Abstract
This study was to compare the sleep quality in people with type 2 diabetes with healthy controls and to investigate the association of diabetes and sleep quality in people with type 2 diabetes without chronic complications. We also explored the effect of pain, nocturia, and obstructive sleep apnea (OSA) on sleep quality. Four hundred and five people were recruited in this case-control study. Sleep quality was assessed in 202 people with type 2 diabetes and was compared with 203 healthy individuals. All diabetic people were free from chronic complications. The Persian version of Pittsburgh Sleep Quality Index (PSQI) was used for the assessment of sleep quality. Poor sleep quality was defined as PSQI score >5. Poor sleep quality was more prevalent in people with type 2 diabetes. The odds ratio for poor sleep quality was 2.63 in people with long-standing diabetes (diabetes duration ≥10 years). Although the PSQI score was slightly better in diabetic people without pain/nocturia (P = 0.03), the prevalence of disturbed sleep showed no significant change after excluding these people from analysis. Diabetes was associated with 1.77-fold increase in risk of OSA, and poor sleep quality was more frequent in diabetic people at high risk for OSA (P = 0.005) independent of body weight. Long-standing diabetes was a significant predictor of poor sleep quality. Furthermore, the effect of diabetes on sleep quality was independent of its complications and other intervening risk factors such as pain/nocturia. Diabetes was also associated with OSA, stronger than the observed association between obesity and OSA.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal average sleep duration has decreased from 8.0–8.9 h per night in 1960 to about 6.9–7.0 h in 2000–2002 [1, 2]. Poor sleep quality and insufficient sleep are common issues for general practitioners, as 46–69 % of patients at primary care level suffer from occasional insomnia [3–6].
Current evidence has shown that poor sleep quality is associated with increased risk of insulin resistance and obesity [7–10]. Furthermore, it has been shown that poor sleep quality and short sleep duration increase the risk of diabetes [11–14]. A close relationship between diabetes and disturbed sleep has been proposed as the incidence of both disorders has dramatically increased during recent years [15–17]. In addition, short sleep duration has been observed to be related to the increased risk of type 2 diabetes [18, 19]. On the other hand, excessive and long sleep could lead to an increased risk of diabetes or may possibly be an early symptom of diabetes [12, 20].
Some epidemiologic studies examined the association between diabetes and sleep. Difficulty initiating sleep, difficulty maintaining sleep, and excessive daytime sleepiness are more common in diabetic patients [18, 21]. Nocturia and neuropathic pain have been proposed as possible causes of decreased sleep quality in diabetes [22]. Moreover, a close connection has been found between obstructive sleep apnea (OSA), diabetes, and worsening sleep quality [23–25]. Although obesity is the most relevant risk factor for OSA, it has been demonstrated that OSA and insulin resistance were present in non-obese subjects as well [26].
Few studies explored the impact of diabetes duration on sleep quality. So, the aim of this study was to compare the sleep quality in people with type 2 diabetes with healthy controls and to investigate the sleep quality in people with type 2 diabetes considering the effect of diabetes duration, nocturia, pain, and OSA.
Materials and methods
Participants
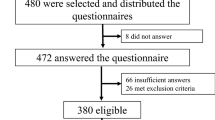
This was a case-control study conducted from September 2012 to February 2013. The study protocol was approved by the Ethics Committee of Iran University of Medical Sciences. Considering 95 % confident coefficient and test power of 80 %, the sample size was calculated on 400 people in both type 2 diabetic subjects and control groups.
A total of 405 people were enrolled; of those, 202 were diagnosed to have type 2 diabetes. The remaining 203 were healthy controls.
People with a diagnosis of type 2 diabetes who were consecutively attended the outpatient diabetes clinic at the Institute of Endocrinology and Metabolism were included. For each case, one matched control regarding age, sex, and body mass index (BMI) was chosen from healthy individuals.
The exclusion criteria were as follows:
-
Newly diagnosed type 2 diabetes with less than 1 year since diagnosis
-
Type 1 diabetes mellitus
-
History of any systemic diseases such as anemia, thyroid disease, liver dysfunction, cardiovascular disease, pulmonary disease, renal impairment, stroke, and peripheral vascular disease
-
Psychological diseases and diagnosed sleep disorders need regular medical treatment
-
Restless leg syndrome
-
Shift workers
-
Use of psychotropic and anticonvulsant medications
-
History of treatment with opioids and routine use of benzodiazepines
-
Known diabetic complications on medical intervention, such as established peripheral neuropathy, diabetic nephropathy, and diabetic retinopathy
-
Alcoholism and habitual smoking
-
Pregnancy and lactation
-
Patients without a hemoglobin A1c (HbA1c) measurement within 3 months prior to enrollment
Study design
All the patients underwent an interview to record information on age, sex, and diabetes duration. Height, weight, and BMI were also measured. Demographic variables and BMI were recorded for the healthy controls as well.
At the end of the visit, each participant was asked to complete a Persian version of the Pittsburgh Sleep Quality Index (PSQI). The reliability and validity of the Persian version of PSQI for assessment of subjective sleep quality were examined previously [27].
The PSQI is a validated 19-item questionnaire used to measure the quality and pattern of sleep in the past 1 month. It produces a global sleep quality score from 0 to 21, derived from seven components. The components include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbances, use of sleeping medications, and daytime dysfunction. People with a global score equal or less than 5 are defined to have “good sleep quality,” while a global score more than 5 identifies those with “poor sleep quality” [28].
We did a subgroup analysis considering those participants who were at high risk for OSA, as well as those who had trouble sleeping because of pain and/or nocturia if their sleep was reported to be disrupted three or more times per week.
OSA was defined when the participants indicated that their sleep was disrupted because of coughing, snoring, or difficulty breathing [29].
Statistical analysis
Analyses were performed for all participants. In case of missing data in questionnaire, total score could not be calculated but the scores of components were calculated. IBM SPSS for Windows Version 19 (IBM Corp., Armonk, NY, USA) was applied for the statistical analysis. The data were analyzed anonymously. Descriptive statistics (means and SDs) were used to describe clinical and demographic characteristics. Categorical data and proportions were compared with χ2 test, and scores of sleep questionnaire components were reported as median (quartile 1 and quartile 3). The intergroup differences involving continuous and the ordinal variables were assessed with the independent sample t test and Mann-Whitney U test, respectively.
Binary logistic regression was used to determine associations between OSA and some other variables. The type 1 error (alpha) level was set at 0.05 for all analyses.
Results
Among the 405 participants, a total of 262 participants, 128 with type 2 diabetes and 134 healthy subjects, filled out the PSQI questionnaire completely. The mean age was 55.45 ± 9.56 years and the mean BMI was 27.89 ± 3.22. In people with diabetes, the mean HbA1c was 7.1 (54) ± 1.4 and the mean duration of diabetes was 7.92 ± 6.42 years.
The people with diabetes and healthy subjects were matched regarding age, sex, and BMI.
Sixty percent of people with diabetes had poor sleep quality compared to 47 % of healthy subjects (P = 0.033). This represents an odds ratio of 1.7 (confidence interval (CI) 1.04–2.78) for poor sleep quality in those people with diabetes. When we considered diabetes duration, the odds ratio for poor sleep quality increased to 2.63 (CI 1.12–6.16, P = 0.02) in people with long-standing diabetes, i.e., people with diabetes duration of more than 10 years, compared to the healthy subjects.
Considering the seven components of the PSQI, there was no statistically significant differences between the groups except for the “duration of sleep” component which represented shorter sleep duration in people with diabetes (P = 0.007). This was due to the fact that people with diabetes reported to get up 14 min earlier on average in the morning, compared to the healthy subjects. The effect of diabetes on sleep quality was independent of age.
When we did the Mann-Whitney analysis for people with long-standing diabetes, we found that the sleep was less efficient (P = 0.05), the reported rates of day dysfunction and use of sleeping medication were higher (P = 0.01 and 0.02, respectively), and the overall quality of sleep was worse (P = 0.04) compared to the healthy subjects (Table 1).
As mentioned before, the mean HbA1c level was 7.1 (54.1) ± 1.4, and 53 % of the people with diabetes had good glycemic control with an HbA1c below 7 (53). However, the people with longer duration of diabetes had poorer glycemic control (r = 0.25, P = 0.000). There was no association between HbA1c level and the global PSQI score (r = −0.16 P = 0.07). However, the logistic regression analysis of the seven components of the PSQI and the level of HbA1c revealed that each point increase in “sleep latency” score leads to a 0.58-fold increase in risk of poor glycemic control (Table 2).
We also separated those people whose sleep was disrupted by frequent pain or nocturia more than three times per week. In the total cohort, there was no change in the duration of sleep (6.57 ± 1.17 h vs 6.49 ± 1.27 h, P = 0.31). However, the global PSQI score was lower (5.36 ± 1.72 vs 6.72 ± 2.32, P = 0.00). In addition, 57 % of people with diabetes still reported to have poor sleep quality compared to 38 % of the healthy subjects (P = 0.015). Although the PSQI score improved slightly by excluding those people with pain and nocturia in diabetic group, the sleep duration and the frequency of poor sleep quality showed no significant changes (Table 3).
When we considered OSA, 54 % of people with diabetes and 39 % of healthy subjects were at high risk for OSA (P = 0.004). In total cohort, BMI was higher in people with OSA (28.65 ± 3.34 vs 27.36 ± 3.09, P = 0.00). Similarly, in diabetic people at high risk for OSA, BMI was significantly higher compared to those without OSA (28.70 ± 3.79 vs 27.47 ± 3.14, P = 0.014). Age, BMI, and diabetes were considered as independent variables in a logistic regression analysis to evaluate their effects on OSA as a dependent variable. The analysis revealed that diabetes was associated with 1.77-fold increase in the risk of OSA independent of body weight (odds ratio 1.77, CI 1.34–2.7, P = 0.01) (Table 4).
Table 5 represents the impact of OSA on sleep quality in people with diabetes and healthy subjects. Poor sleep quality was more frequent in people with diabetes at high risk for OSA (69 vs 37 %, P = 0.005).
Discussion
In this study, we found that poor sleep quality was more prevalent in people with type 2 diabetes not complicated by chronic complications compared to healthy individuals. The effect of diabetes on disturbed sleep was independent of age, sex, and BMI. In addition, long-standing diabetes had a significant impact on sleep quality. The odds ratio for poor sleep quality was 2.63 (CI 1.12–6.16, P = 0.02) in people with diabetes duration of more than 10 years. Moreover, these people had significant problems with regards to sleep efficiency, daily activity, and overall sleep quality. In people with diabetes, we also found that pain and/or nocturia was not responsible for the observed correlation between diabetes and poor sleep quality. On the other hand, diabetes was associated with 1.77-fold increase in risk of OSA.
The literature has demonstrated a reciprocal interaction between sleep and type 2 diabetes [18, 20, 30–34]. The dramatic and simultaneous increase in the incidence of diabetes and poor sleep quality suggests a close relationship between the two [15, 17]. Several studies demonstrated the association of poor sleep quality, diabetes, and its severity [35–37]. However, few studies addressed the impact of diabetes duration on sleep quality, independent of important interfering factors such as diabetes chronic complications, pain, nocturia, and OSA which could bias the observed association.
We also showed that diabetes duration was an important factor determining sleep quality and sleep efficiency in type 2 diabetes.
It could be claimed that frequent pain and or nocturia could play a role in the observed association between diabetes and poor sleep quality [38]. Although the global PSQI score was slightly better in diabetic people without frequent pain and nocturia, the proportion of people with poor sleep quality remained unchanged. This observation supports the hypothesis that diabetes is responsible for disturbed sleep, independent of its complications and subjective symptoms.
On the other hand, in our study, 54 % of people with diabetes were at high risk for OSA, compared to 39 % of the healthy individuals, while Knutson et al. reported a lower prevalence of OSA (20 %) using PSQI [37]. This might be due to the difference in age, BMI, diabetes duration, and glycemic control of the participants in different populations. Obesity is the strongest risk factor for breathing disorders during sleep, and OSA is reported to be present in 40 % of obese individuals [39].
In our study, the effect of obesity on OSA was significantly lower than that of diabetes. The odds ratio for obesity and OSA was 1.14 (CI 1.06–1.23, P = 0.000) while it was 1.77 (CI 1.34–2.74, P = 0.01) for diabetes. So, it seems that both diabetes and obesity could be responsible for breathing disorders during sleep. When we considered OSA in our analysis, we found that the prevalence of poor sleep quality and the global PSQI score were higher in diabetic people at high risk for OSA.
One of the most interesting finding of our study was the lack of association between poor sleep quality and glycemic control. In contrast to our finding, Knutson et al. [37] reported that lower sleep quality is associated with poorer glycemic control. It should be mentioned that in our study, the mean HbA1c was lower (7.1 (54) ± 1.47 vs 8.3 (67) ± 2.1), and 53 % of the people with diabetes had good glycemic control. In another study [40], sleep efficiency was correlated with HbA1c level. We found that out of the seven components of the PSQI, it was the “sleep latency” that correlates well with the level of HbA1c. Further investigations are needed to determine the level of glycemic control that could exert clinically important effect on sleep quality in people with type 2 diabetes. In addition, the impact of every single component of PSQI on overall sleep quality in type 2 diabetes needs to be determined.
To the best of our knowledge, with regards to the very rigorous exclusion criteria, this was the first study to assess sleep quality in a group of people with type 2 diabetes without any chronic complications or any other intervening risk factors, and with an acceptable state of glycemic control. We explored the effect of diabetes duration, pain/nocturia, and OSA on the observed correlation between diabetes and disturbed sleep. However, we had some limitations. The method we used for identifying OSA was not objective, because OSA was defined based on the specific questions in PSQI. Moreover, we did not record the details of treatment or presence of nocturnal hypoglycemia that might affect the sleep quality. In addition, the history of diabetes complications was established only from medical files, and people with established diabetes-related complications were excluded from the study.
References
Martins RC, Andersen ML, Tufik S. The reciprocal interaction between sleep and type 2 diabetes mellitus: facts and perspectives. Braz J Med Biol Res. 2008;41(3):180–7.
Kripke DF, Simons RN, Garfinkel L, Hammond EC. Short and long sleep and sleeping pills. Is increased mortality associated? Arch Gen Psychiatry. 1979;36(1):103–16.
Terzano MG, Parrino L, Cirignotta F, Ferini-Strambi L, Gigli G, Rudelli G, et al. Studio Morfeo: insomnia in primary care, a survey conducted on the Italian population. Sleep Med. 2004;5(1):67–75.
Walsh J, Benca R, Bonnet M, Buysse D, Hauri P, Kiley J, et al. Insomnia: assessment and management in primary care. Am Fam Physician. 1999;59(11):3029–38.
Aikens JE, Rouse ME. Help-seeking for insomnia among adult patients in primary care. J Am Board Fam Pract. 2005;18(4):257–61.
Shochat T, Umphress J, Israel AG, Ancoli-Israel S. Insomnia in primary care patients. Sleep. 1999;22 Suppl 2:S359–65.
Gangwisch JE, Malaspina D, Boden-Albala B, Heymsfield SB. Inadequate sleep as a risk factor for obesity: analyses of the NHANES I. Sleep. 2005;28(10):1289–96.
Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, et al. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27(4):661–6.
Knutson KL, Spiegel K, Penev P, Van Cauter E. The metabolic consequences of sleep deprivation. Sleep Med Rev. 2007;11(3):163–78. doi:10.1016/j.smrv.2007.01.002.
Lou P, Chen P, Zhang L, Zhang P, Yu J, Zhang N, et al. Relation of sleep quality and sleep duration to type 2 diabetes: a population-based cross-sectional survey. BMJ open. 2012;2(4):e000956.
Brooks B, Cistulli PA, Borkman M, Ross G, McGhee S, Grunstein RR, et al. Obstructive sleep apnea in obese noninsulin-dependent diabetic patients: effect of continuous positive airway pressure treatment on insulin responsiveness. J Clin Endocrinol Metab. 1994;79(6):1681–5.
Gottlieb DJ, Punjabi NM, Newman AB, Resnick HE, Redline S, Baldwin CM, et al. Association of sleep time with diabetes mellitus and impaired glucose tolerance. Arch Intern Med. 2005;165(8):863–7. doi:10.1001/archinte.165.8.863.
Kita T, Yoshioka E, Satoh H, Saijo Y, Kawaharada M, Okada E, et al. Short sleep duration and poor sleep quality increase the risk of diabetes in Japanese workers with no family history of diabetes. Diabetes Care. 2012;35(2):313–8. doi:10.2337/dc11-1455.
Hayashino Y, Fukuhara S, Suzukamo Y, Okamura T, Tanaka T, Ueshima H. Relation between sleep quality and quantity, quality of life, and risk of developing diabetes in healthy workers in Japan: the High-risk and Population Strategy for Occupational Health Promotion (HIPOP-OHP) Study. BMC Public Health. 2007;7:129. doi:10.1186/1471-2458-7-129.
Spiegel K, Knutson K, Leproult R, Tasali E, Van Cauter E. Sleep loss: a novel risk factor for insulin resistance and Type 2 diabetes. J Appl Physiol. 2005;99(5):2008–19. doi:10.1152/japplphysiol.00660.2005.
Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18(5):716–38.
Resnick HE, Redline S, Shahar E, Gilpin A, Newman A, Walter R, et al. Diabetes and sleep disturbances: findings from the Sleep Heart Health Study. Diabetes Care. 2003;26(3):702–9.
Sridhar GR, Madhu K. Prevalence of sleep disturbances in diabetes mellitus. Diabetes Res Clin Pract. 1994;23(3):183–6.
Scheen AJ, Byrne MM, Plat L, Leproult R, Van Cauter E. Relationships between sleep quality and glucose regulation in normal humans. Am J Physiol. 1996;271(2 Pt 1):E261–70.
Ayas NT, White DP, Al-Delaimy WK, Manson JE, Stampfer MJ, Speizer FE, et al. A prospective study of self-reported sleep duration and incident diabetes in women. Diabetes Care. 2003;26(2):380–4.
Gislason T, Almqvist M. Somatic diseases and sleep complaints. An epidemiological study of 3,201 Swedish men. Acta Med Scand. 1987;221(5):475–81.
Meier U, Gressner AM. Endocrine regulation of energy metabolism: review of pathobiochemical and clinical chemical aspects of leptin, ghrelin, adiponectin, and resistin. Clin Chem. 2004;50(9):1511–25. doi:10.1373/clinchem.2004.032482.
Tasali E, Mokhlesi B, Van Cauter E. Obstructive sleep apnea and type 2 diabetes: interacting epidemics. Chest. 2008;133(2):496–506. doi:10.1378/chest.07-0828.
Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thorac Soc. 2008;5(2):207–17. doi:10.1513/pats.200708-139MG.
West SD, Nicoll DJ, Stradling JR. Prevalence of obstructive sleep apnoea in men with type 2 diabetes. Thorax. 2006;61(11):945–50. doi:10.1136/thx.2005.057745.
Ip MS, Lam B, Ng MM, Lam WK, Tsang KW, Lam KS. Obstructive sleep apnea is independently associated with insulin resistance. Am J Respir Crit Care Med. 2002;165(5):670–6. doi:10.1164/ajrccm.165.5.2103001.
Farrahi Moghaddam J, Nakhaee N, Sheibani V, Garrusi B, Amirkafi A. Reliability and validity of the Persian version of the Pittsburgh Sleep Quality Index (PSQI-P). Sleep Breath. 2012;16(1):79–82. doi:10.1007/s11325-010-0478-5.
Sleep Medicine Institute. Pittsburgh Sleep Quality Index (PSQI). http://www.sleep.pitt.edu/content.asp?id=1484&subid=2316. Accessed 23 Apr 2013.
Qaseem A, Dallas P, Owens DK, Starkey M, Holty JE, Shekelle P. Diagnosis of obstructive sleep apnea in adults: a clinical practice guideline from theAmerican College of Physicians. Ann Intern Med. 2014;161(3):210–20. doi:10.7326/m12-3187.
Nakajima H, Kaneita Y, Yokoyama E, Harano S, Tamaki T, Ibuka E, et al. Association between sleep duration and hemoglobin A1c level. Sleep Med. 2008;9(7):745–52. doi:10.1016/j.sleep.2007.07.017.
Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354(9188):1435–9. doi:10.1016/s0140-6736(99)01376-8.
Kawakami N, Takatsuka N, Shimizu H. Sleep disturbance and onset of type 2 diabetes. Diabetes Care. 2004;27(1):282–3.
Nilsson PM, Roost M, Engstrom G, Hedblad B, Berglund G. Incidence of diabetes in middle-aged men is related to sleep disturbances. Diabetes Care. 2004;27(10):2464–9.
Yaggi HK, Araujo AB, McKinlay JB. Sleep duration as a risk factor for the development of type 2 diabetes. Diabetes Care. 2006;29(3):657–61.
Song Y, Ye X, Ye L, Li B, Wang L, Hua Y. Disturbed subjective sleep in Chinese females with type 2 diabetes on insulin therapy. PLoS One. 2013;8(1):e54951. doi:10.1371/journal.pone.0054951.
Tsai YW, Kann NH, Tung TH, Chao YJ, Lin CJ, Chang KC, et al. Impact of subjective sleep quality on glycemic control in type 2 diabetes mellitus. Fam Pract. 2012;29(1):30–5. doi:10.1093/fampra/cmr041.
Knutson KL, Ryden AM, Mander BA, Van Cauter E. Role of sleep duration and quality in the risk and severity of type 2 diabetes mellitus. Arch Intern Med. 2006;166(16):1768–74. doi:10.1001/archinte.166.16.1768.
Lamond N, Tiggemann M, Dawson D. Factors predicting sleep disruption in Type II diabetes. Sleep. 2000;23(3):415–6.
Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154(15):1705–11.
Trento M, Broglio F, Riganti F, Basile M, Borgo E, Kucich C, et al. Sleep abnormalities in type 2 diabetes may be associated with glycemic control. Acta Diabetol. 2008;45(4):225–9. doi:10.1007/s00592-008-0047-6.
Acknowledgments
This study was funded and supported by Iran University of Medical Sciences, Grant No. 91-03-122-19195. We also appreciate all the people who contributed to this study.
Conflict of interest
Nothing to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nasseri, R., Malek, M., Aghili, R. et al. Disturbed sleep in type 2 diabetes mellitus independent of chronic complications, pain, and nocturia. Int J Diabetes Dev Ctries 35, 454–459 (2015). https://doi.org/10.1007/s13410-015-0314-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13410-015-0314-3