, restriction digestion and sequencing (ABI prism automatic sequencer, Delhi University, South Campus).
Agroinoculation
The PTR constructs of full length DNA A (pBinA1.6mer) and DNA B (pBinB1.5mer) of MYMIV as described in Surendranath et al. (2005) were used as wild type control. Partial tandem viral constructs of AVI mutants of DNA A of MYMIV and wild type DNA A and DNA B of MYMIV, were mobilized from E. coli strain DH5α to Agrobacterium tumefaciens strain EHA105 using pRK 2013 as a helper plasmid through tri parental mating system.
Agroinoculation was done on 2 days old sprout-seeds of French bean (Phaseolus vulgaris, cv. Selection 9), cowpea (Vigna unguiculata, cv. Pusa Komal), mungbean (V. radiata, cv. Pusa Baisakhi) and blackgram (Vigna mungo cv. T-9). Agroinoculated plants were maintained at National Phytotron Facility (NPF), IARI, in growth chambers set at 28–29°C temperatures, 80% to 90% relative humidity and 22000 lux. Symptom expression was monitored for 30 days.
Genomic DNA extraction and Southern analysis
Total nucleic acid was extracted from the third trifoliate leaves, 21 days post agroinoculation (dpi) from healthy, symptomless and diseased plants by Gem-CTAB method (Rouhibakhsh et al. 2008). Viral replicative forms were analysed by extracting DNA from third trifoliate leaves, DNA was pooled from three plants and about 5 μg of total nucleic acid was electrophoresed in 1.2% agarose gel, and DNA forms were transferred to charged nitrocellulose membrane (NCM Millipore). Southern blot analysis was performed using radiolabelled coat protein (CP) gene for the detection of DNA A components and movement protein (MP) gene for the detection of DNA B component of MYMIV genome. [α-32P]-dCTP labeled CP and MP fragments were prepared by random primer labelling method (Feinberg and Vogelstein 1984). Blots were washed with 2X SSC and 0.1% SDS three times, and signals were detected by phosphorimaging using a Storage Phosphor System, Cyclone Plus (Perkin Elmer).
Whitefly transmission
Non-viruliferous whitefly Bemisia tabaci, cultures were raised from single egg of whitefly and maintained on tobacco (Nicotiana tobaccum cv. Xanthi), brinjal (Solanum melongena) seedlings in an insect proof cage. The seedlings of host plants were replaced at monthly intervals in order to maintain the culture of whiteflies.
The role of ORF AVI of MYMIV in the vector transmission was studied by conducting whitefly transmission in glass house condition using wild type or mutant agroinoculated plants as inoculum source. Inoculations were done on French bean, cowpea, mungbean and blackgram using the respective agroinoculated plants 21 dpi as source of inoculum. Transmission was done with 24 h of acquisition access period (AAP) and inoculation access period (IAP).
Western blot analysis
The protein extraction was carried out as described by Wang et al. (2006). One gram of leaf sample was used as starting material for the detection of the coat protein and protein pellet was finally resuspended in 100 μl SDS sample buffer having 50 mM Tris–HCl (pH 6.8), 2% SDS, 10% glycerol, 1% β-mercaptoethanol, 12.5 mM EDTA. Protein concentrations were determined using spectrophotometer (Nano drop) at 280 nm absorbance and further processed for sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting.
SDS-PAGE and Western blot analysis
Protein sample of 5–10 μg was resolved by 12% SDS-PAGE and were blotted using semi-dry electroblot instrument (ATTO) on nitrocellulose membrane. The western blot was carried out as described by Sambrook and Russel (2001). The transfer was done in the presence of 39 mM glycine, 48 mM Tris base, 0.037% SDS and 20% methanol, at a constant voltage of 14 V/96 mA for 1 h. Coat protein detection was done by using a polyclonal antibody to Squash leaf curl virus (SLCV), alkaline phosphatase-conjugated goat anti-rabbit IgG in 1X PBS with 2% BSA. The colour of protein-antibody complex was developed by using 150 mg/ml nitroblue tetrazolium (NBT) and 75 mg/ml 5-Bromo-4-chloro-3’-indolyl phosphate (BCIP).
ISEM (Immunosorbent electron microscopy)
The immunosorbent electron microscopy was carried out as described by Derrick (1973). The carbon coated grids (carbon side down) were floated over a drop of 10μl of diluted (1:1000) polyclonal antibody in phosphate buffer (pH 6.5, 0.07 M). The drop was put on a piece of dental wax or parafilm placed in humid petriplate and left for 1 h at 37°C. Grids were washed for 10–15 min in phosphate buffer, drained briefly and placed over a drop of 10 μl of leaf extract, left for 30 min at room temperature. Grids were removed, washed with approximately 20 drops of double distilled water and stained with two to four drops of 2% freshly prepared uranyl acetate solution. Grids were drained to dry and examined under EM at 18,000 to 20,000 magnifications. French bean (cv. Sel-9) plants agroinoculated and whitefly transmitted with wild type and mutants were used for immunosorbent electron microscopic observation of virus particles.
Results
Infectivity of coat protein mutants
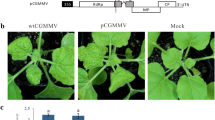
French bean plants agroinoculated with wild type MYMIV, DNA A and DNA B, showed very distinct symptoms of severe stunting and downward leaf curling of cotyledonary leaves. The percentage infection was nearly 100%. The stunting was so severe that shoot elongation was not seen and plants remain aborted in growth and eventually died. The trifoliate leaves if produced showed puckering and were reduced in size (Fig. 2).
In the case of mungbean and blackgram, the plants inoculated with wild type, showed uniformly distributed yellow mosaic in the very first trifoliate leaf 10–12 days post inoculation (dpi). In cowpea, the blackgram isolate of MYMIV produced atypical symptom of leaf curl, darkening of leaves and asymmetry of leaflets, 7–10 dpi.
In French bean, both N′ terminal mutants (AVI-nΔ75 and AVI-nΔ150) and C′ terminal mutants (AVI-cΔ57 and AVI-cΔ108) showed stunting, downward leaf curling and puckering which were less compared to wild type and attenuation of symptoms were prominent. In the case of N′ terminal deletion, mutants produced sparsely distributed yellow mosaic and reduction of leaf lamina.
Unlike wild type the percent infectivity was less in all mutants, in French bean it was 84.9%, 88.5%, 71.4%, 68.3% in the mutants AVI-nΔ75, AVI-nΔ150, AVI-cΔ57, AVI-cΔ108 respectively; in mungbean it was 75.6%, 60% in N′ terminal mutants AVI-nΔ75, AVI-nΔ150 respectively. Contrastingly in plants agroinoculated with C′ terminal mutants AV1-cΔ57 and AVI-cΔ108 symptoms were not seen (Table 2). Cowpea and blackgram plants inoculated with N′ terminal and C′ terminal mutants did not produce any symptom.
Southern blot analysis
Replicative forms of DNA A and DNA B components were studied using coat protein and movement protein gene as a probe. Marked difference was seen in the replicative forms in French bean plants.
When coat protein gene was used as probe, one very interesting results in the case of French bean plants inoculated with mutants AV1-nΔ75 and AVI-nΔ150 was seen. The double stranded (ds), open circular (oc), linear (lin) and super coiled (sc) DNA levels were much higher than wild type, whereas ss DNA was comparatively less than wild type. In plants inoculated with mutants AVI-cΔ57 and AVI-cΔ108 there was no detectable replicative forms of DNA (Fig. 3). In the case of cowpea, mungbean and blackgram plants inoculated with mutants, viral DNA forms could not be detected.
Southern blot analysis of viral replicative forms in different legume hosts agroinoculated with wild type and mutants. Lane 1: 2.7 kb linear DNA as positive control; lane 2: healthy as negative control; lane 3: wild type inoculated French bean; lane 4: wild type inoculated cowpea; lane 5: wild type inoculated mungbean; lane 6: wild type inoculated blackgram; lane 7: mutant AVI-cΔ108 inoculated French bean showing absence of replicative forms of DNA; lane 8: mutant AV1-nΔ150 inoculated French bean (oc, lin and sc DNA levels were much higher than wild type). Radiolabelled coat protein gene used as probe
When movement protein gene was used as probe, there was a clear difference in French bean between different replicative forms and level of DNA B. Single strand DNA level was higher in wild type when compared to AV1-nΔ75 and AV1-nΔ150 in French bean whereas the other replicative forms (oc, lin and sc) of DNA were negligible. While the level of ss DNA in C′ terminal deletions AV1-cΔ57 and AV1-cΔ108 were very less.
Whitefly transmission
Whitefly transmission from wild type agroinoculated plants was very high (88.2%) in French bean and 64% in mungbean. Whereas there was no symptom in all the four legume host plants inoculated with either N′ terminal or C′ terminal mutants as source.
Western blot analysis
Plants inoculated with wild type viral DNA expressed coat protein in readily detectable amounts in the western blot. Rather than the expected 29 kDa of coat protein, dimeric form ~58 kDa band was observed. The sample preparation buffer contained 2% SDS and 5% β mercaptoethanol and samples were boiled for 3 min before loading. There was no detectable protein in French bean agroinoculated or whitefly transmitted with any of the four N′ terminal and C′ terminal mutants, even though the agroinoculated plants were showing very good symptoms and the concentration of crude proteins extracted were also good.
Immunosorbent electron microscopy (ISEM)
French bean plant samples were used for western blot analysis and ISEM study because of high viral concentration compared to cowpea, mungbean and blackgram. Leaf samples from wild type agroinoculated and whitefly transmitted French bean plants, typical geminate particles were observed, whereas in all the four mutants geminate particles could not be seen.
Discussion
Mungbean yellow mosaic India virus affects different grain legumes species blackgram, mungbean, soybean and cowpea in northern and central India incurring considerable economic loss. Management options looked upon in recent years is to develop transgenics with viral derived sequence that may confer RNAi mediated resistance to virus infection. Coat protein or ORF AV1 is considered as ideal candidate gene, as it is highly conserved in whitefly transmitted begomoviruses. Any construct targeting CP region is expected to give broad spectrum resistance to different viruses. Before we attempt to use the CP gene, it is necessary to understand its role in various functions of viral pathogenicity. In the present communication, results of MYMIV CP gene deletion mutation in viral pathogenicity are described. The coat protein is involved in four important functions of viral pathogenesis, replication, cell to cell movement, encapsidation and transmission.
Four deletion mutants of Mungbean yellow mosaic India virus, two in N′ terminal and two in C′ terminal region were generated to ascertain the role of AV1 in viral replication, movement, symptom development and whitefly transmission in four different legume hosts French bean, cowpea, mungbean and blackgram. All the four deletion mutations generated, AVI-nΔ75, AVI-nΔ150, AVI-cΔ57 and AVI-cΔ108 produced symptoms in French bean indicating that coat protein is not required for systemic spread and disease expression in French bean. In the case of mungbean plants in N′ terminal deletion AVI-nΔ75 was tolerated and symptom expression was seen but not with other mutants. In cowpea and blackgram, none of the mutants led to any symptom expression.
Further Southern blot analysis reveal viral replicative forms; in fact in the case of AV1-nΔ75, AVI-nΔ150, the open circular, linear and super coiled replicative forms were five to six folds higher than wild type.
Symptom expression and viral replication are considered to reflect the movement of the viral genome inside the plant. More the number of cells, the virus enters and interferes with regular metabolic status of the plants, the symptoms like yellow mosaic or yellow vein mosaic will be explicit. In this study, symptoms are seen in French bean for all the mutation, which is slightly attenuated, compared to wild type. It can be inferred from above study that cell to cell spread and long distance transport of viruses are not affected in French bean; it is not at all tolerated for infection in cowpea, mungbean and blackgram plants.
The CP of the monopartite begomoviruses mediates movement of viral DNA into the host cell nucleus and ORFs AC4, AV2 and CP co-ordinate movement of virus from cell to cell. In contrast, in bipartite begomoviruses, nuclear export/import and cell to cell spread are mediated by proteins ORF BV1 and BC1 encoded by DNA B. The role played by CP in the intracellular and intercellular movement of virus is very interesting in begomovirus species. However, there is difference in CP requirement for long distance transport, CP independent movement occurs in well adapted permissive host species (Gardiner et al. 1988; Padidam et al. 1995). Wherein non virion forms are transported by CP-vascular protein complex. However in non permissive species, CP is required (Wang et al. 1999). CP is required even in intra cellular movement for facilitating docking of viral genome onto nuclear pore by interacting with α-importin protein as shown for MYMV (Guerra-Peraza et al. 2005). Our results indicate that CP is absolutely essential to systemically spread and produce symptoms in cowpea and mungbean.
In southern blot analysis, absence of CP resulted in reduced level of single stranded DNA with concomitant increase in double stranded replicative form. This phenomenon has been observed in many geminiviruses (Azzam et al. 1994; Rigden et al. 1993). In geminivirus, replication is by rolling circle amplification method in which ss DNA is converted to ds DNA, ds DNA generates ssDNA and ss DNA again enters into replication cycle. Reduction in ssDNA level in mutants can be traced to loss of nuclear localization signals of CP and its inability to bind to ssDNA.
The DNA binding domain in CP is located in Zn finger motif between amino acid residues 1–62 and 65–85 (Kirthi and Savithri 2003; Unseld et al. 2004). It is hypothesized that in the deletion mutations binding domain have been lost resulting in absence of nuclear localization. On the contrary, the production of ss DNA itself might have been blocked, as shown by Malik et al. (2005), who showed, CP not only binds to Rep, it also down regulates nicking and cleaving activity of Rep.
Malik et al. (2005) reported that the central region of MYMIV Rep, including its oligomerization domain, interacts with MYMIV CP. Thus, CP might have a role in controlling the copy number of the geminiviral DNA. The CP not only binds to Rep, it also modulates nicking and closing activity of Rep. It is hypothesized that in the deletion mutation AVI-cΔ57 and AVI-cΔ108, Rep interacting domains are knocked out, CP is not able to interact with rep thereby resulting unregulated replication.
The results of Electron microscopy and whitefly transmission revealed that the deletion mutation affect the transcription and translation of coat-protein gene, therefore assembly of particles and whitefly transmission. In Western blot analysis too coat protein could not be detected due to extreme low concentration.
This preliminary study needs to be extended to finer level to exactly map the domains of CP involved in various events of pathogenicity.
Abbreviations
- MYMIV:
-
Mungbean yellow mosaic India virus
- SLCV:
-
Squash leaf curl virus
- ORF:
-
Open reading frame
- CP:
-
Coat protein
- PTR:
-
Partial tandem repeat
- CTAB:
-
Cetyl trimethyl ammonium bromide
- NBT:
-
Nitroblue tetrazolium
- BCIP:
-
5-Bromo-4-chloro-3’-indolyl phosphate
- ISEM:
-
Immunosorbent electron microscopy
References
Azzam O, Frazer J, Rosa DDL, Beaver JS, Ahlquist P, Maxwell DP (1994) Whitefly transmission and efficient ssDNA accumulation of bean golden mosaic geminivirus require functional coat protein. Virology 204:289–296
Böttcher B, Unseld S, Ceulemans H, Russell RB, Jeske H (2004) Geminate structures of African cassava mosaic virus. J Virol 78:6758–6765
Briddon RW, Watts J, Markham PG, Stanley J (1989) The coat protein of beet curly top virus is essential for infectivity. Virology 172:628–633
Derrick KS (1973) Quantitative assay for plant viruses using serologically specific electron microscopy. Virology 56:652–653
Etessami P, Watts J, Stanley J (1989) Size reversion of African cassava mosaic virus coat protein gene deletion mutants during infection of Nicotiana benthamiana. J Gen Virol 70:277–289
Feinberg AP, Vogelstein B (1984) A technique for radiolabelling DNA restriction endonucleases fragments to high specific activity. Anal Biochem 137:266–267
Gardiner WE, Sunter G, Brand L, Elmer JS, Rogers SG, Bisaro DM (1988) Genetic analysis of tomato golden modaic virus: the coat protein is not required for systemic spread or symptom development. EMBO J 74:899–904
Guerra-Peraza O, Kirk D, Seltzer V, Veluthambi K, Schit AC, Hohn J, Herzog E (2005) Coat proteins of Rice tungro bacilliform virus and Mungbean yellow mosaic virus contain multiple nuclear localisation signals interact with importin. J Gen Virol 86:1815–1826
Kirthi N, Savithri HS (2003) A conserved zinc finger motif in the coat protein of Tomato leaf curl Bangalore virus is responsible for binding to ssDNA. Arch Virol 148:2369–2380
Kittelmann K, Jeske H (2008) Disassembly of African cassava mosaic virus. J Gen Virol 89:2029–2063
Klinkenberg FA, Ellwood S, Stanley J (1989) Fate of African cassava mosaic virus coat protein deletion mutants after agroinoculation. J Gen Virol 70:1837–1844
Malik PS, Kumar V, Bagewadi B, Mukherjee SK (2005) Interaction between coat protein and replication initiation protein of Mungbean yellow mosaic India virus might lead to control of viral DNA replication. Virology 337:273–283
Mandal B, Varma A, Malathi VG (1997) Systemic infection of Vigna mungo using the cloned DNAs of the blackgram isolate mungbean yellow mosaic geminivirus through agroinoculation and transmission of the progeny virus by whiteflies. J Phytopathol 145:503–510
Padidam M, Beachy RN, Fauquet CM (1995) Tomato leaf curl geminivirus from India has a bipartite genome and coat protein is not essential for infectivity. J Gen Virol 76:25–35
Rigden JW, Dry IB, Mullineaux PM, Rezaian MA (1993) Mutagenesis of the virion-sense open reading frames of tomato leaf curl geminivirus. Virology 193:1001–1005
Rouhibakhsh A, Priya J, Periasamy M, Haq QMI, Malathi VG (2008) An improved DNA isolation method and PCR protocol for efficient detection of multicomponents of begomovirus in legumes. J Virol Meth 147:37–42
Sambrook J, Russel DW (2001) Molecular cloning, a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, New York
Surendranath B, Usharani KS, Victoria AK, Malathi VG (2005) Absence of interaction of genomic components and complementation between Mungbean yellow mosaic India virus isolates in cowpea. Arch Virol 150:1833–1844
Unseld S, Frischmuth T, Jeske H (2004) Short deletions in the nuclear targeting sequences of African cassava mosaic virus coat protein prevent geminivirus twined particle formation. Virology 318:89–100
Wang HL, Sudarshana M, Gilbertson RL, Lucas WJ (1999) Analysis of cell-to- cell and long-distance movement of a Bean dwarf mosaic geminivirus-green fluorescent protein reporter in host and nonhost species: identification of sites of resistance. Mol Plant Microb Interact 12:345–355
Wang W, Vignani R, Scali M, Cresti M (2006) A universal and rapid protocol for protein extraction from recalcitrant plant tissues for proteomic analysis. Electrophoresis 27:2782–2786
Zhang W, Olson NH, Baker TS, Faulkner L, Agbandje-McKenna M, Boulton MI, Davies JW, McKenna R (2001) Structure of the Maize streak virus geminate particle. Virology 279:471–477
Acknowledgement
The financial support given by Indian Council of Agricultural Research “ICAR network project on transgenic in crops, cotton and soybean, Code no.21–25” Government of India is gratefully acknowledged. We are thankful to Dr RK. Jain, Division of Plant Pathology and Dr HS. Gupta, Director, Indian Agricultural Research Institute, New Delhi-110012, India, for providing necessary facilities. We are also grateful to the scientists and staff at National Phytotron Facility, Indian Agricultural Research Institute, New Delhi-110012, India for their guidance and support to grow plants under controlled conditions.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haq, Q.M.I., Jyothsna, P., Ali, A. et al. Coat protein deletion mutation of Mungbean yellow mosaic India virus (MYMIV). J. Plant Biochem. Biotechnol. 20, 182–189 (2011). https://doi.org/10.1007/s13562-011-0044-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13562-011-0044-7