Abstract
A high but normal fasting plasma glucose level in adults is a marker for insulin resistance and future development of type 2 diabetes mellitus. We aimed to investigate whether ADIPOR2 variants are associated with increased fasting plasma glucose (FPG) in non-diabetic Chinese Han subjects who had normal FPG levels. This study included 2038 subjects among the non-diabetic Chinese Han population. The ADIPOR2 polymorphisms were genotyped by the TaqMan method. For the analysis, participants were divided into low-normal FPG group (FPG < 4.88 mmol/L) and high-normal FPG group (6.1 mmol/L > FPG ≥ 4.88 mmol/L). We identified five single nucleotide polymorphisms (SNPs) of ADIPOR2 gene: rs10773989, rs2370055, rs9300298, rs10773983, and rs13611594. There was no significant difference in FPG levels between different genotypes of rs10773989, rs2370055, rs9300298, and rs13611594. Our data showed a significant association of rs10773983 with higher FPG levels, fasting insulin levels, and homeostatic model assessment of insulin resistance (HOMA-IR) in A-allele carriers (p = 0.006, p < 0.001, and p < 0.001, respectively). The subjects in high FPG group had higher median fasting insulin level (9.25 vs 8.16 μIU/mL, p = 0.003) and higher HOMA-IR (2.21 vs 1.58, p < 0.001) than those in normal FPG group. Subjects with the A-allele of rs10773983 compared to subjects with G-allele had 1.222-fold higher risk for high-normal FPG (OR = 1.222, 95%CI = 1.010–1.478, p = 0.039). Our findings suggest that ADIPOR2 rs10773983 polymorphism may be associated with an increased risk of high-normal FPG among non-diabetic Chinese Han subjects who have normal FPG levels.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Type 2 diabetes mellitus (T2DM) is a major global health problem, and a continuous increase in the prevalence of type 2 diabetes in China has occurred over a very short time [1]. Early identification of people at high risk of developing type 2 diabetes may help them delay or prevent the dysglycemia. Recent reports have suggested that a high but normal fasting plasma glucose level in adults is a significant risk factor for future development of type 2 diabetes mellitus [2, 3]. Adiponectin is a hormone secreted by adipocytes that acts as an insulin-sensitizing and anti-diabetic adipokine [4, 5]. Two related receptors for adiponectin, termed adiponectin receptor 1 (ADIPOR1) and adiponectin receptor 2 (ADIPOR2), were identified as a mediator in the adiponectin signaling pathway through activation of 5′ AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor alpha (PPARα) [6]; ADIPOR2 is most abundantly expressed in the liver and correlates positively with insulin sensitivity [7]. Several studies have investigated the role of ADIPOR2 variants in the development of T2DM in several human populations, yielding controversial results [8,9,10]. However, it is still not clear whether ADIPOR2 variants are related to the increased fasting plasma glucose (FPG) within the normoglycemic range, which may indicate the earliest stages of insulin resistance or dysglycemic states. The aim of this study is to characterize the association between ADIPOR2 SNPs and FPG levels within the normal range (< 6.1 mmol/L) in non-diabetic Chinese Han population.
Methods
Subjects
This cross-sectional community study initially included 2472 Han Chinese subjects who were referred for health screening at Beijing Shijitan Hospital between January 2012 and December 2013. The physical examination included determination of the height, weight, waist circumference, and seated BP. BMI (kg/m2) was calculated as weight (kg) divided by height (m) [2]. Lipid profile and HbA1c were tested and recorded for all the subjects. Family history of diabetes, according to self-report questionnaire data, was recorded. Exclusion criteria for this study included either diagnosed type 1 or type 2 diabetes; fasting plasma glucose level ≥ 6.1 mmol/L [11]; HbA1c ≥6.0% [12]; medical history of cardiovascular or cerebrovascular diseases; history of cancer; chronic renal, liver, or inflammatory bowel disease; and BMI (in kg/m2) < 20 and > 35. After these exclusions, 2038 subjects were included in this study. The study protocol was approved by the Beijing Shijitan Hospital Ethics Committee, Capital Medical University. Written informed consent was obtained from all the participants before their enrolment in the study.
SNP selection and genotyping
Total genomic DNA samples were obtained from the whole blood samples using the RelaxGene Blood DNA System (Tiangen Biotech, Beijing, China). SNPs located in different haplotype blocks of ADIPOR2 genes for this study were selected based on HapMap using Data Release 27 for the Chinese population (Chinese Han from Beijing-CHB). Tag SNPs were chosen in SNPs with minor allele frequency ≥ 0.1 and Hardy–Weinberg equilibrium (HWE) p value > 0.05, using Tagger in Haploview software (Broad Institute, Cambridge, MA). The criterion for r2 was set at 0.8. We used the pairwise mode and obtained a minimal set of tagging SNPs: rs10773989, rs2370055, rs9300298, rs10773983, and rs13611594. The SNPs were genotyped using the TaqMan real-time PCR assays (Applied Biosystems). The TaqMan genotyping reaction was amplified on a GeneAmp PCR system 7000 (Applied Biosystems) (95 °C for 3 min, followed by 40 cycles of 95 °C for 3 s and 60 °C for 20 s), and fluorescence was detected on an ABI Prism sequence detector (Applied Biosystems).
Biochemical measurements
Blood samples were drawn from subjects after an overnight fast and were immediately centrifuged at 2800 rpm for 15 min at 4 °C. The plasma was kept at − 80 °C. The concentrations of plasma glucose levels, high-density lipoprotein cholesterol (HDL-c), low-density lipoprotein cholesterol (LDL-c), triglyceride (TG), and total cholesterol (TC) were detected by an automatic biochemical analyzer (Hitachi 7080, Tokyo, Japan). HbA1c levels were conducted using automated glycohemoglobin analyzer (TOSOH HLC-723G8, Tokyo, Japan). Quantitative measurements of plasma insulin concentration were measured by a chemiluminescent immunoassay using a Liaison analyzer (DiaSorin, Saluggia, Italy). The homeostatic model assessment of insulin resistance (HOMA-IR) was calculated following a method previously described by Matthews et al.
Statistical analysis
Continuous variables are presented as mean ± standard deviation if symmetrically distributed or as medians and interquartile ranges if distributed asymmetrically (lipid parameters, fasting plasma glucose level, fasting plasma insulin levels). The differences between groups were tested by independent samples t test or Mann-Whitney U tests. Hardy–Weinberg equilibrium test was assessed for each SNP with Pearson’s chi-square test. Demography, metabolic parameters, and genotype distribution of rs10773983 were compared between low-normal FPG and high-normal FPG groups using the chi-square test, independent samples t test, or Mann-Whitney U test as appropriate. Multivariate logistic regression analysis was used to analyze association between rs10773983 polymorphism and high-normal FPG by adjusting for other variables, such as age, sex, family history of diabetes, BMI, triglyceride, total cholesterol, HDL-c, and LDL-c. A two-tailed p value of < 0.05 was considered statistically significant. All statistical analyses were performed with SPSS 22.0 (SPSS Inc. Chicago, IL, USA).
Results
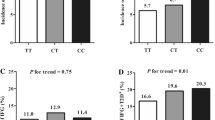
The overall study population consisted of 2038 subjects. The demographic and clinical characteristics of the population studied are shown in Table 1. We genotyped the SNPs (rs10773989, rs2370055, rs9300298, rs10773983, and rs13611594) in ADIPOR2 in the cohort. All of the tested ADIPOR2 SNPs were in Hardy–Weinberg equilibrium (p > 0.05). None of the rs10773989, rs9300298, rs2370055, or rs13611594 was significantly associated with FPG, HDL-c, LDL-c, BMI, triglycerides, and total cholesterol levels. For the sequence variant identified in rs10773983, 707 (34.7%) had homozygote form of G/G, while 338 (16.6%) were homozygote (A/A), and 993 (48.7%) were heterozygotes (G/A). The association between the rs10773983 genotypes and clinical characteristics was analyzed further under three different genetic models as shown in Table 2. No significant difference was observed in age, BMI, HbA1c, TC, family history of diabetes, TG, and LDL-c between different variants of rs10773983. However, there was a significant association of rs10773983 with higher FPG levels, fasting insulin levels, and HOMA-IR in A-allele carriers (p = 0.006, p < 0.001, and p < 0.001, respectively).
Subjects were further divided into two groups according to their FPG levels: low-normal FPG group with FPG < 4.88 mmol/L and high-normal FPG group with FPG ≥ 4.88 mmol/L. The median FPG level in high-normal FPG group was higher than that of normal group (5.37 vs 4.57 mmol/L, p < 0.001). The subjects in high FPG group were significantly older (48.8 ± 12.1 years vs 41.7 ± 12.9 years, p < 0.001) and had higher median total cholesterol level (5.56 vs 4.86 mmol/L, p < 0.001), higher median triglyceride level (1.44 vs 1.09 mmol/L, p < 0.001), higher median LDL-c level (3.22 vs 2.79 mmol/L, p < 0.001), higher medial BMI (24.2 vs 23.3 kg/m2, p < 0.001), higher median fasting insulin level (9.25 vs 8.16 μIU/mL, p = 0.003), higher HOMA-IR (2.21 vs 1.58, p < 0.001) than those in normal FPG group as shown in Table 3. In addition, more individuals in the high-normal FPG group have family history of diabetes than those in normal FPG group (8.54 vs 5.41%, p = 0.036). No significant difference in gender distribution or HDL-c level was observed between the two groups (Table 3). We subsequently investigated the distribution of genotypes for the rs10773983 polymorphisms between the two groups. The frequencies of GA and AA genotypes are significantly higher in subjects with high-normal FPG than those in the low-normal FPG group as shown in Table 3.
The effects of the genotypes of rs10773983 were analyzed further under three different genetic models using logistic tests (Table 4). Multivariate logistic regression analysis showed that after adjustment for age, sex, family history of diabetes, and BMI, the GA and AA genotypes and the combined AA+ GA genotype of ADIPOR2 rs10773983 were significantly associated with a higher risk of high-normal FPG (OR = 1.423, 95%CI = 1.110–1.825; OR = 1.718, 95%CI = 1.211–2.439; OR = 1.491, 95%CI 1.179–1.886 respectively; p < 0.01). The findings showed that subjects with the A-allele of rs10773983 compared to subjects with G-allele had 1.222-fold higher risk for high-normal FPG (OR = 1.222, 95%CI = 1.010–1.478, p = 0.039).
Discussion
The aim of this investigation was to reveal the possible association of ADIPOR2 rs10773983 polymorphisms with high-normal FPG level in the Chinese Han population. Our results provided evidence that rs10773983 was a susceptible genetic variant for high-normal FPG (FPG ≥ 4.88 mmol/L) in Chinese Han people.
Elevated FPG, even within the normal range according to the World Health Organization definition [13], may be associated with insulin resistance and pre-diabetes. Tirosh et al. found that glucose levels within the normal range independently predicted incident diabetes mellitus (DM) during a 6-year follow-up [14]. O'Malley et al data suggested that insulin sensitivity declines when moving from low- to high-normal FPG in pre-pubertal and pubertal obese youth, independently of age, BMI, and sex [15]. The present study showed the similar results for increased fasting insulin levels and decreased insulin sensitivity in subjects with FPG ≥ 4.88 mmol/L in Chinese Han people.
Furthermore, high-normal fasting glucose level may play an important role in the pathophysiology of atherosclerotic disease. Shaye et al. found that elevated fasting glucose level, within the normal range, is an independent predictor of cardiovascular disease (CVD) in men without type 2 DM [16]. Neurologic studies suggested that even in the normal range and in the absence of diabetes, a high-normal fasting glucose level was associated with changes in brain structure and cognitive function [17, 18]. However, the genetic factors contributing to the high-normal fasting glucose level are still incompletely understood. Adiponectin is an adipose tissue hormone secreted by adipocytes and exerts multiple biological effects, such as insulin sensitization, anti-inflammation, and anti-atherogenesis [19], throughout the body mediated by the specific receptors ADIPOR1, ADIPOR2, and T-cadherin. ADIPOR2 is predominantly expressed in the liver and regulates glucose and lipid metabolism by activating AMP-activated protein kinase (AMPK) and peroxisome proliferator-activated receptor alpha (PPARα) pathways [20], leading to increased insulin sensitivity and glucose uptake. In a study of an Amish population diagnosed with type 2 DM, five SNPs (rs1029629, rs12342, rs11061971, rs1044471, and AR2-8f) for ADIPOR2 were found to be associated with type 2 DM and impaired glucose tolerance, while another research on the French population suggested association between SNPs rs767870 of ADIPOR2 and type 2 DM. In this study, we examined the SNPs (rs10773989, rs2370055, rs9300298, rs10773983, and rs13611594) in ADIPOR2 in the non-diabetic Chinese Han population. The results of the present study revealed that only the haplotype containing the A allele of rs10773983 in the ADIPOR2 was significantly associated with the higher normal fasting glucose levels and type 2 diabetes risk in a population consisting of individuals within normal fasting glucose range. There was no association between rs10773983 in ADIPOR2 and BMI. Phenotypic, genotypic, and environmental features of different populations may be the explanation of the discrepancies between our study and past researches [21].
There were a few limitations to our study. One such limitation is the lack of the measurement of the ADIPOR2 expression. Because rs10773983 is an intron variant and does not alter ADIPOR2 amino acid sequence, it might therefore influence ADIPOR2 expression at the transcriptional level rather than altering protein functions. Further studies are required to explain the mechanisms underlying the association between rs10773983 and ADIPOR2 function expression. Also, without routinely testing oral glucose tolerance test of our study population, there might be some participants with impaired blood glucose or even diabetes recruited in our study population and may interfere with the final analysis. Further studies with oral glucose tolerance test, together with HbA1c and FPG, are needed to better verify the relationships between rs10773983 SNPs, high-normal fasting glucose, and insulin resistance in the non-diabetic Chinese Han population.
Conclusions
Our findings suggest that ADIPOR2 rs10773983 polymorphism may be associated with an increased risk of high-normal FPG among non-diabetic Chinese Han subjects who have normal FPG levels.
References
Li R, Lu W, Jiang QW, Li YY, Zhao GM, Shi L, et al. Increasing prevalence of type 2 diabetes in Chinese adults in Shanghai. Diabetes Care. 2012;35(5):1028–30.
Dagogo-Jack S, Askari H, Tykodi G. Glucoregulatory physiology in subjects with low-normal, high-normal, or impaired fasting glucose. J Clin Endocrinol Metab. 2009;94(6):2031–6.
Abdul-Ghani MA, Lyssenko V, Tuomi T, DeFronzo RA, Groop L. Fasting vs postload plasma glucose concentration and the risk for future type 2 diabetes: results from the Botnia study. Diabetes Care. 2009;32(2):281–6.
Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–95.
Weyer C, Funahashi T, Tanaka S, Hotta K, Matsuzawa Y, Pratley RE, et al. Hypoadiponectinemia in obesity and type 2 diabetes: close association with insulin resistance and hyperinsulinemia. J Clin Endocrinol Metab. 2001;86(5):1930–5.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9.
Civitarese AE, Jenkinson CP, Richardson D, Bajaj M, Cusi K, Kashyap S, et al. Adiponectin receptors gene expression and insulin sensitivity in non-diabetic Mexican Americans with or without a family history of type 2 diabetes. Diabetologia. 2004;47(5):816–20.
Rasmussen-Torvik LJ, Pankow JS, Jacobs DR Jr, Steinberger J, Moran A, Sinaiko AR. The association of SNPs in ADIPOQ, ADIPOR1, and ADIPOR2 with insulin sensitivity in a cohort of adolescents and their parents. Hum Genet. 2009;125(1):21–8.
Ferguson JF, Phillips CM, Tierney AC, Pérez-Martínez P, Defoort C, Helal O, et al. Gene-nutrient interactions in the metabolic syndrome: single nucleotide polymorphisms in DIPORQ and ADIPOR1 interact with plasma saturated fatty acids to modulate insulin resistance. Am J Clin Nutr. 2010;91(3):794–801.
Stefan N, Machicao F, Staiger H, Machann J, Schick F, Tschritter O, et al. Polymorphisms in the gene encoding adiponectin receptor 1 are associated with insulin resistance and high liver fat. Diabetologia. 2005;48(11):2282–91.
World Health Organization. Definition and diagnosis of diabetes mellitus and intermediate hyperglycaemia.http://www.who.int/diabetes/publications/Definition%20and%20diagnosis%20of%20diabetes_new.pdf
The International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34.
WHO . Definition and diagnosis of diabetes mellitus and intermediate hyperglycemia. Geneva: World Health Organization; 2006. pp. 1–46. Report of a WHO/IDF Consultation Available at http://www.who.int.
Tirosh A, Shai I, Tekes-Manova D, Israeli E, Pereg D, Shochat T, et al. Normal fasting plasma glucose levels and type 2 diabetes in young men. N Engl J Med. 2005;353:1454–62.
O’Malley G, Santoro N, Northrup V, D'Adamo E, Shaw M, Eldrich S, et al. High normal fasting glucose level in obese youth: a marker for insulin resistance and beta cell dysregulation. Diabetologia. 2010;53(6):1199–209.
Shaye K, Amir T, Shlomo S, Yechezkel S. Fasting glucose levels within the high normal range predict cardiovascular outcome. Am Heart J. 2012;164(1):111–6.
Raizes M, Elkana O, Franko M, Ravona Springer R, Segev S, Beeri MS. Higher fasting plasma glucose levels, within the normal range, are associated with decreased processing speed in high functioning young elderly. J Alzheimers Dis. 2015;49(3):589–92. https://doi.org/10.3233/JAD-150433.
Cherbuin N, Sachdev P, Anstey KJ. Higher normal fasting plasma glucose is associated with hippocampal atrophy: the PATH study. Neurology. 2012;79(10):1019–26.
Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes. 2008;32(Suppl 7):S13–8.
Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13(3):332–9.
Bermúdez VJ, Rojas E, Toledo A, Rodríguez-Molina D, Vega K, Suárez L, et al. Single-nucleotide polymorphisms in adiponectin, AdipoR1, and AdipoR2 genes: insulin resistance and type 2 diabetes mellitus candidate genes. Am J Ther. 2013;20(4):414–21.
Funding
This study was funded by Beijing Municipal Administration of Hospitals’ Ascent Plan (grant number DFL20150701).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study protocol was approved by the Beijing Shijitan Hospital Ethics Committee, Capital Medical University. Written informed consent was obtained from all the participants before their enrolment in the study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Liu, N., Yang, G., Hu, M. et al. ADIPOR2 variant is associated with higher fasting glucose level in non-diabetic Chinese Han population. Int J Diabetes Dev Ctries 38, 403–408 (2018). https://doi.org/10.1007/s13410-018-0620-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-018-0620-7