Abstract
The absorption and fluorescence spectra of laser dye, 10-acetyl-2,3,6,7-tetrahydro-1H,5H,11H-pyrano[2,3-f]pyrido[3,2,1-ij]quinolin-11-one [C-334], are recorded. The ground-state dipole moments (μg) were determined from density functional theory (DFT) computations, Guggenheim’s, and solvatochromic methods. The excited-state dipole moments (μe) were determined from Lippert’s, Bakhshiev’s, Kawski-Chamma-Viallet’s, and McRae’s equations. The μe values are found to be higher than μg values and this suggest that the probe molecule is more polar in the excited state. The absorption maxima and emission maxima of C-334 undergo bathochromic shift as the polarity of the solvent increases and indicates that the transitions involved are π → π*. The change in dipole moment (Δμ) and the angle between μe and μg is calculated. The absorption and fluorescence emission of the probe C-334 were investigated theoretically with the help of Gaussian 09W for all the studied solvents by using time-dependent (TD)-DFT combined with conductor-like polarizable continuum model (CPCM) solvation model and were compared with the experimental results. Further, the ground- and excited-state dipole moments were also estimated for all the studied solvents by using CPCM solvation model and are compared with the experimental results. The HOMO-LUMO energy gaps computed using DFT and from absorption threshold wavelengths are found to be in order with each other. The chemical hardness (η) of the probe molecule is estimated and the results suggest the soft nature of the molecule. Further, the reactive centers like electrophilic site and nucleophilic site were identified with the help of molecular electrostatic potential (MESP) 3D plots using DFT computational analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Coumarin and their derivatives represent a class of well-known laser dyes in the blue-green spectral region, characterized by high-emission quantum yields and find many practical applications in the various fields of science and technology. Since they exhibit fluorescence in the UV-Vis region, they are used as colorants, dye lasers, and non-linear optical fluorophores [1,2,3]. They are used as photo initiators, emission layers in organic light-emitting diodes, probes in the biological study, photodimerization in polar, non-polar solvents, etc. [4,5,6]. They also find applications as fluorescent indicators [7], optical brighteners [8], sunscreens [9], anti-coagulants, biological and chemical sensors [10, 11], in enzymology [12], blood thinners [13], anti-inflammatory [14], anti-tubercular [15], anti-HIV [16], and anti-cancer [17] agents.
Recently, there are reports on using C-334 as an atypical antioxidant [18], electroosmotic flow marker for characterization of carbon quantum dots [19], EOF marker for investigating doxorubicin encapsulation [20], and off–on catalytic chemodosimeter for Cu2+ ions [21], to form single, binary, ternary dye-doped PMMA thin films [22].
The solvatochromic investigations aimed at determination of dipole moments in the ground and excited state are important, as they furnish information about the changes in electronic distribution and symmetry of the molecule in the excited state. The μe value of the molecule is found to be helpful in designing the non-linear optical materials. Further, it also helps in determining the electrophilic and nucleophilic sites, which are useful in photochemical reactions, etc. It is observed that the electronic spectra of molecules are influenced by their immediate environment [2,3,4,5,6, 23,24,25,26,27,28,29,30]. For the determination of dipole moments, there are numbers of methods like electric dichroism [31], electric polarization of fluorescence [32], microwave conductivity [33], and Stark splitting [34] that are available. However, their use is limited as they are considered to be equipment intensive and applicable to relatively simple molecules. On the other hand, Guggenheim’s [35] and solvatochromic methods offer simple techniques to determine the dipole moments of the probe molecules.
Earlier, our research group has reported on resonance energy transfer studies on derivatives of thiophene substituted 1,3,4-oxadiazoles and C-334 laser dye in different media [36]. In the present work, the ground-state dipole moment (μg) and excited-state dipole moment (μe) of C-334 are determined from dielectric, solvatochromic, and DFT studies, the HOMO-LUMO energy gap, chemical hardness, and molecular electrostatic potential (MESP) plots have been studied, and the results obtained are presented and discussed.
2 Theory
2.1 Ground-State Dipole Moment by Guggenheim’s Method
According to Guggenheim’s [35] method, the ground-state dipole moment (μg) is given by
The symbols k, T, N, ɛ, C, and n represent Boltzmann’s constant, absolute temperature, Avogadro’s number, dielectric constant, concentration, and refractive index respectively. The suffixes 12, 1, and 2 correspond to the solution, solvent, and solute respectively. The symbol “Δ” represents the difference in the extrapolated intercepts from the plots (ɛ12 − ɛ1)/C vs. C and (n122 − n12)/C vs. C corresponding to infinite dilution (C → 0). The value of ɛ12 is calculated by using Eq. (3).
where c12 is the capacitance of cylindrical cell with solution and ca is the capacitance of cylindrical cell without solution respectively. c1 represents connecting leads’ capacitance. The suffixes 12, 1, and 2 refer to the solution, solvent, and solute respectively.
2.2 Ground- and Excited-State Dipole Moments by Solvatochromic Method
The μg and μe values are calculated using the solvatochromic equations.
The expression for Stoke’s shift according to Lippert’s [37] is given as
where F(ε, n) is Lippert’s polarity function and is given as
The expression for Stoke’s shift according to Bakhshiev’s [38] is
where F1(ε, n) is Bakhshiev’s polarity function and is given as
According to Kawski-Chamma-Viallet’s [39] equation,
where F2(ε, n) is Kawski-Chamma-Viallet’s polarity function and is given as
According to McRae’s [40] equation,
where F3(ε) is McRae’s polarity function and is given as
From the above Eqs. (4), (6), (8), and (10), it follows that (ῡa − ῡf) vs. F(ɛ,n), (ῡa − ῡf) vs. F1(ɛ,n), 1/2(ῡa + ῡf) vs. F2(ɛ,n), and ῡa vs. F3(ε) should give linear graphs with slopes S, S1, S2, and S3 and are given as
where μe and μg have their usual meaning and h and c correspond to Planck’s constant and velocity of light respectively. The radius of the solute molecule “a0” is of the order of 3.939 Å and its value is determined by using Edward’s [41] atomic increment method.
Assuming that μe and μg are parallel to each other and upon electronic transition, the symmetry of the probe molecule remains same, based on Eqs. (13) and (14), one obtains
If the angles between μe and μg are not parallel, then the angle θ between the two dipole moments can be obtained from Eqs. (16) and (17) and is given by Eq. (19).
2.3 Change in Dipole Moment (Δμ) and Excited-State Dipole Moment (μe) by Solvent Polarity parameter\( \left({E}_T^N\right) \)
This method is based on solvent polarity parameter \( \left({E}_T^N\right) \) to estimate change in dipole moment proposed by Reichardt [42] and developed by Ravi et al. [43]. The expression for spectral band shift with \( \left({E}_T^N\right) \) is given by Eq. (20).
where ΔμB = 9 D represents the change in dipole moment and aB = 6.2 Å denotes Onsager cavity radius of reference betaine dye molecule and Δμ and “a” are the respective quantities of the probe molecule. The Δμ can be determined from Eq. (21).
where m is the slope from the linear plot of Stoke’s shift vs.\( \left({E}_T^N\right). \)
Knowing the value of Δμ and μg (from Eq. (1)), the excited-state dipole moment (μe) can be determined from Eq. (22).
3 Experimental
3.1 Materials Used
The laser dye C-334 is procured from Sigma-Aldrich, USA, and is used without any further purification. The molecular structure of C-334 is given in Fig. 1. All the solvents benzene, tetrahydrofuran (THF), propane-2-ol, acetone, ethanol, methanol, and acetonitrile are procured from S.D. Fine Chem. Pvt. Ltd., India, and are of spectroscopic grade. The various solutions were prepared at a fixed solute concentration of the order of 10−5 M in order to minimize self-absorption and aggregation formation.
3.2 Methods
The ɛ values of the various solutions are determined using a calibrated brass cell by using LCR Data Bridge (Aplab MT-4080D) at 10 kHz frequency. The refractive indices of various dilute solutions are determined by using Abbe’s refractometer. Absorption and emission spectra were recorded using Specord 200 plus spectrophotometer and Hitachi F-7000 spectrofluorometer respectively. Theoretical computations were performed using DFT with basis sets B3LYP/6-31G (d).
4 Results and Discussions
4.1 Determination of Ground- and Excited-State Dipole Moments from Different Methods
The ɛ values of the different solutions (ɛ12) are calculated by using Eq. (3). The refractive indices for various concentrations (n12) are measured using Abbe’s refractometer and the results are given in Table 1.
Then from the knowledge of experimentally measured values of dielectric constant and refractive index of benzene and C-334 solutions, using Eq. (1), the μg value is calculated according to Guggenheim’s method and the result is presented in Table 5.
The absorption and emission maxima, Stoke’s shift, and arithmetic mean of Stoke’s shift values in different solvents are presented in Table 2.
The absorption spectra of C-334 in ethanol and fluorescence spectra of C-334 in different solvents are shown in Figs. 2 and 3 respectively. From Fig. 3, it is observed that the emission maxima undergo a bathochromic shift as the polarity of solvent increases and this indicates the spectral transition to be π → π*.
From Table 2, it is observed that, for different solvents of increasing polarity, the absorption maxima show shifts from 22,522 to 22,075 cm−1 and emission maxima show shift from 20,876 to 19,801 cm−1. Further, it is observed that the spectral shift in the emission spectra is large compared to the absorption spectra. This suggests that in the ground state, the probe molecule is less polar compared to the excited state. It is also noticed from Table 2 that there is a considerable increase in Stoke’s shift (ῡa − ῡf) with increasing solvent polarity from 2291 to 1645 cm−1, which indicates that there is an increase in the dipole moment in the excited state compared to the ground state.
The dielectric constants, refractive indices, and \( \left({E}_T^N\right) \) values along with calculated values of various polarity functions are presented in Table 3.
The plots, Stoke’s shift vs. F(ɛ,n), Stoke’s shift vs. F1(ε,n), arithmetic mean of Stoke’s shift vs. F2(ɛ,n), ῡa vs. F3(ɛ), and Stoke’s shift vs.\( {E}_T^N \) are presented in Figs. 4, 5, 6, 7, and 8 respectively.
The statistical data like slopes, intercepts, and correlation coefficients are reported in Table 4. It is observed that the correlation coefficient values are around 0.8–0.9, which indicates a good linearity for the respective plots.
From the solvatochromic method using the slopes S1 and S2, the value of μg from Eq. (16), the value of μe from Eq. (17), and their ratio μe/μg from Eq. (18) are calculated and are given in Tables 5, 6, and 7 respectively. Further, by substituting the value of μg determined experimentally from Guggenheim’s method in Eqs. (12) to (15), the excited-state dipole moments (μe) according to Lippert’s, Bakhshiev’s, Kawski-Chamma-Viallet’s, and McRae’s methods are calculated and are given in Table 6. Using the slope calculated from Stoke’s shift vs. solvent polarity parameter\( \left({E}_T^N\right) \), the excited-state dipole moment and change in dipole moment are calculated using Eqs. (22) and (21) respectively and the results are tabulated in Tables 6 and 7.
It is observed from Table 5 that there is a good agreement between μg values determined from Guggenheim’s and solvatochromic methods. As is evident from Table 6, the μe determined by using Bakhshiev’s, Kawski-Chamma-Viallet’s, and solvatochromic methods are found to be in good agreement with each other. The μe calculated using solvent polarity parameter \( \left({E}_T^N\right) \) is found to be smaller than the excited-state dipole moment determined using Bakhshiev’s, Kawski-Chamma-Viallet’s, and solvatochromic methods. This may be due to the reason that these methods do not take into account specific solute-solvent interactions like hydrogen bonding, complex formation, and molecular aspects of solvation, whereas they are incorporated in the solvent polarity parameter \( \left({E}_T^N\right) \) method [27]. The excited-state dipole moment (μe) determined from Lippert’s and McRae’s methods is found to be higher compared to the other methods and it may be attributed to non-accountability of polarizability in these methods [5]. From Tables 5 and 6, it is noticed that the excited-state dipole moments (μe) determined from all the methods are found to be higher than the experimental ground-state dipole moment (μg) determined from Guggenheim’s and solvatochromic methods. The higher values of μe indicate that the probe molecule C-334 is more polar or stable in the excited state than the ground state. Further, it is also observed from Table 7 that the Δμ values determined from solvatochromic and \( \left({E}_T^N\right) \) methods are higher. The higher values of μe and Δμ suggest that the probe molecule is more polar or stable in the excited state than the ground state and indicate the existence of more relaxed excited state [41, 42].
From Table 7, the angle between μg and μe is found to be zero. This suggests that the μg and μe are parallel to each other and the symmetry of the molecule remains unchanged upon electronic transition [39].
4.2 Computational Analysis
The absorption and emission spectra of the probe C-334 for all the studied solvents were computed by using Gaussian 09W in order to compare the maxima values with the experimental results. For this purpose, the probe molecule is optimized for the ground and excited state using DFT and TD-DFT with the basis sets B3LYP/6-31G (d) combined with conductor-like polarizable continuum model (CPCM) solvation model. Further, by using the theoretically computed absorption and emission maxima values, the electronic transition energy for absorption as well as emission were calculated and are tabulated in Table 8. The electronic transition energy values were also determined for the experimental absorption and emission maxima of the probe molecule and are given in Table 8.
From Table 8, it is observed that the experimental and theoretical electronic transition energies for both absorption and emission are found to be in good agreement with each other. In case of absorption, the difference in the experimental and theoretical transition energy is of the order of 0.32 to 0.37 eV, where as in case of emission, it is of the order of 0.27 to 0.39 eV respectively. The experimental transition energies undergo a blue shift in case of absorption as well as emission. Further, it is interesting to note that the theoretical transition energies computed using TD-DFT/CPCM solvation model and the experimental energy values exhibit the similar trend. From Table 2, it is observed that as the polarity of the solvent increases, Stoke’s shift increases. From Table 8, it is also observed that as the polarity of the solvent increases, theoretically computed transition energy difference between absorption and emission also increases. From these results, it is noticed that the TD-DFT/CPCM solvation studies reproduce the similar trend as observed experimentally.
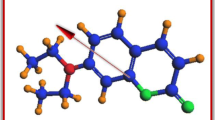
The ground-state dipole moment of the probe molecule in the gaseous state is also estimated theoretically by using DFT with basis sets B3LYP/6-31G (d) and the result is presented in Table 5. The optimized molecular geometry of C-334 molecule along with the direction of dipole moment is shown in Fig. 9.
It is observed from Tables 5 and 6 that the theoretically computed μg value is higher than the experimental μg value. It is to be noted that the experimental methods take solvent and environmental effects like solute-solvent interactions into account, whereas the ab initio computations are based on gaseous phase [27, 43].
Further, in order to analyze the solute-solvent interactions, the ground- and excited-state dipole moments were also estimated theoretically for all the studied solvents by using CPCM solvation model and the results are given in Table 9.
From Table 9, it is noticed that the ground-state dipole moment values for each of the solvents are found to be higher than the ground-state dipole moment value of the probe molecule in the gaseous phase (Table 5). The increase in the dipole moment value is due to the consideration of environmental effects like solute-solvent interactions in the CPCM solvation model. Further, the excited-state dipole moment values were found to be higher than the corresponding ground-state dipole moment values for all the solvents and this suggests that the probe molecule is more polar in the excited state than the ground state. It is interesting to note that the computational studies also reproduce the similar trend as observed experimentally. However, the theoretically computed ground- and excited-state dipole moments were found to be higher than the experimental dipole moments.
The 3D plots of HOMO and LUMO of C-334 molecule are shown in Fig. 10.
The HOMO, LUMO energies and HOMO-LUMO energy band gap (ΔE) value for the probe molecule are presented in Table 10. The optical band gap\( {E}_g^{opt} \) is determined from absorption threshold wavelength and the result is also tabulated in Table 10. It is observed that the HOMO-LUMO energy band gap is in order with the experimental optical energy band gap. The lower values of energy gap for the probe molecule also support the observed higher values of excited-state dipole moments.
The determination of HOMO-LUMO energies also helps in understanding the chemical stability of a molecule in terms of a parameter known as chemical hardness (η). The molecules possessing large HOMO-LUMO energy gap are considered as hard, whereas molecules possessing small HOMO-LUMO energy gaps are considered as soft molecules [41, 42]. The chemical hardness (η) of a molecule [44] is determined from Eq. (23).
where EH and EL are the HOMO and LUMO energies.
The chemical hardness (η) estimated for the probe molecule is given in Table 10. The small values of chemical hardness (η) and HOMO-LUMO energy gaps suggest that the molecule may be considered as soft molecule [41]. These results also support the observed higher values of μe.
The molecular electrostatic potential (MESP) plots provide the information for determining a suitable position for nucleophilic and electrophilic attack along with the hydrogen bonding interactions of solvent. The MESP 3D plot of the probe molecule C-334 is shown in Fig. 11. In this plot, different colors correspond to different values of electrostatic potential at the surface. The red color represents negative phase, which can be related to the electrophilic site, and blue color represents positive phase, which corresponds to nucleophilic site. From Fig. 11, it is observed that the MESP plot of probe molecule shows negative phases around 5,6-dihydropyran-2-one and propan-2-one, whereas positive phases around all hydrogen atoms.
5 Conclusions
In the present study, in order to investigate the solvatochromic behavior and dipole moments, the absorption and fluorescence spectra of C-334 were recorded in different solvents. The μg value of the probe molecule is determined using Guggenheim’s and solvatochromic methods. It is observed that the μg determined from these methods are found to be in agreement with each other. The excited-state dipole moments (μe) are determined by using Lippert’s, Bakhshiev’s, Kawski-Chamma-Viallet’s, solvatochromic, solvent polarity parameter, and McRae’s equations. The μe values determined from Bakhshiev’s, Kawski-Chamma-Viallet’s, and solvatochromic methods are found to be in good agreement with each other. Further, μe values determined from different solvatochromic correlations like Lippert’s, Bakhshiev’s, Kawski-Chamma-Viallet’s, McRae’s, and solvent polarity parameter are found to be higher than the experimental ground-state dipole moments for the probe molecule under investigation. The changes in dipole moment values (Δμ) were found to be higher. This suggests that the probe molecule C-334 is more polar or stable in the excited state than in the ground state and indicates the existence of more relaxed excited state. It is observed that the angle between μg and μe is found to be zero degree, which suggests that μg and μe are parallel to each other and there is no change in the symmetry upon electronic transition.
The absorption and fluorescence emission of the probe C-334 were investigated theoretically with the help of Gaussian 09W for all the studied solvents using TD-DFT combined with CPCM solvation model and were compared with the experimental results. It is observed that there is a good agreement between the theoretical and experimental results. Further, the ground- and excited-state dipole moments were also estimated for all the studied solvents by using CPCM solvation model and are compared with the experimental results.
The HOMO-LUMO energy band gaps determined from DFT computations and from optical energy band gap are found to be in order with each other. The chemical hardness (η) investigations suggest that the molecule exhibits the soft nature. The computational studies performed using DFT imply that the probe molecule exhibits both nucleophilic and electrophilic sites.
References
N.K. Hamdi, M.M. Chebli, H. Grib, M. Brahimi, A.M.S. Silva, Synthesis DFT/TD-DFT theoretical studies and experimental solvatochromic shift methods on determination of ground and excited state dipole moments of 3-(2-hydroxybenzoyl) coumarins. Journal of Molecular Structure 1175, 811–820 (2019)
N. Khanapurmath, M.V. Kulkarni, L. Pallavi, J. Yenagi, J. Tonannavar, Solvatochromic studies on 4-bromomethyl-7-methyl coumarins. Journal of Molecular Structure 1160, 50–56 (2018)
S. Samundeeswari, M.V. Kulkarni, J. Yenagi, J. Tonannavar, Dual fluorescence and solvatochromic study on 3-acyl coumarins. J. Fluoresc. 27, 1247–1255 (2017). https://doi.org/10.1007/s10895-017-2052-z
J. Basavaraj, H.M. Sureshkumar, S.R. Inamdar, M.N. Wari, Estimation of ground and excited state dipole moment of laser dyes C504T and C521T using solvatochromic shifts of absorption and fluorescence spectra. Spectrochim. Acta Part A-Molecular and Biomolecular Spectro 154, 177 (2016)
U.S. Raikar, V.B. Tangod, S.R. Mannopantar, B.M. Mastiholli, Ground and excited state dipole moments of coumarin 337 laser dye. Optics Communication 283, 4289–4292 (2010)
J. Basavaraj, S.R. Inamdar, H.M. Sureshkumar, Solvent effects on the absorption and fluorescence spectra of 7-diethylamino-3-thienoylcoumarin: Evaluation and correlation between solvatochromism and solvent polarity parameters. Spectrochim. Acta Part A-Molecular and Biomolecular Spectro 137, 527 (2015)
E.J. Schitschek, J.A. Trias, P.R. Hammond, R.A. Henry, R.L. Atkins, New laser dyes with blue-green emission. Optics Communication 16, 313–316 (1976)
C. Parkanyi, M.S. Antonious, J.J. Aaron, M. Buna, A. Tine, L. Cissa, Determination of the first excited singlet state dipole moments of coumarins by the solvatochromic method. Spectro. Letters 27, 439–449 (1994)
E. Perez-Rodriguez, J. Aguilera, F.L. Figueroa, J. Expt, Tissular localization of coumarins in the green alga Dasycladus vermicularis (Scopoli) Krasser: a photoprotective role? Botany 54, 1093 (2003)
K.H. Drexhage, Structure and properties of laser dyes, (Springer-Verlag, Berlin, 1973)
J. Thipperudrappa, D.S. Biradar, S.R. Manohara, S.M. Hanagodimath, S.R. Inamadar, R.J. Manekutla, Solvent effects on the absorption and fluorescence spectra of some laser dyes: Estimation of ground and excited-state dipole moments. Spectrochim. Acta Part A- Molecular and Biomolecular Spectro 69, 991–997 (2008)
W.R. Shermon, E. Robins, Fluorescence of substituted 7-hydroxycoumarins. Anal. Chem. 40, 803–805 (1968)
A. Evangelos, P.D. Andrew, Convenient microscale synthesis of a coumarin laser dye analog. Journal of Chemical Education 83(2), 287 (2006)
A. Lacy, R. Kennedy, Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Current Pharmaceutical Design 10, 3797–3811 (2004)
V.U. Jeankumar, R.S. Reshma, R. Janupally, S. Saxena, J.P. Sridevi, B. Medapi, P. Kulkarni, P. Yogeeswari, D. Sriram, Enabling the (3 + 2) cycloaddition reaction in assembling newer anti-tubercular lead acting through the inhibition of the gyrase ATPase domain: lead optimization and structure activity profiling. Organic & Biomolecular Chemistry 13, 2423–2431 (2015)
D. Yu, M. Suzuki, L. Xie, S.L. Morris-Natschke, K.H. Lee, Recent progress in the development of coumarin derivatives as potent anti-HIV agents. Med. Res. Rev. 23, 322–345 (2003)
R.D. Thornes, D.W. Edlow, S. Wood, Inhibition of locomotion of cancer cells in vivo by anticoagulant therapy. I. Effects of sodium warfarin on V2 cancer cells, granulocytes, lymphocytes and macrophages in rabbits. The Johns Hopkins Medical Journal 123, 305 (1968)
D.Z. N’u˜nez, P. Barrias, G.C. Jir’on, M.S.U. Za˜nartu, C.L. Alarc’on, F.E.M. Vieyra, C.D. Borsarelli, E.I. Alarcon, A. Asp’ee, A typical antioxidant activity of non-phenolic amino-coumarins. RSC Advances 8, 1927 (2018)
M. Vaculovicova, S. Dostalova, V. Milosavljevic, P. Kopel, V. Adam, R. Kizek, Characterization of carbon quantum dots by capillary electrophoresis with laser-induced fluorescence detections. J. Metallomics & Nanotechno. 3, 97 (2015)
R. Konecna, H. Viet Nguyen, M. Stanisavljevic, I. Blazkova, S. Krizkova, M. Vaculovicova, M. Stiborova, T. Eckschlager, O. Zitka, V. Adam, R. Kizek, Doxorubicin encapsulation investigated by capillary electrophoresis with laser-induced fluorescence detection. Chromatographia 77, 1469–1476 (2014)
M.H. Kim, H.H. Jang, S. Yi, S.K. Chang, M.S. Han, Coumarin-derivative-based off–on catalytic chemodosimeter for Cu2+ ions. Chemical Communications 4838 (2009)
J. Huang, V. Bekiari, P. Lianos, S. Couris, Study of poly(methyl methacrylate) thin films doped with laser dyes. J. Luminescence 81, 285–291 (1999)
C. Reichardt, Solvents and Solvent Effects in Organic Chemistry, 3rd edn. (Wiley-VCH, New York, 2004)
U.P. Raghavendra, M. Basanagouda, R.M. Melavanki, R.H. Fattepur, J. Thipperudrappa, Solvatochromic studies of biologically active iodinated 4-aryloxymethyl coumarins and estimation of dipole moments. J. Mol. Liq. 202, 9–16 (2015)
S.S. Patil, G.V. Muddapur, N.R. Patil, R.M. Melavanki, R.A. Kusanur, Fluorescence characteristics of aryl boronic acid derivative (PBA). Spectrochim. Acta Part A- Molecular and Biomolecular Spectro 138, 85–91 (2015)
S.K. Patil, M.N. Wari, P.C. Yohannan, S.R. Inamdar, Determination of ground and excited state dipole moments of dipolar laser dyes by solvatochromic shift method. Spectrochim. Acta Part A- Molecular and Biomolecular Spectro 123, 117–126 (2014)
G.V. Muddapur, N.R. Patil, S.S. Patil, R.M. Melavanki, R.A. Kusanur, Estimation of ground and excited state dipole moments of aryl boronic acid derivative by solvatochromic shift method. J. Fluoresc. 24, 1651–1659 (2014)
J.S. Kadadevarmath, G.H. Malimath, N.R. Patil, H.S. Geetanjali, R.M. Melavanki, Solvent effect on the dipole moments and photo physical behaviour of 2,5-di-(5-tert-butyl-2-benzoxazolyl) thiophene dye. Canadian Journal of Physiology and Pharmacology 91, 1107–1113 (2013)
J.R. Manekutla, B.G. Mulimani, S.R. Inamdar, Solvent effect on absorption and fluorescence spectra of coumarin laser dyes: Evaluation of ground and excited state dipole moments. Spectrochim. Acta Part A- Molecular and Biomolecular Spectro 69, 419 (2008)
D.S. Biradar, B. Siddlingeshwar, S.M. Hanagodimath, Estimation of ground and excited state dipole moments of some laser dyes. J. Mol. Struct. 875, 108–112 (2008)
J. Czekella, Two electro optical methods for determination of dipole moments of excited molecules. Chimia 15, 26 (1961)
J. Czekella, Elektrische Fluoreszenzpolarisation: Die Bestimmung von Dipolmomenten angeregter Moleküle aus dem Polarisationsgrad der Fluoreszenz in starken elektrischen Feldern. Z. Elaktrochem. 64, 1221 (1960)
M.P. Hass, J.M. Warman, Photon-induced molecular charge separation studied by nanosecond time-resolved microwave conductivity. Chemistry and Physics of Lipids 73, 35–53 (1982)
J.R. Lombardi, Correlation between structure and dipole moments in the excited states of substituted benzenes. Journal of the American Chemical Society 92, 1831–1833 (1970)
E.A. Guggenheim, The computation of electric dipole moments. Transactions of the Faraday Society 47, 573 (1951)
L. Naik, N. Deshapande, I.A.M. Khazi, G.H. Malimath, Resonance energy transfer studies from derivatives of thiophene substituted 1,3,4-oxadiazoles to coumarin-334 dye in liquid and dye-doped polymer media. Brazilian Journal of Physics 48, 16–24 (2017). https://doi.org/10.1007/s13538-017-0540-x
E.Z. Lippert, Spektroskopische Bestimmung des Dipolmomentes aromatischer Verbindungen im ersten angeregten Singulettzustand. Zeitschrift für Elektrochemie 61, 962 (1957)
N.G. Bakhshiev, Universal intermolecular interactions and their effect on the position of the electronic spectra of molecules in 2-component solutions. Optika i Spektroskopiya 16(5), 821 (1964)
A. Kawski, On the estimation of excited state dipole moments from solvatochromic shifts of absorption and fluorescence spectra. Zeitschrift für Naturforschung 57A, 255 (2002)
U.S. Raikar, V.B. Tangod, B.M. Mastiholli, S. Sreenivasa, Solvent effects and photophysical studies of ADS560EI laser dye. African Journal of Pure and Applied Chemistry 4(9), 188 (2010)
K.B. Akshaya, V. Anitha, L.L. Prajwal, K. Rekakumari, G. Louis, Synthesis and photophysical properties of a novel phthalimide derivative using solvatochromic shift method for the estimation of ground and singlet excited state dipole moments. Journal of Molecular Liquids 224, 247–254 (2016)
A. Roshmy, V. Anita, G. Louis, N. Aatika, Estimation of ground state and excited state dipole moments of a novel Schiff base derivative containing 1,2,4-triazole nucleus by solvatochromic method. J. Mol. Liq. 215, 387 (2016)
S.R. Manohara, V.U. Kumar, G.L. Shivakumaraiah, Estimation of ground and excited-state dipole moments of 1, 2-diazines by solvatochromic method and quantum-chemical calculation. J. Mol. Liq. 181, 97–104 (2013)
R.G. Pearson, Chemical Hardness (Wiley - VCH, Weinheim, 1997)
Acknowledgements
The authors are thankful to the authorities of USIC, KUD, for providing the instrumental facility for our research work. One of the authors (CVM) is thankful to the Principal Prof. B.P. Urakadli and staff, Government First Grade College Hubballi, for their continuous support and encouragement.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maridevarmath, C.V., Naik, L. & Malimath, G.H. Dielectric, Photophysical, Solvatochromic, and DFT Studies on Laser Dye Coumarin 334. Braz J Phys 49, 151–160 (2019). https://doi.org/10.1007/s13538-018-00628-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13538-018-00628-3