Abstract
In the anesthesia automation, an automatic propofol infusion system uses Bi-spectral Index Signal (BIS) as a primary feedback signal to manipulate propofol dose. However, the BIS signal may be suspended for some time due to poor EEG signal quality, noise, and many other factors. Therefore, BIS signal failure may be the main cause of inadequate propofol infusion. This fact motivates the need for integration of multivariable fault tolerance module (MFTM) and fractional-order Smith predictor controller to avoid adverse reactions of inadequate propofol dosing during BIS failure. Smith Predictor control strategy is sufficiently robust to predict feedback BIS during BIS failure via patient pharmacological modeled BIS. However, modeled BIS may not provide a guarantee of adequate propofol infusion during BIS failure and especially in the presence of hypotension and hypertension. Thus, the proposed control strategy is designed with MFTM to detect BIS sensor fault and to estimate feedback BIS during BIS failure. Further, the proposed control strategy is designed with a multivariable pharmacological patient model to analyze the cross effect of propofol infusion on BIS and hemodynamic variables. The robustness of the proposed control strategy is tested in the presence of noxious surgical stimulation, BIS sensor fault and heavy hemodynamic disturbance. The pharmacological parameters and recorded signals of 30 patients during various surgeries have been used to validate simulated results. The performance of the proposed control strategy assures optimization and smooth propofol infusion during BIS failure. The proposed system provides stability for a wide range of physiological parameters range. The proposed scheme maintains smooth BIS and MAP signal despite the delay, BIS sensor fault, and surgical disturbances.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In general anesthesia, it is important to maintain patients’ physical variables BIS, MAP and electromyography (EMG) within their desired range. These variables can be used as a feedback signal in anesthesia automation [1]. In clinical practices, an anesthesiologist uses Aspect Medical BIS monitor (Newton, MA, USA) to identify changes in brain activity with respect to propofol infusion, to reduce the risk of intraoperative awareness and boost recovery time from anesthesia. BIS value decreases with propofol brain concentration increases. Normal BIS range is 100 (fully awake stage)–0 (no electrical activity) and its value within 60–40 indicate adequate DOA. The clinical research shows that BIS feedback based closed-loop control of propofol dosing can have optimistic outcomes to keep vital parameters of the patient in safe limits and to maintain unconsciousness with painless feeling during surgery better than human control [1,2,3].
Many BIS signal based Closed Loop ANnesthesia (CLAN) systems [4] with proportional–integral (PI) and proportional–integral–derivative (PID) controllers have been designed and tested on more than 20 different patients’ model to deliver automatic propofol infusion [5,6,7,8]. They obtained satisfactory BIS response with acceptable oscillations. Later, Model Predictive Controller (MPC) [9, 10], Extended Prediction Self-Adaptive Control (EPSAC) and MPC-EPSAC [11,12,13], Super-Twisting Sliding Mode Control (STSMC) and Sliding Mode Control (SMC) [14, 15], PID with Model Predictive Control [16], Smith Predictor (SP) with PI/PID [17,18,19] and multivariable control scheme [20, 21] have been reported to improve regulation of hypnosis in the presence of surgical disturbances and patient variability. However, frequent modifications in propofol dose during surgery and model dependency are limitations of reported studies.
In an emerging trend, the nonlinear control strategy and fractional calculus have been reported to achieve high accuracy, high speed, perfect setpoint tracking and to compensate for the effect of nonlinearities, dynamic coupling and modeling error [22, 23]. Advance nonlinear controls based on SMC have been reported to [24,25,26] improve the controller performance in the presence of disturbance, setpoint and modeling error. The fractional calculus is beneficial to improve the degree of correctness of the dynamic model as compared to integer-order [22, 27]. Fractional-order PI/PID has an additional tuning parameter facility apart from proportional, integral, and derivative gain. Due to this additional tuning facility, the performance of fractional order control is more robust than integer-order control. Recently, researchers have incorporated the novel tuning and approximation approach of fractional calculus in advance control strategies [22, 27,28,29,30,31]. Most of the tuning methods are based on frequency domain tuning parameters (phase margin, gain crossover frequency, and iso-damping). However, this tuning method is relatively time consuming and required accurate transfer function knowledge. Moreover, this technique needs to solve complex nonlinear equations to obtained tuning parameters. Thus, to avoid the complexity of frequency domain tuning parameters, advance frequency and time domain tuning methods for the delayed system are well discussed in reference literature [32]. Time-domain tuning is more flexible than the frequency domain. Smith predictor with fractional order controller is a model-based robust control scheme for the delay and disturbance effect compensation. Thus, Smith predictor design with fractional order PID controller based on time domain tuning is discussed in the literature [33, 34]. Motivated from these studies, the proposed control strategy is designed with a fraction order Smith predictor structure using two-time domain tuning parameters.
Further, the adaptive nature of control strategy is essential to handle patient variability and to achieve individualized control. In anesthesia automation, Hill function is used to derive the relation between propofol concentration and propofol effect. The value of Hill function parameters changes from one patient to another patient. Thus, to achieve individualized control in all patients and to avoid the complexity of frequent adaption procedures, Hill function parameters based adaption is presented In the literature [18, 35]. They obtained better result compared to other adaption methods. Motivated from these researches, proposed control strategy uses the values of propofol Hill function parameters (EC50 μg/ml and λ) to select optimum gain setting. EC50 (μg/ml) denotes half maximum propofol effect and λ denotes the slope of linearized graph of effect site concentration versus propofol effect (BIS).
Reported studies are notable in anesthesia automation research field. However, these studies are focused on robustness and stability issues of the control system and tacitly neglected serious BIS signal failure problem. BIS signal failure problem arises due to several reasons like monitor malfunction, suspension of the sampling line due to sensor-location changing, frequent appears of the myogenic potential on the electroencephalographic (EEG) signal, high frequency electrical noise, artifacts, body excitement and movement [9]. Inadequate propofol dosing during BIS failure can lead to blood pressure instability and cardiovascular collapse [36]. Over sedation of propofol can cause hypotension, prolonged recovery time, ileus, nausea and immunosuppression. Whereas, under sedation can cause anxiety, agitation and tachycardia [21]. Therefore, cautious management of propofol infusion is essential during normal conditions as well as BIS failure conditions to maintain adequate DOA and propofol infusion. Due to these motives, proposed control strategy is designed with fault tolerance module to detect BIS sensor fault and to provide correct estimated feedback. A few research studies [37,38,39] used MAP signal as a second control variable to deliver adequate propofol dose during BIS failure. Since the significant effect of propofol infusion on the MAP. Propofol administration causes vasodilation, which decreases cardiac output and arterial peripheral resistance and consequently lowers the MAP. The MAP signal within 70–100 mmHg indicates a healthy physiological condition during surgery. However, change in MAP is slow as compared to BIS with a change in the propofol dose. Thus, the approximation of BIS using a single variable (MAP) may be failed to estimate precise feedback BIS during BIS failure in all patients. Yuan Tao et al. [40] reported IMC based state observer-based control scheme to solve BIS failure. They used pharmacological patient modeled BIS to deliver propofol dose. However, patient model-based BIS estimation may be failed to deliver adequate propofol dose in the presence of hemodynamic instability (hypertension and hypotension). BIS regulation may not be enough to maintain a patient’s healthy physical condition during surgery. However, regulation of hemodynamic variables, heart rate, respiratory rate and other vital parameters within the safe range is also essential during surgery [41]. Thus, constraints of reported systems are mainly lacking in the terms of model dependency, frequent prediction complexity, system uncertainty, fixed gain, no faultless solution of BIS failure and small therapeutic advantage. Inspired from the above facts, proposed work is focused on two following main objectives:
-
1.
The first objective is to design an advanced control strategy that has the potential to maintain an adequate level of anesthesia in the presence of BIS failure. Evaluate the performance of the proposed control scheme for hypnosis and hemodynamics regulation during normal conditions as well as BIS failure condition.
-
2.
The second objective is to investigate the effect of a surgical stimulus on BIS, MAP and propofol infusion. Similarly, investigate the robustness of the fault tolerance module in the presence of heavy hemodynamic disturbance.
This proposed work is using the integration of multivariable fault tolerance module with a fractional-order Smith predictor controller to regulate BIS by manipulating propofol dose regimens. The proposed fractional order controller is a cascade structure of fractional filter and integer PID controller. Controller tuning parameters are derived using the Mittag–Leffler function. Further, the proposed control strategy monitors propofol bolus and continuous dosing effect on hemodynamic parameters through multivariable patient model. The MAP signal is derived from the combined effect of propofol concentration at a cardiac output and arterial peripheral resistance. In the case of BIS failure, MFTM detects BIS sensor fault and provides estimated feedback BIS. This estimated BIS is used to manipulate propofol dose. Proposed control strategy has been tested on 30 different patient models. The robustness of control strategy is tested by adding surgical disturbance in simulations. Simulated BIS, MAP signals and propofol dosing are compared with recorded signals. Main motivation behind this research is to design robust automatic propofol infusion system with best fault tolerance ability. We have recorded 30 different patients’ data during a clinical anesthetic study as per our proposed design to validate simulation results. This data was acquired after approval of the Institutional Ethics Committee of the Surat Municipal Institute of Medical Education and Research hospital.
2 The proposed control scheme
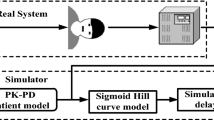
The main objective of the proposed Fractional Order Smith Predictor Controller with MFTM is to regulate hypnosis and to monitor effect of propofol infusion rates μbol1 (t), μcon2 (t) ∈ R+, t ≥ 0 on Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP) and MAP (see Fig. 1). Figure 2 depicts the structure of hypnosis control having the ability to achieve a satisfactory response in a real time clinical environment. The control design needs to achieve the following desired performance criteria.
-
BIS settling time within 1–2.5 min and for MAP within 3–7 min with fewer oscillations.
-
BIS steady-state error with tolerance of ± 5 from set BIS.
-
Integrated Absolute Error (IAE) should be minimum
Here, actual patient model is represented by Gp(s) and reference patient model is represented by \(\overline{Gp} \left( s \right)\). In real clinical environment, a mathematical patient model-based BIS response never matches actual patient BIS response. To mimic this real condition and to produce model mismatch error, the reference patient model is considered along with the actual patient model. The multivariable the patient model is used to analyze propofol infusion effect on BIS and hemodynamic variables. This multivariable patient model is a combination of 3rd order pharmacokinetic (PK) model and three pharmacodynamic (PD) models. Here μbol1 (t) indicates bolus dose and μcon2 (t) represents continuous propofol infusion. Propofol infusion rate μbol1 (t), μcon2 (t) ∈ R, t ≥ 0 are used to maintain DOA in induction and maintenance phase respectively. Propofol infusion leads to sedation which is measured by BIS. The BIS is obtained from the propofol brain concentration using sigmoidal Hill function F1 [see Eq. (18)]. BIS Hill function parameters (EC50 and λ) are estimated from the recorded BIS signal and brain concentration during the induction phase [18]. Further, these estimated parameters are used to select optimum controller gain settings. Hemodynamic variables SBP and DBP are obtained using sigmoidal Hill function F2 from propofol effect on cardiac output and arterial peripheral resistance [see Eqs. (19) and (20)] [42]. MAP signal is derived from SBP and DBP signals [see Eq. (21)]. The estimated value of SBP and DBP Hill function parameters are derived based on minimum IAE error [see Eq. (39)].
There is no sense to connect the controller in the induction phase due to highly nonlinear behaviors of BIS and hemodynamics variables due to large drug transport delay. Lower anesthesia level with continuous infusion in induction phase is painful to the patient. Thus, Propofol bolus dose is essential to achieve adequate DOA within a small time in the induction phase. Moreover, propofol bolus dose reduces BIS within a short time and reduces requirement of propofol in the maintenance phase [16, 18]. To mimic real practices in simulation, proposed control strategy is sent bolus dose (2 mg/kg) to the anesthetic patient model at 10 mg/s infusion rate within first 10 to 25 s. Based on recorded data, propofol bolus dose reduces BIS up to ¼th of the starting value within 56–80 s in all patients. However, propofol bolus dose introduces nonlinearity in BIS and MAP signal due to asymmetric propofol clearance rate. Thus, infusion delay is added in the proposed control strategy design to achieve stable BIS and MAP signal before start controller based propofol infusion. Fixed propofol dose 1.5 mg/kg/h during infusion delay maintains BIS at near 50 against asymmetric propofol clearance rate. Actual and nominal drug transport delays (θp and θr) are produced by the propofol movement from a three-way stopcock to the patient’s body in an intravenous fluid line and distribution of propofol in blood vessels. Optimum delay estimation is required to match actual recorded and simulated signals. The recommended range for actual variable time delay θp s is 80–180 s [9, 43]. The mean value of actual delay (θp) is used as estimated delay (θr) in the proposed control strategy. As mean value always greater than individualized value of delay. These estimated delays are added in simulation.
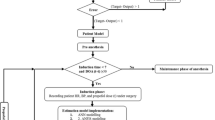
After 80-s infusion delay the value of actual BIS shall be closer to 50. In maintenance phase simulation, a time-based switching manager is used to connect controller after a stipulated time (80 s) period. The proposed Fractional-order Smith Predictor Controller (FSPC) controller will adjust the continuous propofol infusion according to the difference between reference and feedback BIS value. MFTM continuously monitors the feedback BIS signal. In the case of BIS failure, the fault tolerance module will provide an estimated feedback signal to titrate propofol dose. Thus, the proposed Smith predictor design is using MFTM to avoid failure of main feedback path. The proposed control design is compensating the effect of delay; disturbance and model mismatch errors during maintenance phase. Pseudocode of the proposed control strategy is given in “Appendix”.
2.1 Multivariable fault tolerance module design
Generally, the BIS suspension fault produced for a short time period during maintenance phase of anesthesia. However, it may produce undesired actions such as overdosing and under dosing of propofol. In case of the BIS failure, two remedial actions are possible.
One action would be a control strategy estimates feedback BIS based on past recorded value of BIS and deliver the same propofol dose as the one-step before. This estimation may be inaccurate in clinical conditions, hypoglycemia, hypokalemia and hemodynamic instability. As this clinical conditions can disturb MAP and BIS signals. The second would be to switch on the fault tolerance module to tackle BIS sensor fault efficiently. Thus, to solve this issue, switching strategy is used to deal with the sensor fault. The switching strategy mentioned by the subsequent Eqs. (1) and (2). Here, e(t) represents BIS error. The positive value of BIS error indicates that more propofol infusion is required to achieve target BIS and the negative value of BIS error indicates decrement in propofol infusion. In maintenance phase, e(t) = 50 indicates BIS failure condition.
Here, \(e(t) \overset{\wedge}{=}\left| {BIS - BIS_{\bmod el} } \right|\)
When φ = 0, fault is detected. It means \(\bar{\varphi } = 0\). Complete feedback signal Y(t) is depicted by Eq. (2). Here BIS represents the response of the actual patient model and BISmodel represents the response of the reference patient model.
Regression module In faulty condition, the fault tolerance module will switch on multiple linear regression modules to estimate feedback BIS value from MAP and patient model BIS. Analysis of the propofol dosing effect on hemodynamic variables is given in Table 1 based on the recorded signals. A previously reported study [39] suggested that MAP linearly correlates with BIS in the maintenance phase with statistical index R2 = 0.4. A linear relation between BIS and MAP may not be satisfied in all patients due to different drug sensitivity and cross effects of other anesthetic drugs. Therefore, the proposed control strategy is using multiple linear regression to estimate BIS in place of a single variable based linear regression. In our study, the average value of R2 ≥ 0.7. Figure 3 represents regression relation of recorded BIS, estimated BIS and MAP signals. The mathematical relation is depicted by Eq. (3).
The final BIS feedback signal can be estimated using Eqs. (4) and (5). Here, t0= 0 because zero propofol concentration exist in human body before propofol infusion start. t = kT represents propofol concentration at present time. Here, T represents the sampling time period (1 s). The value of regression coefficients η1, η2 and κ for different patients are provided in Table 1.
The BISmodel((k − 1)T) and BIS((k − )T) indicate last recorded BIS values before BIS suspension. Δe((k − 1)T) indicates BIS error based on past recorded values. Consequently, the estimated feedback BISfeedback(kT) is calculated using Eqs. (3) and (5). The proposed fractional order controller (describe in Sect. 4) will titrate propofol dose based on the error of target and estimated feedback BIS signal. When the propofol administrated by non-anesthesiologist (closed loop system), infusion constraint is essential to avoid serious outcomes such as over/under dosing of propofol. Thus Eq. (6) represents infusion constraints of the proposed design.
μth represents the upper constraint value of propofol infusion to avoid over dosing. Lower constraint value of propofol infusion (0.1 mg/kg/h) is used to avoid negative infusion.
3 Mathematical model of patient
In a model-based control strategy, an accurate patient model is an essential to design appropriate control strategy. Moreover, patient model accuracy robustly affects the performance of controller. Thus, inspired by Yoshihito Sawaguchi et al. [9] and Schüttler–Ihmsen et al. [44], the proposed control strategy uses modified PK equations with bolus and continuous infusions and additional 80-s infusion delay. The figure of bolus and continuous propofol infusion with infusion delay is given in Fig. 10 in “Appendix”. Generally, the PK model describes drug absorption, distribution and clearance of the drug in the human body. PK model can be derived from a specific patient’s physiological information like weight and age.
In this paper, the 3rd order modified PK model is used. PD model describes the relation between propofol concentration at effect site and effect of propofol. The complete anesthetic patient model is a combination of PK and PD. This anesthetic patient model measures propofol interaction in the human body and determines the propofol effect on BIS and hemodynamic parameters. However, the PD model parameters (EC50 and λ) are unknown. Most of the reported study had used nominal values of PD parameters in all patients. However, a large difference between nominal and actual values may produce large offset errors in the output BIS signal. Therefore, to reduce offset error and improve the control scheme performance, in proposed control strategy is used estimated PD parameters from brain concentration and BIS signal [18].
Three compartment PK patient model is represented by Eqs. (7) and (9) with bolus and continuous infusion of propofol with infusion delay. Where C1(s) C2(s) C3(s), Ce(s), represents the compartments of the patient model. This study has considered initial value of compartment concentration zero. The C2(s), and C3(s) denote the concentration of residue of the drug in muscles and fat peripheral compartments. Here, Ce(s) represents the brain compartment. Vconi (i = 1, 2, 3) represent volumes (ℓ) of compartments. Kconi (i = 1, 2, 3) represent propofol distribution micro rate constants (ℓ/min−1). Parameter δ = 60 (min/h) for propofol. To convert U (total propofol infusion) in mg/kg/h, it is multiplied by ρ/ω. Where ω is the bodyweight of the patient in kg. ρ represent propofol concentration 10 mg/ml. Td1 is infusion delay. Mathematical equations to derive compartment concentration Ci(s), compartment volume Vconi and drug transport constants Kconi and k12, k21, k13, k31, k1 are given in Table 8 in “Appendix”. The 3rd order PK model is represented by Eq. (10).
+Equation (11) represents simplified form of Eq. (10)
Mathematical relations of aΧ2, aΧ1, aΧ0, bΧ3, bΧ2, bΧ1, bΧ0 are specified in “Appendix” Table 8.
3.1 PD model for brain effect site compartment
The 1st order PD model narrates propofol concentration to BIS. The PD variable, brain concentration is specified by Eq. (12). C1(s) represents plasma propofol concentration. Ce(s) represents brain propofol concentration.
Here, Ke0 = 0.12 ℓ/min−1 analysis pooled from Yelneedi et al. [16]. Delay td represent transport delay between brain and plasma.
3.2 PD model for arterial peripheral resistance and cardiac output effect sites
The proposed control scheme has used the findings of Jeleazcov et al. [42]. They had concluded that two effect sites concentration Ce1 (propofol concentration of arterial peripheral resistance) and Ce2 (propofol concentration of cardiac output) based sigmoidal EMAX model is more effective to derive propofol dosing effect on hemodynamic parameters SBP and DBP [see Eqs. (13)–(16)]. This pharmacodynamics model-based prediction of SBP and DBP is tested and cross-validated by Martín et al. [45], Bergstrand et al. [46] and Bonate et al. [47]. For further details of PD model parameters used in this study see Ref. [42]. The SBP model is suggested by Eqs. (13) and (14).
DBP model is suggested by Eqs. (15) and (16).
Here, the values of Ke01, Ke02, Ke12, Ke13 are pooled from the Jeleazcov et al. [42]. Combination of patient PK model Eq. (11) and First order plus time delay (FOPTD) PD model of BIS, SBP and DBP represents a complete patient model.
The patient model Gp(s) is represented by Eq. (17). The output of patient model is connected with sigmoidal functions to map BIS and hemodynamic variables.
Here, td, td1 and td2 represent drug transport delays between drug infusion and drug effect. The values of these delays are very small value in the maintenance phase due to saturated drug concentration into the patient body.
3.3 Sigmoidal Hill function for BIS
The PD model for BIS is governed by the sigmoidal Hill function. Equation (18) depicts the relationship of effect site (brain) concentration Ce and BIS [1, 9, 16, 17].
where BIS0 indicates an initial value of BIS before propofol infusion and BISmax indicates the maximum intensity effect achieved by the propofol administration. Propofol sensitivity parameters EC50 and λ provide important information about patient body characteristics. The higher value of EC50 and λ indicate the patient’s body required higher propofol dose to achieve BIS = 50 which indicates insensitive patient body characteristics (patient group 3). The smaller value of EC50 denotes a sensitive body due to quick response (patient group 1) [16, 35]. Estimated values of EC50 and λ for all patients are given in Table 9 in “Appendix”. Based on this truth; different patients’ groups’ formation is deliberated.
3.4 Sigmoidal Hill functions for hemodynamic variables
Sigmoidal Hill Eqs. (19) and (20) are used to derive the value of SBP and DBP from two-effect site peripheral compartments [42]. MAP is derived from SBP and DBP by standard Eq. (21).
SBP0 and DBP0 indicate values before propofol infusion. The range of SBP0 ∈ [100 120] and DBP0 ∈ [60 80] is obtained from the recorded data.
The EMAX represents the maximum intensity of drug concentration. The nominal values of sensitivity parameters EC50,1, EC50,2, λ1 and λ2 for SBP and DBP are provided by Jeleazcov et al. [42]. Further, we have modified these nominal values based on minimum IAE error [see Eq. (36)]. Table 2 depicts the mean and standard deviation values of SBP, DBP, MAP and BIS signals which are derived from recorded data. Estimated EC50,1, EC50,2, EMAX1, EMAX2, λ1 and λ2 for SBP and DBP are given in Table 12 in “Appendix”.
4 Design of fraction order Smith predictor controller
The framework reflected the Smith predictor structure with a fractional order controller to regulate hypnosis. Smith predictor with a fractional model based controller is able to capture real patient dynamics accurately in the presence of modeling error, disturbance and delay [22, 27, 28]. Fractional order controllers with new tuning methods are applied in biomedical applications [22, 27, 48]. They concluded that fractional-calculus based regulatory control for a real system is more adequate than integer order control. The fractional order controller uses an additional tuning parameter to optimize the closed-loop robustness to gain deviations. The CRONE controller imposes fraction property on a complete reference close loop in place of only controller structure. These properties make CRONE popular in fractional order controller design. Motivated from fractional order tuning flexibility [28,29,30,31,32,33,34, 50] and CRONE properties, this section discussed CRONE based Smith predictor fractional controller design for an integer order patient model with only two-time domain tuning parameters (γ and τf). These fractional parameters are tuned based on percentage overshoot (PO) and the settling (Ts). The following desires are deliberated for the automatic propofol infusion control system.
-
The system should be designed in such a way that a well damped robust response with percentage overshoot (PO) ≤ 10% should be achieved for set point and disturbance
-
Initial rise time of the system should be around 5 min because surgery cannot be started until the patient acquires an adequate depth of anesthesia. Settling time should be within ± 2%
4.1 CRONE principle
The fractional calculus is a simplification of derivative and integral action to arbitrary orders. The CRONE has two main important properties, (1) phase margin remains constant and (2) insensitive to gain variations. These properties are derived from the analysis of Bode et al. [49]. The ideal shape of the open-loop transfer function for fraction order control concept was suggested by Bode et al. This is denoted by Eq. (22). In Eq. (22), ωgc and τf are gain cross-over frequency and time constant respectively. γ represents the slope of the magnitude curve.
L(s) become an integrator for γ > 0 and become differential for γ < 0. From the bode diagram of L(s), the amplitude of the curve is a constant slope of a straight line with − 20γ dB/dec and phase margin becomes constant at (−γπ/2) rad [33]. Constant phase margin for 1 ≤ γ ≤ 2 indicates robust and stable hypnosis system with gain variation. It means the phase margin is only depending on the value of γ, not on system gain. BIS oscillations are contingent on only the value of γ. Figure 4a, b represent equivalent Smith predictor structure and the desire fractional close loop.
In Eq. (23), CFSPC(s) indicates controller and Gp(s) represents patient model. The system closed-loop transfer function with negative unity feedback is given by Eq. (24).
In the above transfer function as gain changes, gain cross over frequency ωgc also changes but phase margin remains constant at \(\pi (1 - \gamma /2)\) rad. To avoid complexity of frequency domain Castillo-Garcia et al. [34] has been formulated the time domain equations for tuning parameters settling time (Ts) and percentage output (PO) using Mittag–Leffler function. The maximum sensitivity Ms is an analytical method used to measure the robustness and guaranteed stability of the closed-loop system. Maximum sensitivity is represented by Eq. (25). If the value of Ms decreases, robustness increases. Normally the value of Ms preferred in the range of 1.2–2
In our study, the desired value of PO < 10% and Ts equal to 2% are selected respectively. The mathematical formulas for PO and Ts are represented by the Eqs. (26) and (27).
Figure 5a, b depicts the effect of the different values of γ on PO and Ts. The value of percentage overshoot (PO) increases with increases in the value of γ. Settling time Ts normalized with increases the value of γ. In this study, the value of γ is selected in the range of 1.0 to 1.2 to obtain PO less than 10%, maximum sensitivity (Ms) within 1.2 to 2.
4.2 Design procedure of FSPC
Smith predictor structure is shown in Fig. 1. Final transfer function of the Smith predictor from set point to output is denoted by Eq. (28).
To achieve tight control over set point, Y(s) = R(s) condition should be satisfied. The following expression is derived for the Smith predictor controller.
Desire set point response (Y(s)/R(s)) with delay is obtained from Eq. (24) and Fig. 4b.
From Eqs. (29) and (30) final transfer function of fractional order controller is depicted by Eq. (31).
Gp(s) represents the First Order Plus Time Delay (FOPTD) patient model with pade approximation. The proposed fractional order Smith predictor controller can be converted into a cascade form of fraction order filter and integer PID controller using IMC designing. IMC method based fractional order controller design delivers definite closed loop stability by testing the stability of controller and patient model [28]. The final cascade structure of filter and PID controller is derived by Eqs. (32) and (33).
Here, τf signifies the speed of the system response and it also removes model mismatch error normally at high frequencies. Fractional order of the filter is symbolized by γ. IAE function is used as cost functions to reduce performance error see Eq. (39). Small value of IAE indicates fast response and good performance. Table 3 represents optimal values of controller gain and tuning parameters for different patient groups based on propofol sensitivity.
5 Result and discussion
The main goal is to examine the advantages of the proposed feedback control scheme with fault tolerance module as compared to ordinary feedback control system. Our clinical study according to proposed control scheme has considered 30 healthy adult patients of 18–60 years of class ASA-I–II from various surgeries to obtained real propofol infusion rate, BIS and hemodynamic signals. Detail of premedication drug dosing and other anesthetic drug dosing used during surgeries are given in Table 11 in “Appendix”.
These recorded signals are used to examine simulated signals. Simulating each patient in a realistic manner, the proposed control strategy uses real PK parameters of the specific patient and estimated PD parameters from recorded data. Mainly simulation study focuses on the performance evolution of the proposed control strategy in the presence of BIS continuous and heavy hemodynamic surgical disturbances during BIS failure period. Therefore, this study has considered two different cases to examine the robustness of the proposed control scheme.
Case 1 Artifacts in the BIS signal reflects a sudden change in the BIS signal. The sudden change in BIS strongly affects the hemodynamic variables and propofol dosing. Mimic this condition in simulation, by adding sudden disturbance with ± d units in the BIS signal.
Case 2 Artifacts in the blood pressure signal indicate a sharp change in MAP signal (hypertension and hypotension circumstance). Heavy hemodynamic disturbance during BIS failure period directly affects the performance of the fault tolerance module. This critical condition created in simulation by adding sudden disturbance with ± d in MAP signal to test the robustness of fault tolerance module. Hemodynamic surgical stimulation can produce MAP < 65 mmHg. This value of MAP indicates critical incidents. The simulated result validated by comparing with real recorded data of the manual controller. To quantify the performance of the proposed system with manual and previously reported studies, we have used the median performance error (MDPE), median absolute performance error (MDAPE), Wobble and DIVERGENCE [22, 51].
The instantaneous performance error (PE) is defined by Eq. (30). The median performance error (MDPE) provides the control bias and is calculated by Eq. (35).
The median absolute performance error (MDAPE) reflects magnitude of the control inaccuracy.
MDAPE less than 5% is tolerable from a clinical point of view.
The Wobble measure oscillations in output response and is expressed as a percentage (%).
DIVERGENCE indicates divergence of output response with relation to set point and time. Negative value indicates approaching toward set point [22]. Positive value indicates approaching far from set point. Large value of IAE indicate sluggish response and less preferable.
In our proposed strategy, two different propofol infusions are used to reduce BIS settling time and oscillations during the induction phase. In a simulation, the time band t ∈ [0, 3) minute is considered as induction phase. The maintenance phase starts at t ∈ [3, 57] minute. Figure 6a, b represent a comparison of real recorded BIS and MAP response with simulated signals. It should be noted that the proposed control strategy demonstrates robust performance.
However, from the perspective of patient safety, the proposed control strategy provides balanced management of sedation to regulate BIS and MAP within the desire limit. The performance evaluation measure for the proposed control strategy and other methods are given in Table 4. Sign of MDPE reflect direction of (over and under control) performance error. The proposed scheme with bolus and continuous propofol infusion is able to maintain BIS at desired set point and also maintains a MAP with in the desired range. Comparative analysis with similar reported closed loop control studies show that an average 1.2% improvement in MDPE and 5.4% improvement in MDAPE. Performance indexes MDPE and MDAPE of the proposed strategy are found less than manual control.
Case 1 In order to further investigate the effect of BIS surgical stimulus on the regulation of BIS, propofol infusion and MAP signal, two scenarios are presented. (1) BIS(t), t ≥ 0 for normal condition (2) BIS(t) + d, 20 < t<50 for disturbance in BIS signal. Here, d represents magnitude of continuous disturbance. Standard noxious disturbance pattern is represented by Fig. 7a. Detail about trajectory of disturbance profile is discussed in article [22]. Noxious disturbance pattern A indicates the event of arousal reaction due to the surgical incision. B represents continuous surgical disturbance. C pretends the withdrawal of stimulations during the skin-closing. The goal of proposed control strategy is to provide the adequate propofol dose to maintain minimum set point error in BIS and MAP signals.
Figure 7b–f represent the proposed FSPC controller performance on 30 different patient models in the presence of continuous disturbance to maintain DOA and effect of propofol dosing on hemodynamic variables. Sudden changes in BIS cause the controller to fully open the propofol infusion pump to achieve target BIS within a short time. However, the average propofol dose delivered by the proposed system during set point control and BIS disturbance are respectively: 2.5 mg/kg/h and 3.55 mg/kg/h. It signifies that proposed scheme provides safety against over and under dosing of propofol in the presence of BIS disturbance. Fractional order Smith predictor provides fast response with good disturbance rejection due to the small values of tuning parameters τf and γ. The proposed controller ensures robustness in terms of gain uncertainty and disturbances while fulfilling desire time domain criteria such as percentage overshoot and settling time. Moreover, maintaining SBP, DBP and MAP within an acceptable physiological range which guarantees adequate perfusion of internal organs.
Adequate propofol dose is delivered by the system with acceptable overshoot during surgical disturbance. Tables 5 and 6 show that proposed FSPC leads PO within 10% in all patients during the disturbance. The average settling time for BIS 50 ± 2 is 250 s and MAP 87 ± 18.73 is 4 to 7 min. The minor difference in BIS offset error is produced due to the inter patients’ variability and different values of propofol sensitivity parameters. Clearly, there is some inconsistency in the measured MDAPE errors but it is limited and doesn’t meaningfully affect the set-point tracking. Small values of the wobble represent bump less performance. In presence of disturbance, negative value of divergence directs aggressive reaction to reach zero BIS error. Simulation results reflect that this proposed approach has the potential to overcome the effect of time delays, patient variability and disturbances. Proposed may lead to sudden change in MAP signal. This critical transition was documented by Frei et al. [38].
Based on this information proposed study is analyzed three different scenarios for MAP signal (1) MAP(t), t > 0 indicates normal condition (2) MAP(t) + d, t > 25 min indicate hypertension with magnitude of heavy disturbance (+d) = 13 and (3) MAP(t)−d, t > 40 min indicate hypotension with pleasant disturbance magnitude (−d) = 8. Figure 8a represents incidence of BIS failure during septoplastic surgery. Figure 8b, c represents comparisons of three different methods to estimate feedback BIS during BIS failure.
From the result, multivariable based estimated feedback BIS is more precise as compared to only patient modeled BIS or MAP signal. The only MAP based BIS estimation may imprecise due to sluggish response of MAP with a change in propofol and insignificant value of a statistical index. Only modeled BIS based feedback BIS estimation could not be able to capture hemodynamic surgical stimulation effect in estimated BIS. Thus, there is no significant variation in propofol dosing during the BIS failure period (see Fig. 8c).
As per medical practices, propofol dose should be changed to suppress hemodynamic disturbance adverse effect on BIS and hemodynamic variables. Based on this reason only, modeled BIS based propofol infusion may not be considered as an adequate infusion in the presence of hemodynamic disturbance. Thus, only modeled BIS or MAP signal-based feedback BIS estimation may not trustworthy in the presence of MAP disturbance during BIS failure. The result shows that multivariable based estimation of feedback BIS is providing precise result as compare to existing methods.
In Fig. 9a–d simulation results show the effect of positive and negative MAP signal variations on estimated feedback BIS and propofol dosing during BIS sensor failure. MAP variation depends on non-measurable variables such as intensity and location of stimulation. Height of the MAP signal can deviate 60% from the actual values in the presence of hemodynamic disturbance. The evolution of controller performance in the presence of hemodynamic disturbance during the BIS suspension period is essential to test robustness. From simulation results, obviously the proposed algorithm can handle heavy and pleasant hemodynamic disturbance more robustly than reported study during BIS failure condition. Statistical analysis of the proposed scheme (see Table 7) shows that the average value of IAE equal to 480 and 170 s average time is required to compensate MAP disturbance. This performance index reflects the satisfactory performance of the proposed controller. Small values of DIVERGENCE indicate tight control toward a set point. The proposed control scheme also maintains acceptable range of MDPE, MDAPE and propofol infusion constrains. The proposed FSPC controller output has never saturated between upper and lower infusion constrain limits. It means smooth and optimum propofol infusion is delivered by the system. The proposed control strategy has not calculated cross effect of an analgesic drug on BIS and hemodynamic variables. In future work, the proposed controller design will be used with the combination of analgesic drugs and ensure robustness with the variability in an analgesic infusion. The results evidently validate the efficacy of a proposed system in the presence of surgical disturbances and BIS failure. Based on the obtained result the following considerations are prepared.
Result validation of proposed FSPC controller in the presence of heavy hemodynamic disturbance during BIS failure condition for four different patients. BIS, MAP and propofol infusion rate for a case 1 (patient 1), b case 2 (patient 2), c indicates case 3 (patient 3) and d indicates case 4 (patient 4)
-
1.
The proposed fractional Smith predictor controller with fault tolerance concept provides fast transient in the induction phase and limited BIS oscillations in the maintenance phase. The performance of the proposed FSPC system provides good results in order to maintain hemodynamic stability during faulty conditions than recently reported fault tolerant control strategy [40].
-
2.
The desired feature of the proposed control strategy is to obtain fault identification, automatic situation assessment and calculation of appropriate corrective actions to avoid certain consequences of a BIS sensor failure fault.
-
3.
Integration of fault handling concept with control scheme improves safety against the malfunction of the control scheme and also increases robustness, stability and patient safety.
6 Conclusion
In this paper, the proposed control strategy is designed with the integration of a multivariable fault tolerance module and a fractional-order control strategy to tackle BIS failure problem more effectively. Simulation results using 30 different patient models with varying pharmacological parameters show that the proposed control strategy is promising in design to regulate propofol sedation, BIS and hemodynamic parameters within the desired range. Further simulation results show that estimated feedback BIS using multivariable fault tolerance module is able to catch patient body dynamics accurately than single variable based estimation in the presence of hemodynamic instability. Results show that the proposed control strategy is able to provide stable performance in the presence of BIS sensor fault and heavy surgical disturbance. Further, simulation results are validated with recorded signals during surgery. The proposed control strategy satisfies global requirements like, to maintain adequate propofol sedation, small settling time and smooth BIS and hemodynamic signals during surgery. To develop automatic propofol infusion system in clinical intravenous anesthesia is plausible with the proposed control system and it has the potential to provide stability and satisfactory performance in automatic propofol infusion in most of the patients during surgery.
References
Struys MM, De Smet T, Versichelen LF, Van De Velde S, Van den Broecke R, Mortier EP. Comparison of closed-loop controlled administration of propofol using bispectral Index as the controlled variable versus standard practice controlled administration. Anesthesiology. 2001;95(1):6–17.
Ilyas M, Butt MFU, Bilal M, Mahmood K, Khaqan A, Riaz, RA. A review of modern control strategies for clinical evaluation of propofol anesthesia administration employing hypnosis level regulation. Hindawi BioMed Research International, 2017;2017:1–12.
Neckebroek M, De Smet T, Struys M. Automated drug delivery in anesthesia. Curr Anesthesiol Rep. 2013;3:18–26.
Absalom A, Sutcliffe N, Kenny G. Closed-loop control of anesthesia using Bi spectral index: performance assessment in patients undergoing major orthopedic surgery under combined general and regional anesthesia. Anesthesiology. 2002;96:67–73.
Reboso JA, Méndez JA, Reboso HJ, León AM. Design and implementation of a closed-loop control system for infusion of propofol guided by bispectral index (BIS). Acta Anaesthesiol Scand. 2012;56(8):1032–41.
Heusden KV, Dumont GA, Soltesz K, et al. Design and clinical evaluation of robust PID control of propofol anesthesia in children. IEEE Trans Control Syst Technol. 2014;22(2):491–501.
Padula F, Ionescu C, Latronico N, et al. Optimized PID control of depth of hypnosis in anesthesia. Comput Methods Programs Biomed. 2017;144:21–35.
Merigo L, Beschi M, Padula F, Latronico N, Paltenghi M, Visioli A. Event-based control of depth of hypnosis in anesthesia. Comput Methods Programs Biomed. 2017;147:63–83.
Yoshihito S, Eiko F, Gotaro S, Mituhiko A, Kazuhiko F. A model predictive hypnosis control system under total intravenous anesthesia. IEEE Trans Biomed Eng. 2008;55(3):874–87.
Naşcu I, Krieger A, Ionescu CM, Pistikopoulos EN. Advanced model-based control studies for the induction and maintenance of intravenous anesthesia. IEEE Trans Biomed Eng. 2015;62(3):832–41.
Ionescu C, Machado JT, De Keyser R, Decruyenaere J, Struys M. Nonlinear dynamics of the patient’s response to drug effect during general anesthesia. Commun Nonlinear Sci Numer Simul. 2015;20:914–26.
Ionescu CM, Copot D, Keyser R. Anesthesiologist in the loop and predictive algorithm to maintain hypnosis while mimicking surgical disturbance. IFAC Pap Online. 2017;50(1):15080–5.
Neckebroek M, Ionescu CM, van Amsterdam K, De Smet T, De Baets P, Decruyenaere J, De Keyser R, Struys MMRF. A comparison of propofol-to-BIS post-operative intensive care sedation by means of target-controlled infusion, Bayesian-based and predictive control methods: an observational open-label pilot study. J Clin Monit Comput. 2019;33(4):675–686.
Ilyas M, Iqbal J, Ahmad S, et al. Hypnosis regulation in propofol anesthesia employing super-twisting sliding mode control to compensate variability dynamics. IET Syst Biol. 2020;14(2):59–67.
Ilyas M, Khaqana A, Iqbalb J, Riaz RA. Regulation of hypnosis in propofol anesthesia administration based on non-linear control strategy. Braz J Anesthesiol. 2017;67(2):122–30.
Yelneedi S, Samavedham L, Rangaiah GP. A comparative study of three advanced controllers for the regulation of hypnosis. J Process Control. 2009;19(9):1458–69.
Méndez JA, Torres S, Reboso JA, Reboso H. Adaptive computer control of anesthesia in humans. Comput Methods Biomech Biomed Eng. 2009;12(6):727–34.
Bhavina P, Hiren P, Pragna V, Divyang S, Alpesh S. Adaptive Smith predictor controller for total intravenous anesthesia automation. Biomed Eng Lett. 2019;9(1):127–44.
Abdulla SA, Wen P. Robust internal model control for depth of anesthesia. Int J Mechatron Autom. 2011;1(1):1–8.
Mendez JA, Leon A, Marrero A, Gonzalez-Cava JM, Reboso JA, Estevez JI, Gomez-Gonzalez JF. Improving the anesthetic process by a fuzzy rule based medical decision system. Artif Intell Med. 2018;84:159–70.
Padmanabhan R, Meskin N, Haddad WM. Closed-loop control of anesthesia and mean arterial pressure using reinforcement learning. Biomed Signal Process Control. 2015;22:54–64.
Dumont GA, Martinez A, Ansermino JM. Robust control of depth of anesthesia. Int J Adapt Control Signal Process. 2009;23:435–54.
Ajwad SA, Iqbal J, Ullah MI, et al. A systematic review of current and emergent manipulator control approaches. Front Mech Eng. 2015;10:198–210.
Iqbal J, et al. Nonlinear control systems—a brief overview of historical and recent advances. Nonlinear Eng. 2017;6(4):301–12.
Ullah S, Mehmood A, Khan Q, et al. Robust integral sliding mode control design for stability enhancement of under-actuated quadcopter. Int J Control Autom Syst. 2020;18:1671–8.
Jamshed Iqbal. Modern control laws for an articulated robotic arm: modeling and simulation engineering. Technol Appl Sci Res. 2019;9(2):4057–61.
Ionescu CM, Lopes A, Tenreiro Machado J, Bates J. The role of fractional calculus in modeling biological phenomena: a review. Commun Nonlinear Sci Numer Simul. 2017;51:141–59.
Muresan CI, Dutta A, Dulf EH, Pinar Z, Maxim A, Ionescu CM. Tuning algorithms for fractional order internal model controllers for time delay processes. Int J Control. 2016;89(3):579–93.
De Keyser R, Muresan CI, Ionescu C. A novel auto-tuning method for fractional order PI/PD controllers. ISA Trans. 2016;62:268–75.
De Keyser R, Ionescu CM, Muresan CI. Comparative evaluation of a novel principle for PID auto tuning. In: 11th the Asian control conference gold coast convention Centre Australia, 2017; p. 1164–9.
Muresan CI, Birs IR, Prodan O, Nascu I, De Keyser R. Approximation methods for FO-IMC controllers for time delay systems. In: E3S web of conferences, 2019; p. 115.
Birs IR, Muresan CI, Nascu I, Ionescu CM. A survey of recent advances in fractional order control for time delay systems. IEEE Access. 2019;7:30951–65.
Marzieh S, Saeed T. Smith predictor based fractional-order control design for time-delay integer-order systems. Int J Dyn Control. 2017;6(1):180–7.
Castillo-Garcia FJ, Feliu-Batlle V, Rivas-Perez R. Time domain tuning of fractional order controllers combined with a Smith predictor for automation of water distribution in irrigation main channel pools. Asian J Control. 2013;15:819–33.
Ionescu CM. A computationally efficient Hill curve adaptation strategy during continuous monitoring of dose–effect relation in anaesthesia. Nonlinear Dyn. 2018;92(3):843–52.
Fan SZ, Wei Q, Shi PF, Chen YJ, Liu Q, Shieh JS. A comparison of patient’s heart rate variability and blood flow variability during surgery based on the Hilbert Huang transform. Biomed Signal Process Control. 2012;7(5):465–73.
Frei CW. Fault tolerant control concepts applied to anesthesia. Ph.D. thesis, ETH Zurich; 2000.
Yu Y-N, Doctor F, Fan S-Z, Shieh J-S. An adaptive monitoring scheme for automatic control of anesthesia in dynamic surgical environments based on bispectral index and blood pressure. J Med Syst. 2018;42(5):95.
Sang WL, Soo EC, Jin Hee H, Sung-Wook P, Wha Ja K, Young KC. Effect of beach chair position on Bi spectral index values during arthroscopic shoulder surgery. Korean J Anesthesiol. 2014;67(4):235–9.
Tao Y, Fang M, Wang Y. A fault tolerant closed loop anesthesia system based on internal model control and extended state observer. In: 25th Chinese control and decision conference (CCDC), 2013; p. 4910–4.
Neckebroek M, Boldingh JW, De Smet T, Struys MM. Influence of remifentanil on the control performance of the bispectral index controlled Bayesian-based closed-loop system for propofol administration. Anesth Analg. 2020;130(6):1661–9.
Jeleazcov C, Lavielle M, Schüttler J, Ihmsen H. Pharmacodynamics response modeling of arterial blood pressure in adult volunteers during propofol anesthesia. Br J Anesth. 2015;115(2):213–26.
Ionescu CM, Hodrea R, Keyser R. Variable time-delay estimation for anesthesia control during intensive care. IEEE Trans Biomed Eng. 2011;58(2):363–9.
Schuttler J, Ihmsen H. Population pharmacokinetics of propofol: a multicenter study. Anesthesiology. 2000;92(3):727–38.
Martín-Mateos I, Pérez JM, Morales JR, Gómez-González JF. Adaptive pharmacokinetic and pharmacodynamics modeling to predict propofol effect using BIS-guided anesthesia. Comput Biol Med. 2016;75:173–80.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Bonate PL. Pharmacokinetic-pharmacodynamics modeling and simulation. 2nd ed. New York: Springer; 2011.
Birs I, Copot D, Muresan CI, De Keyser R, Ionescu CM. Robust fractional order PI control for cardiac output stabilization. IFAC Papers Online, Florianópolis - SC, Brazil, 2019:52(1):994-99.
Bode HW. Network analysis and feedback amplifier design. New York: Van Nostrand; 1945.
Chevalier A, Francis C, Copot C, Ionescu CM, De Keyser R. Fractional-order PID design: towards transition from state-of-art to state-of-use. ISA Trans. 2018. https://doi.org/10.1016/j.isatra.2018.09.017.
Struys MM, Smet T, Greenwald S, Absalom AR, Bing S, Mortier EP. Performance evaluation of two published closed-loop control systems using bi spectral index monitoring: a simulation study. Anesthesiology. 2004;100(3):640–7.
Acknowledgements
The authors would like to thank to anesthesia department team of the SMIMER hospital, Surat for providing the clinical environment facility and drug dose combination as per proposed scheme. The authors are grateful to anonymous reviewers for their useful suggestions to improve the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in study involving human participants were in accordance with the ethical standards of the Surat Municipal Institute of Medical Education and Research (SMIMER), India and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards or comparable ethical standards. Informed consent: Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
See Tables 8, 9, 10, 11, 12 and Figs. 10 and 11.
Rights and permissions
About this article
Cite this article
Patel, B., Patel, H., Shah, D. et al. Control strategy with multivariable fault tolerance module for automatic intravenous anesthesia. Biomed. Eng. Lett. 10, 555–578 (2020). https://doi.org/10.1007/s13534-020-00169-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13534-020-00169-2