Abstract
Objective
Metabolic syndrome (MetS) and insulin resistance (IR) are associated with diabetes. Insulin therapy in type 1 diabetes (T1DM) may complicate the diagnosis of both these conditions. Therefore, investigation of the diagnostic efficacy of MetS and IR components is important in paediatric population with type 1 diabetes mellitus (T1DM).
Methods
SCOPUS, Web of Science, and PubMed were searched for studies that have MetS and IR in paediatric populations with T1DM. We assessed the strength of association for MetS and IR components. A random effect model was used for the meta-analysis and the effect size was reported in terms of Hedge’s g.
Results
A total 30 studies were identified relevant to our systematic search. Insulin dosage and HbA1c, markers for glycemic condition showed very small effect on MetS with T1DM. In the lipid profile, triglyceride (TG) and low-density lipoprotein (LDL) showed better effect size than high-density lipoprotein (HDL). In case of IR, heterogeneous nature of studies made it difficult to carry out a meta-analysis. A descriptive review of existing and novel markers is thus provided.
Conclusion
In children with T1DM, lack of association between markers of glycemic condition suggested that MetS may develop independent of glycemic level. Other than TG and HDL, LDL may be used in the diagnosis of MetS. A universally accepted diagnosis protocol would enhance accuracy and comparability across research and clinical settings, as observed in the descriptive review.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Type 1 diabetes mellitus (T1DM) is an autoimmune condition which results in the loss of pancreatic beta cells. This leads to dependency of the person on exogenous insulin therapy. The peak age for T1DM diagnosis is 5-9yrs and 10-14yrs with the prevalence increasing among young individuals [1, 1,2,3,4,5]. People with recently diagnosed T1DM generally have a lower body mass index; however, obesity rates has risen in this population [6]. Notably, metabolic syndrome (MetS) and insulin resistance (IR) can also be observed in lean individuals with T1DM [7,8,9]. MetS and IR are the risk factors for cardiovascular diseases (CVD). Therefore, diagnosis and management of MetS and IR are crucial for the prevention of cardio metabolic risks.
The prevalence of MetS in people with T1DM is suggested to be 23.7% and is increasing [10, 11]. The diagnosis of MetS is based on three different criteria that are laid down by the World health Organization (WHO), the National Cholesterol Education Programme Adult Treatment Panel III (NCEP ATP III), and the International Diabetes Federation (IDF) [12]. These criteria are based on anthropometric measurements such as waist circumference (WC), hypertension (HTN) and biochemical parameters such as the lipid profile (Table 1).
Most of the cut-offs for the diagnosis of MetS are developed for the adult population [15,16,17]. These parameters are modified only by changing the threshold for use in paediatric population. Along with MetS, the prevalence of IR in children with T1DM termed as double diabetes is also risen in children [18,19,20,21,22].The term double diabetes has been used to refer to individuals with T1DM who are overweight, have family history of diabetes, and have clinical features of insulin resistance [18]. Factors such as food habits, reduced physical activity, gender, age, and genetic predisposition may contribute to the development of IR in children with T1DM [23, 24]. Presence of IR in children with T1DM increases the risk of development of various macro and microvascular complications [25]. Hence, the diagnosis of IR may help clinicians to implement preventive measures or add an adjuvant therapy.
The diagnosis of IR in Type 2 diabetes (T2DM) depends on measurement of fasting insulin levels which are negligible in T1DM. Therefore, the indices used for the diagnosis of IR in T2DM have little use in T1DM. The gold standard method for the diagnosis of IR in children with T1DM is the Hyperinsulinemia Euglycemic Clamp (HEC) in which the glucose concentration is maintained by variable infusion of exogenous glucose and insulin [26]. However, the HEC technique is expensive, and space and time consuming. Therefore, various alternate methods have been developed for the diagnosis of IR that rely on indirect markers such as estimated glucose disposal rate (eGDR) [27,28,29,30], Insulin Sensitivity Score (ISS) [31] and, insulin sensitivity equation (eIS) [32] provided by Epidemiology of Diabetes Complications (EDC), Search for diabetes in youth (SEARCH), and Coronary Artery Calcification in T1DM (CACTI) respectively (Table 2).
The indices for IR in T1DM have been validated by direct comparison with HEC [8, 31, 35, 36]. There are no threshold or cut-offs provided for these indices. However, many authors have provided cohort based thresholds. Most of these studies include adults with T1DM (Supplementary Table 1) [37,38,39,40,41]. The exogenous insulin administration and pubertal age may interfere with the existing parameters of MetS and IR. Therefore, a systematic review and meta-analysis is needed for both these conditions.
Methods
This is an exploratory meta-analysis and follows the PRISMA (Preferred Reporting of Systematic Review and Meta-analysis) guidelines.
Search strategy, and Inclusion and exclusion criteria
Two authors independently searched for the relevant keywords in three databases (PubMed, SCOPUS, Web of Science) for identification of research articles related to MetS and IR in children, adolescents, and young adults with T1DM. The search was performed till May 5, 2023. The articles were from 1982 to 2023. The search for the relevant keywords was as follows.
((("Type 1 Diabetes" OR "IDDM" OR "insulin dependent diabetes" OR "T1DM") AND ("insulin resistance" OR "IR" OR "Metabolic syndrome" OR "MetS" OR "insulin sensitivity" OR "IS")) AND ("Molecular markers" OR "markers" OR "Biological markers" OR "Clinical markers" OR "gene expression markers")) AND ("Paediatric" OR "child" OR "children" OR "adolescent" OR "adolescence" OR "young adult").
The search was limited to peer reviewed English articles. Only original research articles were included for this review. Studies that had type 1 diabetes population with the age group < 25yrs were retained. The studies were then imported to a Rayyan software for screening and removal of duplicates [42]. Studies using animal models, cell lines, and organ tissue samples were excluded. Studies including children with complications associated with diabetes and on medication other than insulin therapy were excluded.
Selection of studies and data extraction
We segregated the studies based on presence or absence of MetS and IR in the T1DM population. The studies that provide markers for such conditions, either standard (insulin dose, eGDR for IR, IDF criteria for MetS) or surrogate (body mass index: BMI, WC etc.), were included in this review. Meta-analysis was performed only if multiple studies with similar parameters were available. Other studies were utilized for descriptive review. Parameters such as duration of diabetes, insulin dosage, HbA1c, and lipid profile were assessed in each study. The sample size, mean, and standard deviation (sd) for each parameter were recorded accordingly. If median and interquartile range were provided they were converted to estimated mean and variance depending on sample size [43]. Author names, publication year, ethnicity, and gender details of the population were also recorded for the studies that were part of the systematic review (Table 3).
Statistical analysis and evaluation
Meta-analysis was performed when two or more studies reported mean, standard deviation, and sample size. Metaphor package was applied for the analysis [69]. Standard Mean Difference (SMD) was calculated using R (version 4.1.1). We calculated the effect size (ES) in terms of hedges g that corrects for the sample size providing unbiased adjusted ES. Random effects model (REM) was used for quantitative meta-analysis. A forest plot was used to visualize summary of results [70]. Chi-squared test was used to measure heterogeneity (p val < 0.1). The I2 statistic was used to estimate if the heterogeneity was considerable (I2 > 40%) [71]. The strength of relationship between parameters and traits was estimated based on the effect size (0–0.2: no effect; 0.2–0.5: small; 0.5–0.8: moderate; 0.8–1: large; > 1: very large effect) [72].
Assessment of Sensitivity and publication bias
Funnel plots were used for visualization of publication bias [73]. The pooled results were analysed for their sensitivity by sequential removal of individual studies and their effect on heterogeneity.
Results
Identification of studies for diagnostic markers of MetS and IR
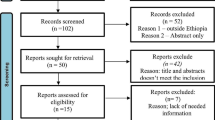
We identified 67 research articles on PubMed, 930 on SCOPUS, and 88 on Web of Science by searching keywords in titles and abstracts. After applying the filters for language and exclusion criteria, 66, 739, and 86 articles were retained. Manual search provided 3 additional studies. These articles were then imported in Rayyan [42]. In this software 78 duplicate articles were removed and 816 unique original research articles were retained. Based on the screening of abstracts and titles, 743 articles were omitted. Full text scrutiny identified 73 research articles, and 30 research articles were retained based on inclusion and exclusion criteria (Fig. 1).
The general nature of these research articles is mentioned in Table 3. All were observational studies with a cross-sectional or longitudinal design. The data in the studies was either prospectively collected or used retrospectively from registries and hospitals.
Qualitative summary and characteristics of studies
As mentioned earlier, we limited our search to observational studies. There were a total of 30 studies with standard and surrogate markers of MetS and IR in T1DM. 12 studies were based on case–control and 18 studies were cohort based. Six studies provided novel markers for IR whereas, 24 studies used existing parameters for IR and MetS. Information about ethnicity was not available for 15 studies (Table 3). Five of the 30 studies compared children with T1DM with children whereas, another 5 studies compared children with T1DM to children with T2DM. Four studies assessed MetS in children with T1DM by grouping them according to IDF criteria. The grouping of studies for IR was difficult as only two studies have classified the children with T1DM on the basis of IR indices (eGDR) [45, 65] (Table 4).
Assessment of markers for MetS in T1DM
Four studies out of thirty have grouped T1DM children as being MetS positive and MetS negative (Table 4). The parameters such as units of insulin, HbA1c, WC and lipid profile were selected for our meta-analysis. Summary statistics for fasting glucose and hypertension were not available.
Random Effect Model (REM) was used where, WC (d = 1.34, [95% CI: 0.79–1.90]) and TG (d = 0.85, [95% CI: 0.14–1.55]) showed significantly large effect size whereas, HbA1c (d = 0.75, [95% CI: −0.20–1.71]), and LDL (d = 0.73, [95% CI: 0.15–1.32]) showed a moderate effect on MetS. The effect size was significant for LDL but not for HbA1c. On the other hand, HDL (d = 0.37, [95% CI: −0.65–−0.10]) showed a significantly small negative effect. Units of insulin dosage (d = 0.17, [95% CI: −0.06–0.4]) also showed no significant effect on MetS (Fig. 2).
Assessment of publication bias
No heterogeneity was observed for insulin dose and HDL, however; a heterogeneity was observed for HbA1c, LDL, TG, and WC in the identified datasets (Fig. 2). Since, the latter showed a significant heterogeneity, we decided to assess the publication bias. A funnel plot analysis was performed for all the markers mentioned above (Supplementary Fig. 1). HDL and the units of insulin did not show any outliers. The publication bias was assessed for the remaining parameters such as HbA1c, WC, TG, and LDL by sequential removal of each study. The study by Monika Grabia et al. 2022 strongly contributed to the heterogeneity for HbA1c, TG, and LDL. Removal of this dataset removed the heterogeneity and improved the effect size of TG (from 0.85 to 1.18) and LDL (from 0.73 to 1). The effect size of HbA1c (from 0.75 to 0.32) on the other hand, reduced. In case of WC, strong heterogeneity was contributed by the study by Soliman et al. 2019. Removal of this dataset improved the effect size of WC (from 1.34 to 1.63). The possible sources of heterogeneity are discussed later. In summary, TG, LDL, and WC seem to have a significantly large effect on MetS (Supplementary Fig. 2).
Assessment of markers for IR in T1DM
Out of the 30 studies, only two studies had grouped the participants based on presence or absence of IR [45, 65]. In both these studies the measurement of IR was performed by using eGDR. However, Nisthala et al., 2020, divided the children having T1DM by eGDRBMI and the association of eGDRBMI with different clinical parameters was observed. The study suggested that the population in lower quartiles of eGDRBMI had significantly higher levels of total cholesterol and triglycerides. Dabelea et al., 2011 attempted to segregate the population of children with T1DM and T2DM based on eGDR. The study found stronger association of IR in children with T2DM than in T1DM. The parameters to calculate eGDR and the study design were not consistent between these two studies (Table 4). As a result, we provide a descriptive review of other markers for IR. Some of the markers that include Volume of Oxygen uptake during peak exercise (VO2peak), Free Fatty Acid (FFA), Leptin, cIMT (carotid intima media thickness), have been validated using HEC. A few others markers have been validated using indices such as eGDR, SEARCH, and CACTI (Table 3).
Quantitative markers frequently used by clinicians include measurement of insulin dosage in combination with HbA1c [46], central obesity [54], and body fat [8]. Along with HbA1c, family history for T2DM is an important parameter. Central obesity measured by waist to height ratio > 0.5 and BMI > 95 percentile are also suggested parameters for IR [60]. Body fat estimated by thickness of triceps and subscapular skin fold have been used to predict body fat [8]. A qualitative marker: acanthosis nigricans is also used as an indicator of IR; however, it is more related to obesity than IR [45, 52, 53, 55].
Some of the novel quantitative markers such as adiponectin, leptin, fetuin A, and kisspeptin are being investigated for the assessment of IR. A longitudinal study in T1DM children suggested that levels of adiponectin, (a hormone produced by adipocytes with a role in insulin sensitization) were strongly related to WC and insulin dose in 20 yr old adults with T1DM [59]. Adiponectin and leptin (another hormone produced by adipose tissue involved in maintenance of normal body weight), both have been studied in association with IR [48]. It has recently been suggested that leptin may act as a potential biomarker for the detection of IR in T2DM. In case of T1DM, the association of leptin with IR is not very well studied. However, a few reports suggest that fluctuations in leptin levels are observed in children and adolescents with T1DM [48, 62]. Increase in fetuin A, a hepatokine and an adipokine, is associated with IR and obesity. In T1DM, this association was limited to glycemic levels and as a risk predictor for complications of diabetes. Further studies to assess the role of fetuin A in IR are needed. Another hormone, Kisspeptin (produced in the hypothalamus) inversely associates with adiponectin levels and in turn, to insulin sensitivity [75]. However, the association was only studied in reproductive age female population. Further studies will be required to conclude kisspeptin as a marker for IR. Two studies have shown an association of IR markers (increased insulin dose, increased BMI and, increased Dehydroepiandrosterone sulphate (DHEAS) with increased micro-albuminuria [54, 58]. The DHEAS is a precursor for sex hormones and is known to act as an insulin sensitizer. VO2peak which is a measure of cardiovascular and skeletal muscle oxidative function shows a significant moderate positive correlation with HEC (reduced GDR by HEC indicate IR) [44].
Other less studied novel indicators include carbohydrate (CHO) oxidation and Delta 6 desaturase (D6D) activity. The CHO oxidation which estimates the capacity to oxidise a meal in the form of differential 13C/12C enrichment in the expired air using flow isotope mass spectrometry, has been associated with IR. The CHO oxidation showed a moderate correlation with eGDR in T1DM [64]. A high activity of D6D, a rate limiting enzyme in production of long chain Poly Unsaturated Fatty Acids (PUFA) has been associated with decreased insulin sensitivity therefore, increased activity of D6D has been suggested to be a strong marker for IR in T1DM adolescents [66] and non-diabetic adults [76]. All the novel quantitative markers are still under investigation and are not part of routine clinical applications.
Discussion
Metabolic syndrome (MetS) and Insulin resistance (IR) in combination and independently can be the risk factors for CVD. Usually surrogate markers are used for the diagnosis of MetS and IR in children with T1DM. We performed a systematic review and a meta-analysis to study the effect size of the parameters for the diagnosis of MetS in children with T1DM. Participants with T1DM aged < 25yrs were included due to the lack of experimental evidence for the cutoffs in this age group. To focus specifically on metabolic syndrome, we excluded participants with complications related to T1DM or those taking medication other than insulin therapy. Inconsistency in measurement methods made it challenging to perform a similar meta-analysis for IR (Table 4).
In our meta-analysis, insulin dosage and HbA1c showed low effect size suggesting that the MetS appears independent of glycemic condition in children with T1DM (Fig. 2a, b). WC was strongly associated (with large effect size) with MetS in T1DM (Fig. 2c). Since, all four studies made use of the IDF criteria which require central obesity as a mandatory component for the assessment of MetS, this association was expected. However, this association was observed with a considerable heterogeneity that was contributed by Soliman et al. (2019). The study cohort was from Egypt and the population has been shown to have a different cut-off for WC for obesity [77]. Removal of this study removed the heterogeneity and increased the effect size (Supplementary Fig. 2). Our results fall in line with previous studies where WC predicted MetS in adults with T1DM [78] and was significantly associated with MetS in children who did not have diabetes [79].
Increased TG and LDL were also associated (large and moderate effect size respectively) with MetS in children with T1DM (Fig. 2f). The source heterogeneity contributed to this association may have been from the attempt to convert median and interquartile range provided by Monika Grabia et al. (2021) to mean and standard deviation [43]. The omission of this study did not alter the effect size for TG whereas, effect size for LDL improved from moderate to large (Supplementary Fig. 2). TG are already a part of IDF criteria and together with WC provide a better diagnostic efficiency for MetS [80]. Considering that LDL is not a part of the IDF criteria for MetS, the strong association of LDL with MetS is noteworthy. Increased LDL is suggested to be a risk factor for CVD [81]. Significantly increased LDL was observed in children who do not have diabetes but, had predisposition to MetS [82]. Moreover, reduction in LDL levels are suggested as a treatment strategy by the IDF [83]. This reflects the significance of LDL in MetS. Therefore, increased LDL can be used as one of the parameters to screen for MetS in children with T1DM. However, LDL alone might be an insufficient indicator and may thus be used along with other parameters in the assessment of MetS [84]. HDL is one of the parameters proposed by the IDF, WHO, and NCEP III to screen MetS. HDL is known to have a negative association with MetS which was reflected in our analysis. All datasets showed homogeneity for HDL; however, the cumulative effect size of HDL was moderate. Other than lipid profile, some inflammatory markers such as adiponectin and leptin are under investigation for their association with cardiometabolic risk in children with MetS [68].
For IR, we came across only two studies where young people having T1DM were classified based on presence or absence of IR. Diverse designs and varying parameters to test IR made the compilation of studies difficult. We came across a large number of non-invasive and invasive parameters used to assess IR in T1DM. Most of them are quantitative in nature (Supplementary Table 2). Routinely used quantitative measures include BMI and waist-to-height ratio. Increased BMI was one of the components for IR detection. However, with recent observations of IR in lean children with T1DM [46], it has become evident that people especially of Asian ethnicity may follow a ‘thin fat’ phenotype with low normal BMI, and high percent fat [55]. Therefore, waist-to-height ratio may be a better marker than BMI for IR detection. Increased dose of insulin is observed in children having T1DM with IR. Insulin dosage may vary depending on the meal type, physical activity etc. Thus, insulin dose may not represent the accurate status of IR in children with T1DM. A qualitative marker-Acanthosis Nigricans (AN) may be observed as a result of abnormal proliferation of keratinocytes due to excessive binding of insulin to insulin like growth factor receptor rather than insulin receptor [85]. Acanthosis is observed to be associated with obesity more than IR.
Among the novel markers, breath test and cIMT offer least invasive methods for detection of IR. The breath test assesses the capacity to oxidize exogenous carbohydrates which directly correlate with eGDR and ISS significantly. This is presented by enriched C12/13 in expired breath [64]. This method being non-invasive can be more applicable to large paediatric cohorts. The cIMT (carotid intima media thickness), an early sign of atherosclerosis correlates moderately with insulin sensitivity is not a direct measure for IR. Its use in assessing the cardiovascular risk is limited. Moreover, the test is expensive and difficult to add in to a routine check-up.
Investigations of hormones involved in the pathogenesis of IR could provide valuable insights. Most of these hormones are novel and under investigation. These hormones actively participate in metabolic regulation and include adiponectin, leptin, fetuin A, kisspeptin etc. Adiponectin an insulin sensitizer produced by adipose tissue, involved in regulation of gluconeogenesis is suggested to be reduced in participants with T1DM [48] (Table 3). Adiponectin showed a strong discriminatory power for detection of IR in adolescents who did not have diabetes [86, 87]. Leptin, an appetite suppressing hormone, plays a role in energy balance by reducing energy uptake and increasing energy expenditure. Similar to adiponectin, leptin it is produced by white adipose tissues and shows negative correlation with insulin sensitivity. The evaluation of the ratio of both these hormones has been limited in adolescents who do not have diabetes [88]. Fetuin A, an inhibitor of insulin receptor tyrosine kinase activity is a suggested marker for IR in adolescents with no signs of diabetes [89]. Kisspeptin was observed to be higher in people with IR [75]. All these hormones lack assessment of their role as marker in children with T1DM and validation against HEC. An understanding of the pattern of these hormones with respect to IR provides a window for development of novel indices for the diagnosis of IR.
Other markers that are least understood and are under investigation include reduced D6D activity. Erythrocyte D6D activity has been suggested to be a strong marker of IR in T1DM [66]. D6D is a desaturase enzyme that introduces a double bond in a specific position of long chain fatty acids. Reduced activity of D6D can interfere with the fatty acid composition. The detailed explanation of this reduced activity is beyond the scope of our review. However, to consider D6D as an IR marker, more detailed studies are required.
Strengths and limitations of the study
To the best of our knowledge, this is the first systematic review and meta-analysis for assessment of surrogate markers for MetS and a systematic review for IR in children with T1DM. However, for the IR, the studies are reported in different forms of indices which made it difficult for us to compile them for the assessment of IR markers. Also, this systematic review could not assess the effect of age and pubertal status on the accuracy of markers of MetS and IR. The number of studies available for meta-analysis are very small hence, with increasing reports there are chances that the results may improve in future.
Conclusion
From the results it can be concluded that in the children with T1DM, markers of glycemic levels are not associated with MetS. Other than TG and HDL, LDL may also be considered in the diagnostic criteria for MetS. A combination of WC and TG may increase the efficacy of MetS diagnosis in paediatric population living with T1DM. Many novel markers currently under investigation for the diagnosis of IR need evaluation against HEC. These markers may be used in combination to increase the accuracy of IR diagnosis.
Data Availability
No new data generated. Data sharing not applicable.
Code availability
References
Tuomilehto J. The emerging global epidemic of type 1 diabetes. Curr Diab Rep. 2013;13(6):795–804. https://doi.org/10.1007/s11892-013-0433-5.
Id AG, et al. Variation in the incidence of type 1 diabetes mellitus in children and adolescents by world region and country income group: a scoping review. PLOS Glob PUBLIC Heal. 2022;2(11):1–18. https://doi.org/10.1371/journal.pgph.0001099.
Thunander M, et al. Incidence of type 1 and type 2 diabetes in adults and children in Kronoberg, Sweden. Diabetes Res Clin Pract. 2008;82(2):247–55. https://doi.org/10.1016/j.diabres.2008.07.022.
Mobasseri M, Shirmohammadi M, Amiri T, Vahed N, Fard HH, Ghojazadeh M. Prevalence and incidence of type 1 diabetes in the world: a systematic review and meta-analysis. Heal Promot Perspect. 2020;10(2):98–115. https://doi.org/10.34172/hpp.2020.18.
Tung JY, et al. Increasing incidence of type 1 diabetes among Hong Kong children and adolescents: The Hong Kong Childhood Diabetes Registry 2008 to 2017. Pediatr Diabetes. 2020;21(5):713–9. https://doi.org/10.1111/pedi.13016.
De Vries L, et al. Changes in weight and BMI following the diagnosis of type 1 diabetes in children and adolescents. Acta Diabetol. 2013;4(24). https://doi.org/10.1007/s00592-013-0524-4.
Reinehr T, et al. Insulin resistance in children and adolescents with type 1 diabetes mellitus: relation to obesity. Pediatr Diabetes. 2005;6(1):5–12. https://doi.org/10.1111/j.1399-543X.2005.00093.x.
Szadkowska A, et al. Insulin sensitivity in Type 1 diabetic children and adolescents. Diabet Med. 2008;25(3):282–8. https://doi.org/10.1111/j.1464-5491.2007.02357.x.
Šebeková K, Gurecká R, Csongová M, Koborová I, Repiská G, Podracká Ľ. Lean insulin-resistant young adults display increased cardiometabolic risk: a retrospective cross-sectional study. Diabetes Res Clin Pract. 2022;185: 109217. https://doi.org/10.1016/j.diabres.2022.109217.
Belete R, Ataro Z, Abdu A, Sheleme M. Global prevalence of metabolic syndrome among patients with type I diabetes mellitus: a systematic review and meta-analysis. Diabetol Metab Syndr. 2021;13(1):1–13. https://doi.org/10.1186/s13098-021-00641-8.
Grabia M, Markiewicz-Żukowska R, Socha K. Prevalence of metabolic syndrome in children and adolescents with type 1 diabetes mellitus and possibilities of prevention and treatment: a systematic review. Nutrients. 2021;13(6):1–15. https://doi.org/10.3390/nu13061782.
Grundy SM, et al. Diagnosis and management of the metabolic syndrome. Circulation. 2005;112(17):285–90. https://doi.org/10.1161/circulationaha.105.169405.
Alberti KGMM, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO consultation. Diabet Med. 1998;15(7):539–53. https://doi.org/10.1002/(SICI)1096-9136(199807)15:7%3c539::AID-DIA668%3e3.0.CO;2-S.
Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12(6):295–300. https://doi.org/10.5551/jat.12.295.
Soliman HM, Mosaad YO, Ibrahim A. The prevalence and the clinical profile of metabolic syndrome in children and adolescents with Type 1 diabetes. Diabetes Metab Syndr Clin Res Rev. 2019;13(3):1723–6. https://doi.org/10.1016/j.dsx.2019.03.036.
Köken ÖY, Kara C, Yılmaz GC, Aydın HM. Prevalence of obesity and metabolic syndrome in children with type 1 diabetes: a comparative assessment based on criteria established by the international diabetes federation, world health organisation and national cholesterol education program. JCRPE J Clin Res Pediatr Endocrinol. 2020;12(1):55–62. https://doi.org/10.4274/jcrpe.galenos.2019.2019.0048.
Barros BSV, et al. Genomic ancestry and metabolic syndrome in individuals with type 1 diabetes from an admixed population: a multicentre, cross-sectional study in Brazil. Diabet Med. 2021;38(2):1–9. https://doi.org/10.1111/dme.14400.
Cleland SJ, Fisher BM, Colhoun HM, Sattar N, Petrie JR. Insulin resistance in type 1 diabetes: what is ‘double diabetes’ and what are the risks? Diabetologia. 2013;56(7):1462–70. https://doi.org/10.1007/s00125-013-2904-2.
Kietsiriroje N, Pearson S, Campbell M, Ariëns RAS, Ajjan RA. Double diabetes: a distinct high-risk group? Diabetes Obes Metab. 2019;21(12):2609–18. https://doi.org/10.1111/dom.13848.
DeFronzo RA, Hendler R, Simonson D. Insulin resistance is a prominent feature of insulin-dependent diabetes. Diabetes. 1982;31(9):795–801. https://doi.org/10.2337/diab.31.9.795.
Minges KE, Whittemore R, Grey M. Overweight and obesity in youth with type 1 diabetes. Annu Rev Nurs Res. 2013;31:47–69. https://doi.org/10.1891/0739-6686.31.47.
Polsky S, Ellis SL. Obesity, insulin resistance, and type 1 diabetes mellitus. Curr Opin Endocrinol Diabetes Obes. 2015;22(4):277–82. https://doi.org/10.1097/MED.0000000000000170.
Pozzilli P, Guglielmi C, Caprio S, Buzzetti R. Obesity, autoimmunity, and double diabetes in youth. Diabetes Care. 2011;34(SUPPL. 2). https://doi.org/10.2337/dc11-s213.
Wolosowicz M, Lukaszuk B, Chabowski A. The causes of insulin resistance in type 1 diabetes mellitus: is there a place for quaternary prevention? Int J Environ Res Public Health. 2020;17(22):1–13. https://doi.org/10.3390/ijerph17228651.
Adeva-Andany MM, Martínez-Rodríguez J, González-Lucán M, Fernández-Fernández C, Castro-Quintela E. Insulin resistance is a cardiovascular risk factor in humans. Diabetes Metab Syndr Clin Res Rev. 2019;13(2):1449–55. https://doi.org/10.1016/j.dsx.2019.02.023.
DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol Endocrinol Metab Gastrointest Physiol. 1979;6(3). https://doi.org/10.1152/ajpendo.1979.237.3.e214.
Epstein EJ, Osman JL, Cohen HW, Rajpathak SN, Lewis O, Crandall JP. Use of the estimated glucose disposal rate as a measure of insulin resistance in an urban multiethnic population with type 1 diabetes. Diabetes Care. 2013;36(8):2280–5. https://doi.org/10.2337/dc12-1693.
Chillarón JJ, et al. Estimated glucose disposal rate in assessment of the metabolic syndrome and microvascular complications in patients with type 1 diabetes. J Clin Endocrinol Metab. 2009;94(9):3530–4. https://doi.org/10.1210/jc.2009-0960.
Nyström T, Holzmann MJ, Eliasson B, Svensson AM, Sartipy U. Estimated glucose disposal rate predicts mortality in adults with type 1 diabetes. Diabetes Obes Metab. 2018;20(3):556–63. https://doi.org/10.1111/dom.13110.
Bîcu ML, et al. Estimated glucose disposal rate (eGDR)–A marker for the assessment of insulin resistance in type 1 diabetes mellitus. Rom J Diabetes Nutr Metab Dis. 2016;23(2):177–82. https://doi.org/10.1515/rjdnmd-2016-0021.
Dabelea D, et al. Development, validation and use of an insulin sensitivity score in youths with diabetes: the SEARCH for diabetes in youth study. Diabetologia. 2011;54(1):78–86. https://doi.org/10.1007/s00125-010-1911-9.
Duca LM, et al. Development and validation of a method to estimate insulin sensitivity in patients with and without type 1 diabetes. J Clin Endocrinol Metab. 2016;101(2):686–95. https://doi.org/10.1210/jc.2015-3272.
Kilpatrick ES, Rigby AS, Atkin SL. Insulin resistance, the metabolic syndrome, and complication risk in type 1 diabetes: ‘double diabetes’ in the diabetes control and complications trial. Diabetes Care. 2007;30(3):707–12. https://doi.org/10.2337/dc06-1982.
Teixeira MM, et al. Insulin resistance and associated factors in patients with type 1 diabetes. Diabetol Metab Syndr. 2014;6(1):1–10. https://doi.org/10.1186/1758-5996-6-131.
Snell-Bergeon JK, Maahs DM, Schauer IE, Bergman BC, Rewers M (2010) A method for estimating insulin sensitivity in adults with type 1 diabetes. In: 70th Annual Meeting of the American Diabetes Association, vol 25, p 29
Williams KV, Erbey JR, Becker D, Arslanian S, Orchard TJ. Can clinical factors estimate insulin resistance in type 1 diabetes? Diabetes. 2000;49(4):626–32. https://doi.org/10.2337/diabetes.49.4.626.
Ferreira-Hermosillo A, Ibarra-Salce R, Rodríguez-Malacara J, Molina-Ayala MA. Comparison of indirect markers of insulin resistance in adult patients with Double Diabetes. BMC Endocr Disord. 2020;20:1–9. https://doi.org/10.1186/s12902-020-00570-z.
Cano A, et al. Utility of insulin resistance in estimating cardiovascular risk in subjects with type 1 diabetes according to the scores of the steno type 1 risk engine. J Clin Med. 2020;9:1–12. https://doi.org/10.3390/jcm9072192.
Uruska A, Zozulinska-ziolkiewicz D, Niedzwiecki P, Pietrzak M, Wierusz-wysocka B. TG/HDL-C ratio and visceral adiposity index may be useful in assessment of insulin resistance in adults with type 1 diabetes in clinical practice. J Clin Lipidol. 2018;12:734–40. https://doi.org/10.1016/j.jacl.2018.01.005.
Oza CM, Khadilkar V, Kadam S, Khadilkar A. Response to sirolimus in a case of diffuse congenital hyperinsulinaemic hypoglycaemia due to homozygous KCNJ11 mutation. BMJ Case Rep. 2022;15:10–3. https://doi.org/10.1515/jpem-2022-0076.
Zheng X, et al. A new model to estimate insulin resistance via clinical parameters in adults with type 1 diabetes. Diabetes Metab Res Rev. 2017;33(4). https://doi.org/10.1002/dmrr.2880.
Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan-a web and mobile app for systematic reviews. Syst Rev. 2016;5(1):1–10. https://doi.org/10.1186/s13643-016-0384-4.
Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:1–10. https://doi.org/10.1186/1471-2288-5-13.
Nadeau KJ, et al. Insulin resistance in adolescents with type 1 diabetes and its relationship to cardiovascular function. J Clin Endocrinol Metab. 2010;95(2):513–21. https://doi.org/10.1210/jc.2009-1756.
Dabelea D, et al. Etiological approach to characterization of diabetes type: the SEARCH for diabetes in youth study. Diabetes Care. 2011;34(7):1628–33. https://doi.org/10.2337/dc10-2324.
Davis NL, Bursell JDH, Evans WD, Warner JT, Gregory JW. Body composition in children with type 1 diabetes in the first year after diagnosis: relationship to glycemic control and cardiovascular risk. Arch Dis Child. 2012;97(4):312–5. https://doi.org/10.1136/archdischild-2011-300626.
Rathsman B, Rosfors S, Sjöholm Å, Nyström T. Early signs of atherosclerosis are associated with insulin resistance in non-obese adolescent and young adults with type 1 diabetes. Cardiovasc Diabetol. 2012;11:1–7. https://doi.org/10.1186/1475-2840-11-145.
Safai N, et al. Levels of adiponectin and leptin at onset of type 1 diabetes have changed over time in children and adolescents. Acta Diabetol. 2015;52(1):167–74. https://doi.org/10.1007/s00592-014-0630-y.
Chan CL, Pyle L, Morehead R, Baumgartner A, Cree-Green M, Nadeau KJ. The role of glycemia in insulin resistance in youth with type 1 and type 2 diabetes. Pediatr Diabetes. 2017;18(6):470–7. https://doi.org/10.1111/pedi.12422.
Cree-Green M, et al. Youth with type 1 diabetes have adipose, hepatic, and peripheral insulin resistance. J Clin Endocrinol Metab. 2018;103(10):3647–57. https://doi.org/10.1210/jc.2018-00433.
Gourgari E, et al. The association of low-density lipoprotein cholesterol with elevated arterial stiffness in adolescents and young adults with type 1 and type 2 diabetes: the SEARCH for Diabetes in Youth study. Pediatr Diabetes. 2020;21(5):863–70. https://doi.org/10.1111/pedi.13021.
Hamed N, et al. Clinical and metabolic characteristics of children with hybrid diabetes mellitus (Hd) compared to children with type 2 diabetes mellitus (t2dm): a preliminary comparative study. Acta Biomed. 2021;92(5):5–10. https://doi.org/10.23750/abm.v92i5.11598.
Calcaterra V, et al. Acanthosis nigricans in children and adolescents with type 1 diabetes or obesity: the potential interplay role between insulin resistance and excess weight. Children. 2021;8(8):1–9. https://doi.org/10.3390/children8080710.
Stone ML, Craig ME, Chan AK, Lee JW, Verge CF, Donaghue KC. Natural history and risk factors for microalbuminuria in adolescents with type 1 diabetes: a longitudinal study. Diabetes Care. 2006;29(9):2072–7. https://doi.org/10.2337/dc06-0239.
Mazumder R, Sarkar D, Chowdhury BR, Chowdhury UR, Chowdhury S. Clinical assessment of obesity and insulin resistance in type 1 diabetes subjects seen at a center in Kolkata. J Assoc Physicians India. 2009;57(7):511–5 ([Online]).
Girgis CM, Scalley BD, Park KEJ. Utility of the estimated glucose disposal rate as a marker of microvascular complications in young adults with type 1 diabetes. Diabetes Res Clin Pract. 2012;96(3):e70–2. https://doi.org/10.1016/j.diabres.2012.02.004.
Valerio G, et al. Abdominal adiposity and cardiovascular risk factors in adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2012;97(1):99–104. https://doi.org/10.1016/j.diabres.2012.01.022.
Ješić M, Ješić M, Sajić S, Bogićević D, Buljugić S, Maglajlić S. The effect of metabolic and hormonal parameters on microalbuminuria in adolescents with type 1 diabetes mellitus. Srp Arh Celok Lek. 2013;141(5–6):315–9. https://doi.org/10.2298/SARH1306315J.
Lecaire TJ, Palta M. Longitudinal analysis of adiponectin through 20-year type 1 diabetes duration. J Diabetes Res. 2015;2015:18–20. https://doi.org/10.1155/2015/730407.
Cedillo M, et al. Obesity, islet cell autoimmunity, and cardiovascular risk factors in youth at onset of type 1 autoimmune diabetes. J Clin Endocrinol Metab. 2015;100(1):E82–6. https://doi.org/10.1210/jc.2014-2340.
Şiraz ÜG, Doğan M, Hatipoğlu N, Muhtaroğlu S, Kurtoğlu S. Can fetuin-A be a marker for insulin resistance and poor glycemic control in children with type 1 diabetes mellitus? JCRPE J Clin Res Pediatr Endocrinol. 2017;9(4):293–9. https://doi.org/10.4274/jcrpe.4532.
Bjornstad P, et al. Leptin is associated with cardiopulmonary fitness independent of body-mass index and insulin sensitivity in adolescents with type 1 diabetes: a brief report from the EMERALD study. J Diabetes Comp. 2017;31(5):850–3. https://doi.org/10.1016/j.jdiacomp.2017.02.019.
Sevaliev N, Strich D, Avnon-Ziv C, Levy-Khademi F. The metabolic consequences of overweight in a cohort of children with type 1 diabetes. J Pediatr Endocrinol Metab. 2019;32(7):715–9. https://doi.org/10.1515/jpem-2018-0483.
Marigliano M, et al. 13C/12C breath test ratio after the ingestion of a meal naturally enriched with (13C)carbohydrates is a surrogate marker of insulin resistance and insulin sensitivity in children and adolescents with type 1 diabetes. Diabetes Res Clin Pract. 2020;169: 108447. https://doi.org/10.1016/j.diabres.2020.108447.
Nishtala R, Kietsiriroje N, Karam M, Ajjan RA, Pearson S. Estimated glucose disposal rate demographics and clinical characteristics of young adults with type 1 diabetes mellitus: a cross-sectional pilot study. Diabetes Vasc Dis Res. 2020;17(5):147916412095232. https://doi.org/10.1177/1479164120952321.
Morandi A, et al. Long chain fatty acids metabolism and cardiovascular risk factors in youth with type 1 diabetes. Nutr Metab Cardiovasc Dis. 2021;31(1):297–305. https://doi.org/10.1016/j.numecd.2020.08.023.
Gomes MB, et al. Overweight/obesity in adolescents with type 1 diabetes belonging to an admixed population. A Brazilian multicenter study. Diabetol Metab Syndr. 2022;14(1):1–10. https://doi.org/10.1186/s13098-021-00759-9.
Khadilkar A. Adiponectin – leptin ratio as a marker of cardio- metabolic risk in Indian children and youth with type 1 diabetes. J Pediatr Endocrinol Metab. 2023;1–7. https://doi.org/10.1515/jpem-2023-0087.
Viechtbauer W. Conducting meta-analyses in R with the metafor package. J Stat Softw. 2010;36(3):1–48. https://doi.org/10.18637/jss.v036.i03.
Chang Y, et al. The 5 min meta-analysis: understanding how to read and interpret a forest plot. Eye. 2022;36(4):673–5. https://doi.org/10.1038/s41433-021-01867-6.
Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA (eds). Cochrane Handbook for Systematic Reviews of Interventions. 2nd Edition. Chichester (UK): John Wiley & Sons, 2019.
Cohen J (1988) Statistical power analysis in the behavioral sciences, 2nd ed. Routledge, New York [Online]. Available: https://doi.org/10.4324/9780203771587
Sterne JAC, Harbord RM. Funnel plots in meta-analysis. Stata J Promot Commun Stat Stata. 2004;4(2):127–41. https://doi.org/10.1177/1536867x0400400204.
Grabia M, et al. Prevalence of metabolic syndrome in relation to cardiovascular biomarkers and dietary factors among adolescents with type 1 diabetes mellitus. Nutrients. 2022;14(12):1–18. https://doi.org/10.3390/nu14122435.
Calcaterra V, et al. Insulin resistance and potential modulators of ovarian reserve in young reproductive-aged women with obesity and type 1 diabetes. Gynecol Endocrinol. 2021;37(9):823–30. https://doi.org/10.1080/09513590.2021.1940127.
Moriyama K, et al. Estimated Elovl6 and delta-5 desaturase activities might represent potential markers for insulin resistance in Japanese adults. J Diabetes Metab Disord. 2022;21(1):197–207. https://doi.org/10.1007/s40200-021-00958-1.
Ibrahim MM, Elamragy AA, Girgis H, Nour MA. Cut off values of waist circumference & associated cardiovascular risk in egyptians. BMC Cardiovasc Disord. 2011;11(1):53. https://doi.org/10.1186/1471-2261-11-53.
Ferreira-Hermosillo A, Ramírez-Rentería C, Mendoza-Zubieta V, Molina-Ayala MA. Utility of the waist-to-height ratio, waist circumference and body mass index in the screening of metabolic syndrome in adult patients with type 1 diabetes mellitus. Diabetol Metab Syndr. 2014;6(1):1–7. https://doi.org/10.1186/1758-5996-6-32.
Hirschler V, Aranda C, De Luján Calcagno M, Maccalini G, Jadzinsky M. Can waist circumference identify children with the metabolic syndrome? Arch Pediatr Adolesc Med. 2005;159(8):740–4. https://doi.org/10.1001/archpedi.159.8.740.
de Cuevillas B, Alvarez-Alvarez I, Riezu-Boj JI, Navas-Carretero S, Martinez JA. The hypertriglyceridemic-waist phenotype as a valuable and integrative mirror of metabolic syndrome traits. Sci Rep. 2021;11(1):1–10. https://doi.org/10.1038/s41598-021-01343-x.
Jung E, Kong SY, Ro YS, Ryu HH, Do Shin S. Serum cholesterol levels and risk of cardiovascular death: a systematic review and a dose-response meta-analysis of prospective cohort studies. Int J Environ Res Public Health. 2022;19(14):8272. https://doi.org/10.3390/ijerph19148272.
Katsa ME, Ioannidis A, Sachlas A, Dimopoulos I, Chatzipanagiotou S, Gil APR. The roles of triglyceride/high-density lipoprotein cholesterol ratio and uric acid as predisposing factors for metabolic syndrome in healthy children. Ann Pediatr Endocrinol Metab. 2019;24(3):172–9. https://doi.org/10.6065/apem.2019.24.3.172.
Powell EE, Jonsson JR, Clouston AD. Metabolic factors and non-alcoholic fatty liver disease as co-factors in other liver diseases. Dig Dis. 2010;28(1):186–91. https://doi.org/10.1159/000282084.
Paredes S, Fonseca L, Ribeiro L, Ramos H, Oliveira JC, Palma I. Novel and traditional lipid profiles in Metabolic Syndrome reveal a high atherogenicity. Sci Rep. 2019;9(1):1–7. https://doi.org/10.1038/s41598-019-48120-5.
Phiske M. An approach to acanthosis nigricans. Indian Dermatol Online J. 2014;5(3):239. https://doi.org/10.4103/2229-5178.137765.
de Cassia da Silva C, et al. Homeostatic model assessment of adiponectin (HOMA-Adiponectin) as a surrogate measure of insulin resistance in adolescents: comparison with the hyperglycaemic clamp and homeostatic model assessment of insulin resistance. PLoS One. 2019;14(3):1–12. https://doi.org/10.1371/journal.pone.0214081.
Shafiee G, et al. Association of adiponectin and metabolic syndrome in adolescents: the caspian- III study. J Diabetes Metab Disord. 2015;14(1):89. https://doi.org/10.1186/s40200-015-0220-8.
Agostinis-Sobrinho C, et al. Is the leptin/adiponectin ratio a better diagnostic biomarker for insulin resistance than leptin or adiponectin alone in adolescents? Children. 2022;9(8):1193. https://doi.org/10.3390/children9081193.
Shim YS, Kang MJ, Oh YJ, Baek JW, Yang S, Hwang IT. Fetuin-A as an alternative marker for insulin resistance and cardiovascular risk in prepubertal children. J Atheroscler Thromb. 2017;24(10):1031–8. https://doi.org/10.5551/jat.38323.
Acknowledgements
SBK and VK thank SIU for research fellowships.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
SBK and VK performed the systematic literature search. SBK performed the statistical analysis. SBK and SPK wrote the manuscript. AK and SPK contributed to conceptual design of the study.
Corresponding authors
Ethics declarations
Systematic review registration
PROSPERO CRD42023418954.
Ethics declaration
No ethical approval was needed as the data was collected from previous published studies in which the informed consent was obtained by primary investigators.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Khandagale, S.B., Kamble, V.S., Oza, C. et al. Surrogate markers of metabolic syndrome and insulin resistance in children and young adults with type 1 diabetes: a systematic review & meta-analysis (MetS and IR in T1DM). Int J Diabetes Dev Ctries 44, 450–464 (2024). https://doi.org/10.1007/s13410-023-01284-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-023-01284-3