Abstract
In the last two decades, several noninvasive physical methods based on the interactions of electromagnetic radiation with matter have been developed to evaluate the food quality. Some of these methods uses ionizing radiation or radiation that strongly interacts with chemical compound limiting the analysis to the food surface. Nuclear magnetic resonance (NMR) is based on radiofrequency radiation, so it does not use ionizing radiation and is not strongly attenuated by food or package composition; therefore, it can be used in noninvasive analysis of intact and packaged foods. Although high-resolution NMR spectroscopy and imaging have been used in food analysis, they are usually based on expensive and bulky instruments that limit their practical application. Therefore, most noninvasive NMR-based analyses of intact food have been performed using low-cost, low-field, time-domain NMR instruments (TD-NMR). Instrumental requirements, pulse sequences, applications and limitation of TD-NMR in noninvasive analysis of fresh and industrialized food products will be reviewed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Brazil is one of the largest agricultural producers and exporters in the world and the sector is a significant part of national economy. Therefore, there is an urgent demand for rapid, accurate and nondestructive or noninvasive analytical methods for quality control and quality assurance of products and process, from the farmer to the consumers.
In the last two decades, several noninvasive physical methods based on the interactions of electromagnetic and sound waves with matter have been used to evaluate the food quality [1]. Some of these methods uses the electromagnetic waves from ultraviolet to infrared regions and are rapid and simple, but most of them are based on surface analysis, restricting the applications to monitor internal quality parameters. In nuclear magnetic resonance (NMR) spectroscopy, relaxometry or imaging methods, that uses very low energy electromagnetic waves, in the radiofrequency range, it is not strongly attenuated by biological tissues and can be used for internal nondestructive analysis of intact fresh and industrialized food products, direct in the glass, plastic, paper and other nonmetallic packages.
Although high-resolution NMR spectroscopy and imaging have been used in food analysis, they are usually based on expensive and bulky instruments that limit their practical application in farms, packing houses and industries. Thus, most of noninvasive NMR analyses of intact food have been performed using low-field (B0 < 0.7 T), low-resolution (\(\Delta B\) > 100 ppm) magnets, known as low-resolution or time-domain NMR instruments (TD-NMR).
In this paper, a review of the fundamental theory, instrumental requirements, pulse sequences, applications and limitation of TD-NMR in noninvasive analysis of fresh and industrialized food products is present.
2 Nuclear Magnetic Resonance
In 1938, Isidor Rabi made the first observation of the nuclear magnetic resonance (NMR) phenomenon in molecular beams and was awarded by Physics Nobel Prize in 1944. In 1946, Felix Bloch and Edward Mills Purcell independently observed the NMR phenomenon in condensed matter and shared the Nobel Prize in Physics in 1952 [2]. Since then, various applications were developed in physics, chemistry, biology and medicine and three other Nobel prizes have been awarded to applications of NMR in chemistry, Richard Ernst in 1991 and Kurt Wuthrich 2002 and medicine, Paul Lauterbur and Peter Mansfield in 2003.
Nowadays, there are several equipment and methods to observe the effects of nuclear magnetism, with different characteristics and spectral resolution; thus, NMR applications can be divided in some groups, as: i) high-resolution NMR (HR-NMR) spectroscopy, including special techniques and instrumentation for analysis of solid or liquids samples, mainly focuses on determination of chemical structural information, ii) NMR tomography or magnetic resonance imaging (MRI), specially used in medicine applications; and iii) low magnetic field (< 1 T) time-domain NMR (TD-NMR), which has no spectral or spatial resolution and is based mainly on the relaxation measurements.
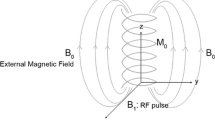
The NMR signal is observed when a sample containing a nucleus with spin \(\overrightarrow{I}\) >0 (nuclear magnetic moment \(\overrightarrow{\mu }=\gamma \hslash \overrightarrow{I}\)) is placed in a static magnetic field \({\overrightarrow{B}}_{0}={B}_{0}\widehat{z}\), resulting in the Zeeman splitting [2, 3], and is irradiated with an oscillating magnetic field (B1) with the Larmor frequency given by Eq. 1.
where \(\gamma\) is the magnetogyric ratio which is a constant for each isotope. Therefore, NMR is an isotope-selective technique since the values of \(\gamma\) differ significantly among the nuclei.
For 1H, nuclear spin ½, the Zeeman energy levels α and β have a spin population rate of
where \({N}_{\alpha }\) represents the population at the lower energy level, \({N}_{\beta }\) represents the population at the higher energy level, k is Boltzmann’s constant, \(\hslash =h/2\pi\) is the Planck’s constant, T is temperature (Kelvin), and \(\mathrm{\Delta E}={E}_{\beta }-{E}_{\alpha }={\hslash \gamma B}_{0}\).
Equation (2) shows that there is a small excess of spin population at the lower energy level, which produces a macroscopic magnetization \({\overrightarrow{M}}_{0}=\sum \overrightarrow{\mu }\) in the sample, parallel to the magnetic field direction \({\overrightarrow{B}}_{0}\). Irradiating the sample with a pulsed oscillating magnetic field (Pulsed NMR spectroscopy), \({\overrightarrow{B}}_{1}={B}_{1}cos(\omega t+\varphi )\), at the resonance frequency, and perpendicular to the direction of \({\overrightarrow{B}}_{0}\), the macroscopic magnetization \({\overrightarrow{M}}_{0}\) can be rotated to a direction perpendicular to the applied pulse. After the pulse, the \({\overrightarrow{M}}_{0}\) precess and returns to the \({\overrightarrow{B}}_{0}\) direction, which can be detected as a respond signal induced in the probe coil, a time-domain voltage signal, so-called free induction decay (FID).
In the context of TD-NMR applications, the isotope 1H is usually the choice to be observed, due the high natural abundance of 99.98%, high sensibility in comparison with other nuclei, due to its largest \(\gamma =26.752 \cdot {10}^{7} rad .{T}^{-1} .{s}^{-1}\), so in an external magnetic field of 1 Tesla, the Larmor frequency \((\upsilon ={\omega }_{0}/2\pi )\) is 42.578 MHz; the spins transition in the energy levels can be done with radiofrequency (rf) pulses in the megahertz range (MHz).
3 NMR Instruments for Noninvasive Analysis in Intact Food Products
Figure 1 shows a block diagram of a NMR equipment composed by: i) static magnetic field B0 to split the Zeeman levels; ii) transmitter to produce the radiofrequency (rf) oscillating ( Larmor frequency) magnetic field pulses; to excite the transitions; iii) probe to converted the rf in oscillating B1 and detect the induced NMR signal from the sample; iv) receiver to amplify and digitalized the NMR signal [4, 5].
The magnet field strength (B0), homogeneity (ΔB0) and the coil ring down are the main experimental factors that limit the applications of a NMR instrument. High-cost superconducting magnets generate the most intense and homogeneous magnetic field; however, for industrial application the cost of the instrument and its maintenance is prohibitive for general application, so permanent magnets have been the obvious choice [6]. Recently, middle resolution benchtop NMR spectrometer based in 1 to 2.5 T permanent magnets has been developed and used to obtain chemical information based on chemical shift and others spectral parameters [7–10]. However, these high magnetic fields are obtained in small gap or bore, limiting the applications to liquids or solutions with 5 mm in diameter that restricts the applications in noninvasive analysis of intact food products. Therefore, the noninvasive food analysis is based on NMR instruments with permanent magnets with wider bore of gap, with B0 < 0.7 T and ΔB0 > 100 ppm. These instruments have been used to measure signal intensity that is proportional to concentration of single products or the relaxation and diffusion measurements that give information about food physical and chemical properties.
Figure 2 shows five designs of permanent magnets used in TD-NMR. Figure 2a and b present the classical “H” and “C” shaped magnets built with a yoke made with soft or low carbon content steel. Figure 2c shows the yokeless Halbach magnet composed with several blocks of permanent magnets in specific orientation. The individual magnet blocks can be moved for a mechanical shim of the magnetic field and has null magnetic field outside the magnet. Halbach is normally the magnet with smallest volume and mass because there is no need for steel yoke. Figure 2d shows the hollow cylindrical magnetic (HCM) with an external soft, low carbon steel. This magnet is composed by a single permanent magnet hollow cylinder with axial magnetization. It is a much simpler design than the Halbach but is limited to lower magnetic field [11].
Illustration of TD-NMR magnets with a) H-shaped b) C-shaped, c) Halbach, d) hollow cylinder magnet (HCM). In magnets a to d, the sample needs to fit in the bore or gap of the magnets, e) the unilateral geometry where the sample can be much bigger than the magnet. The analysis is performed in a spot few millimeters inside the sample
In all magnets of Fig. 2a to d, the sample has to be placed inside of the magnet with the probe coil and consequently limit the application in larger samples. Figure 2e shows one of the unilateral NMR sensors (magnet and probe coil) designs where the sample is not limited by the magnet/probe dimension. In this type of magnet, the sample is place in front of the sensor and a small volume of the sample at a defined distance is analyzed. The slice is selected by the resonance frequency that is proportional to B0. The unilateral magnets seem to be an ideal design for analysis of intact food products. However due to strong magnetic field gradient (in order of 107 T.m−1) [12], the unilateral sensor only detects the signal from a very small sample volume and consequently has very low intensity signal, then requiring long experiments to collect several single scans. It also can strong attract any ferromagnet material as it is an open magnet that is not safe for general applications.
4 NMR At Inhomogeneous Magnetic Field
As the TD-NMR instruments are based on permanent and inhomogeneous magnets (cheaper for industrial applications), the spectral resolution in general is not enough to observe the difference in the molecular chemical shift. In TD-NMR, the spectrum of any sample is always a single and broad line and the width is determined by magnetic field inhomogeneity. Therefore, the TD-NMR analysis is performed exploring the signal intensity, the relaxation times or diffusion which have been used in several food science applications.
The behavior of the net magnetization in the usual time-domain NMR experiments can be successful described by classical models [13, 14], where the magnetic moments \(\overrightarrow{\mu}\) vectors precess around the direction of \({\overrightarrow{B}}_{0}\) (z-direction), with the Larmor frequency \({\omega }_{0}\) (Eq. 1). A pulse of radiofrequency, \({\overrightarrow{B}}_{1}\), promotes the transitions of the nuclei spins in the energy levels, and in a macroscopic view, produces a rotation of the net magnetization that depends on the intensity B1, phase φ, frequency ω and time duration of the rf pulse tp, so the total magnetization can be rotated by a flip angle of \(\theta =\gamma {B}_{1}{t}_{p}\) from the z-axis.
A 90° pulse is obtained when the net magnetization is rotated to the xy plane, so after the pulse, the net magnetization precess with the Larmor frequency and returns to the thermal equilibrium position along the z-axis. This return occurs with the relaxation mechanisms described by T1 relaxation time.
The longitudinal relaxation time (T1) is related to process involving energy transfer from the spin to the lattice — also called spin–lattice relaxation times — and the transversal relaxation times (T2) does not depend on energy transfer to the lattice but is related to the loss of coherence of the magnetization in the xy plane [2] — also called spin–spin relaxation times. These relaxation processes can be estimated by the Bloch Eq. (15)
where M0 represents the component of the magnetization on thermal equilibrium, \({M}_{xy}\) is the component of the magnetization in the xy-plane, and \({M}_{z}\) is the component along the direction of the static magnetic field B0. It takes a time of 5T1 for 99.33% of the magnetization to reach the thermal equilibrium (\({\overrightarrow{M}}_{0}\widehat{z}\)).
Relaxation is a process that depends on the random spins interactions due random local fields (magnetic noise), so relaxation times are particularly sensitive to molecular motion (correlation time τc) [16]. Therefore, determination of T1 and T2 relaxation times provides information about the molecular mobility and can be correlated with viscoelasticity and mechanical properties, as viscosity, solid–liquid content, porosity, etc.
In the presence of an inhomogeneous magnetic field ΔB0, after an 90° pulse, the dephasing of the spins is accelerated due the differences in their resonance frequencies; thus, the total magnetization observed decays with an effective relaxation times T2* given by:
Figure 3a shows an illustration of a 90° pulse, followed by the dead time period (dt), and then by the NMR signal acquisition, that decays with \({T}_{2}^{*}\). The time evolution of the NMR signal after a single pulse is called free induction decay (FID), and the data acquisition only starts after a dead time, which arrives from the instrumental limitation and a consequence of the required time to open the receiver.
a) Free induction decay (NMR signal) after a 90° pulse. The dead time (dt) is approximately 10–20 μs in TD-NMR spectrometers. Signal acquisition starts after the dead time, and FID decays with time constant of T2*. b) Inversion recovery (IR) pulse sequence to measure T1 relaxation time. c) Spin-echo pulse sequence refocuses the spins phases caused by the external magnetic field inhomogeneity and produces an echo where the intensity matches the amplitude of the T2 decay curve. Appling several 180°y pulses spaced by τ times (CPMG sequence), the T2 curve can be obtained. d) pulsed field gradient—spin-echo pulse sequence
The basic pulse sequence to determine longitudinal T1 relaxation times is showed in Fig. 3b, that consists of a 180° pulse followed after a variable time τ by a 90° pulse to read the signal intensity. Performing these measurements in function of the τ time, the exponential T1 recovery signal can be obtained; this pulse sequence is called inversion recovery (IR). There are several others sequences to measure T1, and recently, we developed a single-shot one based on continuous wave free precession (CWFP) regime [17].
Spin-echo pulse sequence (90°—τ—180°—echo), Fig. 3c, used a 180° pulse to refocus the spins dephasing due to the external magnetic field inhomogeneity after the first excitation; therefore, it produces an echo with amplitude that depends only on T2 and not with ΔB0 (for short τ values) or T2*. The T2 in this sequence is also measured in function of a series of experiments with variable τ time. For liquid sample, in longer τ values the echo amplitude is also dependent of spin diffusion. However, Carr–Purcell, in 1954 [18], proposed a single-shot sequence composed by several 180° pulse ( 90°x—( τ—180°x—τ—echo)n) that can use only short τ times, reducing the effect of diffusion in echo amplitude. This sequence was improved by Meiboom–Gill, in 1958 [19], by shifting by 90° the phase of 180° pulses ( 90° x—(τ—180y°—τ—echo)n), being called CPMG that is the standard method to measure T2. Besides relaxation times, another important parameter that can be measured is the self-diffusion coefficient (D). Figure 3d shows the illustration of a pulsed field gradient — spin-echo (PFG-SE) pulse sequence. In this sequence, the pulsed magnetic field gradient accelerates the dephasing process and the echo amplitude does not depend only on T2, but also on D. Measuring the intensity of the echoes as function of PFG intensities, the diffusion coefficient can be determined [6]. These sequences can also be combined to perform two-dimension measurements to obtain correlation maps between the relaxation times and between relaxation times and diffusion [20,21,22].
5 TD-NMR Applications in Intact Food Products At Embrapa Instrumentation
Embrapa Instrumentation is one of the research centers of the Brazilian Agricultural Research Corporation (Embrapa) located at São Carlos/SP, Brazil, that was created by Professor Sérgio Mascarenhas. It has a long tradition in developing TD-NMR instruments and methods for analysis of agri-food products [22,23,24,25,26,27,28]. These research and developments are in line with the recent studies around the world [29–32].
Figure 4 shows pictures of the firsts TD-NMR spectrometers constructed at Embrapa Instrumentation in the late 1980s and early 1990s, under supervision of Professor Sérgio Mascarenhas. Figure 4a shows the first TD-NMR prototype built 1988. It was not controlled by computer and the maximum intensity of the NMR signal (spin echo) was measured using an analogue oscilloscope. Figure 4b shows the second version built in 1990 that included a computer for single datapoint acquisition and data analysis. This instrument was developed for the Department of Genetic of the Luiz de Queiroz College of Agriculture of the University of São Paulo (Esalq-USP). It was used to noninvasive analysis of the oil content in seeds and was used in the maize high oil breeding program [33]. Figure 4c presents the 1994 prototype that was fully controlled by a computer. The technology of this version was transfer to Gil Fabricação e Projetos Especiais company and the instrument in Fig. 4d is the photograph of the product placed in market in 1996. Since then, other TD-NMR instruments and methods were developed and transferred to Brazilian private companies. Actually, one of these companies ( Fine Instrument Technology — FIT [34]) have been commercializing TD-NMR for agroindustry applications in more than 20 countries [34, 35]. The major application of this instrument is the nondestructive analysis of oil content in palm fruits and in several by-products of palm oil industries [36], applications in high oleic acid peanuts breeding programs [37, 38] and Brazil nuts [39].
TD-NMR spectrometers constructed at Embrapa Instrumentation under supervision of Professor Sérgio Mascarenhas. a) Photograph of the first non-computerized TD-NMR constructed in 1988. b) Photograph of the second prototype built in 1990 that included a computer for single datapoint acquisition and data analysis. c) Photograph of the 1994 prototype, fully controlled by a computer. d) Photograph of the commercial TD-NMR instrument produced and commercialized by company Gil Fabricação e Projetos Especiais, in 1996
Figure 5a, c shows the Halbach permanent magnets, with 10 cm bores used in the noninvasive analysis of intact and processed food products at Embrapa Instrumentation. The longer TD-NMR Halbach magnet shown in Fig. 5c has a pre-polarizing magnet to speed up online measurement. Figure 5b shows the rear of the Halbach magnet of Fig. 5c, displaying some orange samples in a conveyor belt used for measurement in flux. The velocity of the conveyor belt in TD-NMR using the Halbach magnet of Fig. 5c is typically in the range of 10–30 cm/s. The sampling velocity depends on the length of pre-polarize magnet and T1 relaxation time of the samples. Figure 5d shows a unilateral TD-NMR magnet, which is able to detect the NMR signal from a spot some millimeter inside the sample.
a) to c) Halbach magnets with bore of 10 cm used in noninvasive analysis of intact and processed food products at Embrapa Instrumentation. b) Views of the rear of the Halbach magnet of Fig. 5c showing the samples in a conveyor belt for measurement in flux. d) Unilateral TD-NMR magnet that measure a spot some millimeter inside the sample
Using the magnets of Fig. 5, several noninvasive TD-NMR methods have been developed for the analysis of fresh and processed food products. We have developed TD-NMR methods for noninvasive analysis of fruits and vegetable measuring the total soluble content (related to the sugar content) in plums [40], oranges [41, 42] and tomatoes [43], differences in ripening process [44], enzymatic activity in cassava roots [45] and physiological disorder in mangoes [46]. In fresh meat, TD-NMR has been used to measured moisture and fat contents [47], meat color [48], aging [49], tenderness, water holding capacity in beef and physiological disorder (myopathies) in chicken breasts [50].
Figure 6 shows an example of the application of TD-NMR in noninvasive analysis of fresh food product. This figure shows the inverse Laplace transform (ILT) [51] of the CWFP-T1 signals of fresh beef samples with different content of intramuscular fat content (3%, 20% and 62%) named lean (LE), low fat (LF) and high fat (HF) samples, respectively, and a sample composed only by subcutaneous material, without meat (FA) containing 85% of fat. With these relaxation measurements, it is possible to quantify the fat content using the signal between 0.3 and 0.4 s (T1a) related to water (or nonfat content) and the signal at approximately 0.1 s (T1b) to fat content. It was shown that the fat content (FC) obtained by solvent extraction method and TD-NMR method is correlated with equation FC = 106B/(A + B) + 0.3, where A and B are the areas of T1a and T1b signals, respectively [49].
T1 distribution times obtained by CWFP-T1 pulse sequence. LE is the lean beef sample; LF is the low‐fat beef sample; HF is the high‐fat beef sample; and FA is the subcutaneous fat only sample. CWFP-T1 was acquired with 12,000 points, τ = 300 μs and 64 scans, resulting in a total experimental time of less than 4 min for each sample. Adapted from Publication [52], Copyright (2003), with permission from Wiley
TD-NMR has also been used for noninvasive analysis of processed food products direct in the commercial nonmetallic packages (glass, plastic, paper, etc.). Noninvasive TD-NMR methods to detect adulteration of olive oil with polyunsaturated cheaper oil like soybean oil [53], the fat content in commercial packaged mayonnaise [54] and salad dressing, that range from approximately 5 (light) to 50% (regular) moisture [54], dry matter content in mustard sauces [55] and total soluble content in jams and jellies [56] have been developed.
Figure 7 shows an example of the application of TD-NMR in package food. Figure 7 shows the principal component analysis (PCA) of CPMG signal of 37 commercial oil bottles of authentic olive oils (open circles); polyunsaturated corn, canola, sunflower and soybean oils (closed circle); and oils adulterated with polyunsaturated oil (open squares). The closed square in the middle of authentic olive oil is a hazelnut oil that has chemical composition similar to olive oils. PCA score plot of Fig. 7 shows three well-separated cluster assigned to authentic olive oil, adulterated olive oil, polyunsaturated oils and hazelnut oils.[53].
PCA score plot of the full T2 relaxation curves of (●) polyunsaturated, (○) olive, (◼) hazelnut and (◻) adulterated olive oil samples. Adapted from Publication [53], Copyright (2003), with permission from Sociedade Brasileira de Química
The Embrapa NMR group has also developed some new and fast pulses sequences for food analysis, based on continuous wave free precession (CWFP) regime. CWFP sequences have been applied to monitor liquid flow parameters [24], fast determination of oil content of seeds [25], fast determination of T1 and T2 relaxation times [26], reduction of probe heating problems in flow analysis [57]. Recently, two new and fast two-dimension (2D) sequences using single-shot CWFP-T1 sequence were proposed. These sequences have been used to obtain T1–T2 and T1–D correlation maps [20–22] of complex food samples.
6 Conclusions
This paper shows some of the applications of time-domain nuclear magnetic resonance (TD-NMR) in agro-food products that was introduced by Professor Sérgio Mascarenhas at Embrapa Instrumentation, São Carlos/SP. In the last decades, this research helped to obtain new scientific results, new instruments and methods that have been used not only in research and development but to support breeding programs such as new high oleic peanut cultivars available to Brazilian farmers and, a method for fast and nondestructive determination of oil content in palm fruits that have been used in agroindustry around the world. Therefore, the seed planted and cultivated by Prof. Mascarenhas is another example of his contribution to advancement of science and technology.
References
M.D. Ferreira, Instrumentação pós-colheita em frutas e hortaliças. 1 ed. Brasilia, DF: Embrapa Instrumentação. 284 (2017)
M.H. Levitt, Spin dynamics: basics of nuclear magnetic resonance: Wiley. 710 (2001)
B. Blümich, S. Haber-Pohlmeier, W. Zia, Compact NMR: De Gruyter. (2014)
S. Bai, Nuclear Magnetic Resonance Instrumentation. EAC. 1–26.
NMR Hardware and Software, NMR Spectroscopy Explained. 74–134 (2007)
J. Mitchell, L.F. Gladden, T.C. Chandrasekera, E.J. Fordham, Low-field permanent magnets for industrial process and quality control. Prog. Nucl. Magn. Reson. Spectrosc. 76, 1–60 (2014)
Magritek. https://magritek.com/: Spinsolve; 2021
Bruker. https://www.bruker.com
Nanalysis. https://www.nanalysis.com/
P. Blümler, F. Casanova, Chapter 5: Hardware Developments: Halbach Magnet Arrays. Mobile NMR and MRI: Developments and Applications: Spec. Publ. R. Soc. Chem. (2015)
C.M.S. Lobo, G. Tosin, J.E. Baader, L.A. Colnago, Complementary analyses of hollow cylindrical unioriented permanent magnet (HCM) with high permeability external layer. J. Magn. Reson. 283, 79–88 (2017)
B. Blümich, J. Anders, When the MOUSE leaves the house. Magnetic Resonance open access. 2, 149–60 (2021)
L.G. Hanson, Is quantum mechanics necessary for understanding magnetic resonance? Concepts in Magnetic Resonance Part A. 32A(5), 329–340 (2008)
D.I. Hoult, B. Bhakar, NMR signal reception: Virtual photons and coherent spontaneous emission. Concepts in Magnetic Resonance. 9(5), 277–297 (1997)
F. Bloch, W.W. Hansen, M. Packard, Nuclear induction. Phys. Rev. 69(3–4),127 (1946)
A. Abragram, The principles of nuclear magnetism, Oxford University, Londres, (1961)
T.B. Moraes, T. Monaretto, L.A. Colnago, Rapid and simple determination of T1 relaxation times in time-domain NMR by Continuous Wave Free Precession sequence. J. Magn. Reson. 270, 1–6 (2016)
H.Y. Carr, E.M. Purcell, Effects of diffusion on free precession in nuclear magnetic resonance experiments. Phys. Rev. 94(3), 630–638 (1954)
S. Meiboom, D. Gill, Modified Spin-Echo method for measuring nuclear relaxation times. Rev. Sci. Instrum. 29(8), 688–691 (1958)
E.T. Montrazi, T. Monaretto, T.J. Bonagamba, L.A. Colnago, New and rapid pulse sequences for two-dimensional D-T1 correlation measurements. J. Magn. Reson. 315 (2020)
T. Monaretto, E.T. Montrazi, T.B. Moraes, A.A. Souza, C. Rondeau-Mouro, L.A. Colnago, Using T1 as a direct detection dimension in two-dimensional time-domain NMR experiments using CWFP regime. J. Magn. Reson. 311 (2020)
T. Monaretto, T.B. Moraes, L.A. Colnago, Recent 1D and 2D TD-NMR Pulse Sequences for Plant Science. Plants-Basel. 10(5) (2021)
R.B.D. Azeredo, L.A. Colnago, M. Engelsberg, Quantitative analysis using steady-state free precession nuclear magnetic resonance. Anal. Chem. 72(11), 2401–2405 (2000)
R.B.D. Azeredo, M. Engelsberg, L.A. Colnago, Flow sensitivity and coherence in steady-state free spin precession. Phys. Rev. E 64(1), 4 (2001)
R.B.V. Azeredo, L.A. Colnago, A.A. Souza, M. Engelsberg, Continuous wave free precession - Practical analytical tool for low-resolution nuclear magnetic resonance measurements. Anal. Chim. Acta 478(2), 313–320 (2003)
T. Venancio, M. Engelsberg, R.B.V. Azeredo, N.E.R. Alem, L.A. Colnago, Fast and simultaneous measurement of longitudinal and transverse NMR relaxation times in a single continuous wave free precession experiment. J. Magn. Reson. 173(1), 34–39 (2005)
L.A. Colnago, F.D. Andrade, A.A. Souza, R.B.V. Azeredo, A.A. Lima, L.M. Cerioni et al., Why is Inline NMR Rarely Used as Industrial Sensor? Challenges and Opportunities. Chem. Eng. Technol. 37(2), 191–203 (2014)
T.B. Moraes, T. Monaretto, L.A. Colnago, Applications of Continuous Wave Free Precession Sequences in Low-Field, Time-Domain NMR. Applied Sciences-Basel. 9(7) (2019)
H.T. Pedersen, L. Munck, S.B. Engelsen, Low-field H-1 nuclear magnetic resonance and chemometrics combined for simultaneous determination of water, oil, and protein contents in oilseeds. J. Am. Oil Chem. Soc. 77(10), 1069–1076 (2000)
T.M. Osan, J.M. Olle, M. Carpinella, L.M.C. Cerioni, D.J. Pusiol, M. Appel et al., Fast measurements of average flow velocity by Low-Field H-1 NMR. J. Magn. Reson. 209(2), 116–122 (2011)
B.P. Hills, Applications of Low-Field NMR to Food Science. In: Webb GA, editor. Annual Reports on Nmr Spectroscopy, Vol 58. Annual Reports on NMR Spectroscopy. 58. San Diego: Elsevier Academic Press Inc. 177–230 (2006)
J. van Duynhoven, A. Voda, M. H. Witek Van As, Chapter 3 - Time-Domain NMR Applied to Food Products. Annual Reports on NMR Spectroscopy. 69: Academic Press. 145–97 (2010)
T.H. Aramendiz, Seleção para alto teor de óleo na semente de milho e seus efeitos sobre caracteres agronômicos, Universidade de São Paulo, Piracicaba, (1990)
F.I. Technology, https://www.fitinstrument.com/FITbr/FineInstrumentTechnology.html: SpecFit; (2021)
D. Consaler, L.A. Colnago, Challenges and Accomplishments of TD-NMR in Industry 4.0. Ann. Chem. Sci. 1(2) (2019)
D.W.M. Flores, A.S. Carvalho, S.P. Azevedo, L.A. Colnago, D.M. Consalter, Uso de resonancia magnética en la investigación del potencial de aceite en frutas, su composición y pérdidas. Revista Palmas. 40, 20–8 (2019)
T.D.F. Suassuna, N.D. Suassuna, J.C. Bogiani, F. Perina, D.D. Fragoso, V. Sofiatti et al., BRS 425: the first runner peanut cultivar related to wild ancestral species. Crop Breed. Appl. Biotechnol. 19(3), 373–377 (2019)
T.D.F. Suassuna, N.D. Suassuna, E.P. de Medeiros, J.C. Bogiani, F.J. Perina, D.D. Fragoso et al., “BRS 421” and “BRS 423”: high oleic peanut cultivars for production in Brazil. Crop Breed. Appl. Biotechnol. 20(1), 4 (2020)
U.A. Ribeiro, L.C. Queiroz, A.G. Marassi, A.S. Carvalho, G.A. Barros, D.M. Consalter et al., Development of a TD-NMR Method to Monitor Brazil Nuts Oil Content: A Green and Low-Cost Based Approach. J. Braz. Chem. Soc. 32(7), 1405–1412 (2021)
F.M.V. Pereira, A.D. Carvalho, L.F. Cabeca, L.A. Colnago, Classification of intact fresh plums according to sweetness using time-domain nuclear magnetic resonance and chemometrics. Microchem. J. 108, 14–17 (2013)
D.W.M. Flores, L.A. Colnago, M.D. Ferreira, M.H.F. Spoto, Prediction of Orange juice sensorial attributes from intact fruits by TD-NMR. Microchem. J. 128, 113–117 (2016)
M. Bizzani, D.W.M. Flores, L.A. Colnago, M.D. Ferreira, Non-invasive spectroscopic methods to estimate orange firmness, peel thickness, and total pectin content. Microchem. J. 133, 168–174 (2017)
K.R. Borba, F.C.A. Oldoni, T. Monaretto, L.A. Colnago, M.D. Ferreira, Selection of industrial tomatoes using TD-NMR data and computational classification methods. Microchem. J. 164, 8 (2021)
F.Z. Ribeiro, L.V. Marconcini, I.B. de Toledo, R.B.D. Azeredo, L.L. Barbosa, L.A. Colnago, Nuclear magnetic resonance water relaxation time changes in bananas during ripening: a new mechanism. J. Sci. Food Agric. 90(12), 2052–2057 (2010)
J.D. Ferreira, T.B. Moraes, L.A. Colnago, F.M.V. Pereira, Enzymatic Activity Prediction Using Time-Domain Nuclear Magnetic Resonance (TD-NMR) and Multivariate Analysis: A Case Study Using Cassava Roots. Appl. Magn. Reson. 49(7), 653–664 (2018)
M. Bizzani, D.W.M. Flores, T.B. Moraes, L.A. Colnago, M.D. Ferreira, M.H.F. Spoto, Non-invasive detection of internal flesh breakdown in intact Palmer mangoes using time-domain nuclear magnetic resonance relaxometry. Microchem. J. 158 (2020)
F.M.V. Pereira, L.A. Colnago, Determination of the Moisture Content in Beef Without Weighing Using Benchtop Time-Domain Nuclear Magnetic Resonance Spectrometer and Chemometrics. Food Anal. Methods 5(6), 1349–1353 (2012)
L. Moreira, A.C. Ferrari, T.B. Moraes, R.A. Reis, L.A. Colnago, F.M.V. Pereira, Prediction of beef color using time-domain nuclear magnetic resonance (TD-NMR) relaxometry data and multivariate analyses. Magn. Reson. Chem. 54(10), 800–804 (2016)
N.R.B. Cônsolo, J. Silva, V.L.M. Buarque, L.M. Samuelsson, P. Miller, P.H. Maclean et al., Using TD-NMR relaxometry and 1D 1H NMR spectroscopy to evaluate aging of Nellore beef. Meat Sci. 181:108606 (2021)
N.R.B. Consolo, L.M. Samuelsson, L. Barbosa, T. Monaretto, T.B. Moraes, V.L.M. Buarque et al., Characterization of chicken muscle disorders through metabolomics, pathway analysis, and water relaxometry: a pilot study. Poult. Sci. 99(11), 6247–6257 (2020)
T.B. Moraes, Transformada Inversa de Laplace para análise de sinais de Ressonância Magnética Nuclear de Baixo Campo. Quim. Nova 44(8), 7 (2021)
T. Monaretto, A. Souza, T.B. Moraes, V. Bertucci-Neto, C. Rondeau-Mouro, L.A. Colnago, Enhancing signal-to-noise ratio and resolution in low-field NMR relaxation measurements using post-acquisition digital filters. Magn. Reson. Chem. 57(9), 616–625 (2019)
P.M. Santos, F.V.C. Kock, M.S. Santos, C.M.S. Lobo, A.S. Carvalho, L.A. Colnago, Non-Invasive Detection of Adulterated Olive Oil in Full Bottles Using Time-Domain NMR Relaxometry. J. Braz. Chem. Soc. 28(2), 385–390 (2017)
F.M.V. Pereira, A.P. Rebellato, J.A.L. Pallone, L.A. Colnago, Through-package fat determination in commercial Samples of mayonnaise and salad dressing using time-domain nuclear magnetic resonance spectroscopy and chemometrics. Food Control 48, 62–66 (2015)
F.M.V. Pereira, J.C. Hashimoto, J.A.L. Pallone, L.A. Colnago, Determination of Quality Parameters for Mustard Sauces in Sealed Packets Using Time-Domain Nuclear Magnetic Resonance Spectroscopy and Chemometrics. Food Anal. Methods 8(1), 122–125 (2015)
P.M. Santos, L.A. Colnago, Comparison Among MIR, NIR, and LF-NMR Techniques for Quality Control of Jam Using Chemometrics. Food Anal. Methods 11(7), 2029–2034 (2018)
F.D. de Andrade, A.M. Netto, L.A. Colnago, Qualitative analysis by online nuclear magnetic resonance using Carr-Purcell-Meiboom-Gill sequence with low refocusing flip angles. Talanta 84(1), 84–88 (2011)
Acknowledgements
The authors would like to dedicate this article to the memory of Prof. Sergio Mascarenhas. We would like to thank Embrapa Instrumentação, Pró-Reitoria de Pesquisa da Universidade Federal de Minas Gerais (PRPq—ADRC—07/2020), Fundação de Amparo à Pesquisa do Estado de São Paulo—FAPESP processes # 2019-13656-8 and 2021-12694-3 and CNPq Process # 302866/2017-5 for financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest with any organization regarding the material discussed in this manuscript.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Moraes, T.B., Colnago, L.A. Noninvasive Analyses of Food Products Using Low-field Time-domain NMR: A Review of Relaxometry Methods. Braz J Phys 52, 43 (2022). https://doi.org/10.1007/s13538-022-01055-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13538-022-01055-1