Abstract
In this paper, a facile approach has been developed to synthesize the novel multifunctional Ag@Fe3O4@C-Au magnetic core-shell microspheres that display a highly efficient surface-enhanced Raman scattering (SERS) substrate with high stability and reproducibility. The morphology, size, chemical component, and magnetic property of as-prepared composite microspheres were characterized by scanning transmission electron microscopy (SEM), transmission electron microscopy (TEM), X-ray diffraction (XRD), thermogravimetric analysis (TGA), X-ray photoelectron spectra (XPS), and vibrating sample magnetomerter (VSM), respectively. The enhancement effect on the Raman active was investigated by using rhodamine-b (RdB) as a probe molecule. The result shows that the well-designed Ag@Fe3O4@C-Au core-shell microspheres have uniform sphere size and high magnetization, and the SERS signals of RdB on the Ag@Fe3O4@C-Au microspheres were much stronger than those on both Ag@Fe3O4 and Fe3O4@C-Au microspheres.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surface-enhanced Raman scattering spectroscopy (SERS) is a highly sensitive and selective tool that provides fine details of molecules through high-quality spectra, primarily due to the large electromagnetic enhancement (EM) of the localized surface plasmon resonance (LSPR) in nanostructures. Since its first observation in the 1970s [1], SERS has been employed increasing toward microanalytical applications in various fields due to it being 106–108 times more sensitive than conventional Raman. Generally, substrates based on metals such as Au, Ag, and their composites are required to realize a substantial SERS effect [2–5]. But, it is widely known that surface plasmon efficiency is greater for Ag than it is for Au [6]. In addition, Ag is more mobile and more easily oxidized than Au. If Ag nanostructures can be stabilized by a chemically and electrically inert shell, these weaknesses can be managed. So, Kim et al. developed an Ag-silica-Au hybrid device that displayed a long-range plasmon transfer of Ag to Au leading to enhanced Raman scattering of molecules largely separated from the optically excited Ag surface [7]. On the other hand, despite there are many advantages of long-range plasmon transfer of Ag to Au leading to enhanced Raman scattering, it must be emphasized that most of these SERS substrates are thrown away after detecting probably. Thus, it is of considerable importance to develop an efficient SERS substrate that can not only provide strong enhancement factors but also show high stability and reproducibility. To resolve this problem, magnetic materials such as Fe3O4 were introduced usually with Au or Ag nanoparticles to form composite structures for SERS substrates. In the past few years, various research groups have shown that such magnetic-core noble metal-shell composites not only are biocompatible but also facilitate large-scale Raman scattering enhancements [8–11]. For instance, Shen et al. report a novel multifunctional Fe3O4@ Ag/SiO2/Au core-shell nanoparticles that display long-range plasmon transfer of Ag to Au leading to enhanced Raman scattering [6]. Wang et al. synthesized Fe3O4/Ag/Au composites for immunoassay based on surface plasmon resonance biosensor [12].
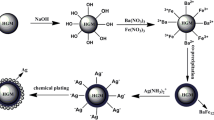
In this work, we report the fabrication of a new kind of multifunctional Ag@Fe3O4@C-Au magnetic core-shell microspheres. The detailed protocol for the preparation of the Ag@Fe3O4@C-Au magnetic core-shell microspheres and detection of the rhodamine-b (RdB) is shown in Scheme 1. The well-designed microspheres have high magnetization and uniform sphere size. The SERS activities of these microspheres have been tested by using RdB as a probe molecule. The unique nanostructure makes the microspheres novel stable, reproducible, and a high-enhancement effect for Raman detection.
Experimental
Materials
Ethylene glycol (EG), anhydrous sodium acetate (NaOAc), iron nitrate (Fe(NO3)3·9H2O), silver nitrate (AgNO3), poly(vinyl-pyrrolidone) (PVP MW = 30,000), and polyetherimide (PEI) were obtained from the Tianjin Guangfu Fine Chemical Research Institute. Chloroauric acid (HAuCl4·4H2O), ethanol, and RdB were purchased from Sinopharm Chemical Reagent Co.; 1-[3-(dimethylamino)propyl]-3-ethylcarbodii-mide hydrochloride (EDC), and n-hydroxy-succinimide (NHS) were purchased from Sigma Aldrich Co. All reagents were used as received without further purication. The pure water was obtained from a Milli-Q synthesis system.
Synthesis of Ag@Fe3O4 nanospheres
Functionalized Ag@Fe3O4 nanoparticles were synthesized via a versatile solvothermal reaction reported with a slight modification [13]. Briefly, Fe(NO3)3·9H2O (1.5 g), NaOAc (3.5 g), PVP (1.0 g), and AgNO3 (0.15 g) were dissolved in EG (70 ml) with magnetic stirring, followed by the transfer of the resulting mixture into a 100-ml Teflon-lined stainless-steel autoclave and heated at 200°C for 8 h. Finally, the products were collected with a magnet and dried in a vacuum oven at 60°C for further use.
Synthesis of Ag@Fe3O4@C nanospheres
The carboxyl and negative charge-functionalized magnetic Ag@Fe3O4@C nanospheres were obtained following the method reported [14]; 0.1 g Ag@Fe3O4 nanoparticles and 1.0 g glucose were immersed in 70 ml deionized water by ultrasonication for 30 min. Then, the solution was transferred to a 100-ml Teflon-sealed autoclave for treated at 200°C for 12 h. The products were separated by a magnet, and washed several times with ethanol and deionized water. Last, the as-obtained products were dried at 60°C for further use.
Loading Au nanoparticles to obtain Ag@ Fe3O4@C-Au nanospheres
Citrate-stabilized Au nanoparticles of about 16 nm were prepared according to the reported methods [15]. The deposition of Au nanoparticles onto Ag@Fe3O4@C was performed as follows. First, 0.1 g of Ag@Fe3O4@C was dispersed into 100-ml (0.5 mg/ml) PEI aqueous solution under magnetic stirring for 2 h; then, EDC (0.83 mmol) and NHS (0.83 mmol) were added into the solution under magnetic stirring for 12 h. The residual PEI was removed by magnetic separation, and the composites were rinsed with water at least three times. Last, Ag@Fe3O4@C-PEI microspheres was dispersed into 100-ml Au NP solution (0.48 mmol/l), and the dispersion was stirred for 4 h. Then, the precipitate was collected by magnetic separation, washed several times with deionized water.
Measurements of SERS activity of the Ag@Fe3O4@C-Au microspheres
RdB was used as a model molecule and Raman label for examination of the SERS activity of the Ag@Fe3O4@C-Au, Ag@Fe3O4, and Fe3O4@C-Au microspheres, respectively. In a typical experiment, 10 mg of the as-prepared microspheres were dispersed in 10-ml ethanol solution of 10−8 M concentrations of RdB aqueous solution for 12 h under ambient temperature with vigorous shaking. Then, in situ SERS activity of the solution at the position of the magnet was detected, and a 633-nm laser was used for the Raman measurements.
Characterization
Transmission electron microscopy (TEM) images were obtained on a JEM-2100 TEM (Jeol Ltd., Tokyo, Japan). X-ray powder diffraction (XRD) analysis was performed using a Dmax-2500 (CuKα = 1.5406 Å; Rigaku Corporation, Tokyo, Japan). Magnetic characteristics were studied using a vibrating sample magnetometer (VSM) (Lake Shore Company, Westerville, OH, USA) at room temperature. Scanning transmission electron microscopy (SEM) was carried out on an S-4800 microscope (Hitachi Limited, Tokyo, Japan). Thermogravimetric analysis (TGA) of nanocomposite was performed on the TGA Q500 from TA Instruments (New Castle, DE, USA). Analyzed samples were heated from 100 to 800°C at a heating rate of 10°C/min. X-ray photoelectron spectra (XPS) were recorded by Thermo ESCALAB 250XI X-ray photoelectron spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA) with nonmonochromatized Al Kα radiation as excitation source. Raman spectra were recorded on a HR800 Raman microprobe (Horiba Jobin Yvon LabRAM, France) with 633-nm laser excitation.
Results and discussion
The shape and size of the as-prepared microspheres were examined by SEM and TEM. Figure 1a shows SEM image, which clearly indicates that the as-prepared Ag@Fe3O4 nanocomposite has a typical spherical nanostructure. All the particles are uniform and well dispersed without any large aggregations. The average diameter of the nanospheres is around 90 nm, and the surface of the particles is rough. The morphology of the Ag@Fe3O4 nanospheres is also clearly demonstrated from the TEM image. As shown in Fig. 1d, the Ag@Fe3O4 composite nanospheres are composed of two distinct components: the Fe3O4 shell composed of many fine primary magnetite nanocrystals was about 40 nm, and the black of Ag cores was about 45 nm. These nanostructures of Ag@Fe3O4 can overcome the aggregation of Ag nanoparticles, because the magnetic shell acts as a physical barrier to protect the Ag cores from irreversible aggregation [6]. As seen in Fig. 1b, e, it is obvious that the carbon-coated Ag@Fe3O4 composite nanospheres are perfectly spherical in shape with smooth surfaces and the shell layer of carbon is about 8 nm in thickness. The surface of the obtained Ag@Fe3O4@C composite microspheres was further modified by PEI to form sufficient amounts of amine functional groups, and the strong chemical bonding between Au atoms and N atoms in the amino groups ensures the grafting of Au NPs on the surface of Ag@ Fe3O4@C. After loading Au nanoparticles, the corresponding SEM and TEM images were shown in Fig. 1c, f; the whole Ag@Fe3O4@C-Au composite microspheres with the rough surface (a size of approximately 150 nm) are coated with about 16 nm Au nanoparticles. Thus, this triple core-shell-structured Ag@ Fe3O4@C-Au has a Fe3O4 magnetic embedded Ag core, an amorphous carbon coat, and a layer of Au nanoparticle shell.
The TGA curves of naked Ag@Fe3O4 and Ag@Fe3O4@C nanospheres were shown in Fig. 2. For naked Ag@Fe3O4, the TGA curve showed that the weight loss over the temperature range from 100 to 800°C was about 7.2%. This might be due to the loss of the removal of the remaining water and agents. Compared with the TGA curves of the naked Ag@Fe3O4, the Ag@Fe3O4@C nanospheres show that the main mass of the as-synthesized occurred about 40.0% decrease attributed to the decomposition of carbon coated on the surface of the Ag@Fe3O4 nanospheres.
The X-ray diffraction (XRD) patterns of the synthesized naked Fe3O4 nanospheres, Ag@Fe3O4, Ag@Fe3O4@C, and Ag@Fe3O4@ C-Au composite microspheres are shown in Fig. 3. For all the samples, six diffraction peaks at 30.08°, 35.42°, 43.08°, 53.56°, 57.0°, and 62.7° were indexed to the (220), (311), (400), (422), (511), and (440) planes of the Fe3O4 cubic inverse spinel phase. As shown in Fig. 3b for Ag@Fe3O4@C composite microspheres, four extra diffraction peaks at 38.2°, 44.3°, 64.4°, and 77.6° were indexed to the (111), (200), (220), and (311) planes of the Ag cubic phase (JCPDS no. 04-0783). As shown in Fig. 3c, d, these characteristic peaks can be seen in the composite magnetic nanoparticles, while the broad peak at 17–27° was ascribed to an amorphous carbon. According to the literature [6], the characteristic peaks for Au and Ag were too close to distinguish.
In order to further identify the formation of Ag@Fe3O4@C-Au microsphere composite, the samples were analyzed by electron mapping image analysis (Fig. 4). As can be seen in Fig. 4a–e, the Au, Ag, Fe, C, and O are distributed over the entire structure: Ag atoms are located in the core, Fe and O atoms are located in the shell of the Ag@Fe3O4 core-shell nanostructure, and C and Au nanoparticles are located in the outer surface of the Fe3O4 microspheres, respectively. Energy-dispersive X-ray spectroscopy (EDX) of an individual nanosphere in Fig. 4g shows that Ag, Au, Fe, C, and O peaks are presented in the spectra.
XPS analysis was performed on the Ag@Fe3O4@C-Au core-shell composite microspheres to gain a better understanding of the phase surface chemistry. Figure 5a shows the full scan spectra, which reveals the presence of silver, iron, oxygen, carbon, and nitrogen elements in the Ag@Fe3O4@C-Au composite microspheres. In the spectrum of Fe 2p (Fig. 5b), the peaks of Fe 2p1/2 and Fe 2p3/2 are located at 724.6 and 711.2 eV, respectively. In the Ag 3d spectrum (Fig. 5c), the binding energies of Ag 3d3/2 and Ag 3d5/2 with a peak splitting of 6 eV are 374.1 and 368.1 eV, respectively, which matches the standard reference XPS spectrum of metallic Ag. The 4f5 and 4f7 peaks of Au at 87.7 and 84.1 eV in Fig. 5d can be assigned to the metal Au.
Figure 6 shows the UV-vis spectroscopy of Au, Ag@Fe3O4, and Ag@Fe3O4@C-Au composite microspheres. The Ag@Fe3O4 microspheres have an absorption peak at 409 nm because of the typical surface plasmon resonance of silver nanoparticles; the peak at 524 nm observed on Au nanoparticles was caused by the surface plasmon coupling between closely spaced Au nanoparticles. The absorption peak leads to a redshift to 570 nm after coating Au nanoparticles on the Ag@Fe3O4@C microspheres. Thus, SERS measurements with 633-nm excitation have been extended to adsorbents on Au surfaces.
In addition, to gain a better understanding of the magnetic properties of the as-synthesized microspheres, the room-temperature magnetization saturation of the as-prepared Ag@Fe3O4, Ag@Fe3O4@C, and Ag@Fe3O4@C-Au particle values were measured in Fig. 7. The values of Ms of Ag@Fe3O4, Ag@Fe3O4@C, and Ag@Fe3O4@ C-Au particles were decreased to 76.3, 65.8, and 53.3 emu/g, successively. These phenomena can be explained by the diamagnetic contribution of the carbon shell and the Au nanoparticles surrounding Ag@Fe3O4@C microspheres.
On the other hand, the Ag@Fe3O4@C-Au microspheres can be readily used as SERS substrates for molecular sensing with sensitivity and specificity. Because the core-shell microspheres have the Fe3O4 with magnetic properties and the novel metals were well-known SERS active materials, the Ag@Fe3O4@C-Au microspheres would be collected by magnet and also have SERS activity. In order to evaluate their performance as SERS substrates, we used RdB as the model SERS marker. Figure 8 presents the SERS spectra of 10−6 M RdB adsorbed on the surface of the Ag@Fe3O4, Fe3O4@C-Au, and Ag@Fe3O4@C-Au microspheres, respectively. As shown in Fig.8(A–C), the characteristic bands including νCH(ip) (1193 cm−1) and νC-C (1356, 1503, 1580, and 1652 cm−1) are observed, which is the Raman spectra of RB [6]. The Ag@Fe3O4 core-shell microspheres exhibit weak enhancement because of the electromagnetic damping of Fe3O4 shells on Ag (Fig. 8(B)), and the Fe3O4@C-Au microspheres possess weaker enhancement, which display that surface plasmon efficiency is greater for Ag than that for Au (Fig. 8(C)). The Ag@Fe3O4@C-Au microspheres cause a large increase in the intensity, which is larger than the other structure microspheres (Fig. 8(A)). In addition, compared with the Fe3O4@C-Au microspheres and Ag@Fe3O4 microspheres, the levels of SERS enhancement of these microspheres were measured. As shown in Fig. 9, it is clearly seen that the low concentrations of RdB (10–9) in the solution can be detected by the Ag@Fe3O4@C-Au microspheres, but the other samples under the same concentration was almost undetectable. And the results are consistent with Fig. 8, which shows the SERS signals of RdB on the Ag@Fe3O4@C-Au microspheres were much stronger than those on both the Ag@Fe3O4 and Fe3O4@C-Au microspheres.
Conclusion
In conclusion, we have successfully synthesized novel multifunctional Ag@Fe3O4@C-Au magnetic core-shell microspheres that exhibit good sensitivity performance in SERS. The high SERS activities of the Ag@Fe3O4@C-Au composite microspheres have been utilized for the detection of low concentrations of RdB. Furthermore, due to their excellent magnetic properties, the microspheres can be reused with convenient magnetic separability.
References
Fleischmann M, Hendra PJ, Mcquillan AJ (1974) Raman spectra of pyridine adsorbed at a silver electrode. Chem Phys Lett 26:163–166. doi:10.1016/0009-2614(74)85388-1
Fan M, Brolo AG (2009) Silver nanoparticles self assembly as SERS substrates with near single molecule detection limit. Phys Chem Chem Phys 34:7381–7390. doi:10.1039/b904744a
Zhang YY, Walkenfort B, Yoon JH, Schlucher S, Xie W (2015) Gold and silver nanoparticle monomers are non-SERS-active: a negative experimental study with silica-encapsulated Raman-reporter-coated metal colloids. Phys Chem Chem Phys 33:21120–21126. doi:10.1039/C4CP05073 H
Qi JW, Li D, Yang M, Wu Q, Chen ZQ, Wang WD, Lu WQ, Yu XY, Xu JJ, Sun Q (2013) Large-area high-performance SERS substrates with deep controllable sub-10-nm gap structure fabricated by depositing Au film on the cicada wing. Nanoscale Res Lett 8:437–482. doi:10.1186/1556-276X-8-437
Zhao Y, Zeng WC, Tao ZC, Xiong PH, Qu Y, Zhu YW (2015) Highly sensitive surface-enhanced Raman scattering based on multi-dimensional plasmonic coupling in Au-graphene-Ag hybrids. Chem Commun 51:866–869. doi:10.1039/c4cc07937j
Shen JH, Zhu YH, Yang XL, Zong J, Li CZ (2013) Multifunctional Fe3O4@Ag/SiO2/Au core-shell microspheres as a novel SERS-activity label via long-range plasmon coupling. Langmuir 29:690–695. doi:10.1021/la304048v
Kim KH, Back YK, Jeon H, Srinivasarao M, Jung HT (2012) Cylindrical posts of Ag/SiO2/Au multi-segment layer patterns for highly efficient surface enhanced Raman scattering. Nanotechnology 31:1–5. doi:10.1088/0957-4484/23/31/315302
An Q, Zhang P, Li JM, Ma WF, Guo J, Hu J, Wang CC (2012) Silver-coated magnetite-carbon core-shell microspheres as substrate-enhanced SERS probes for detection of trace persistent organic pollutants. Nanoscale 4:5210–5216. doi:10.1039/c2nr3 1061a
Zhang XQ, Zhu YH, Yang XL, Zhou Y, Yao YF, Li CZ (2014) Multifunctional Fe3O4@ TiO2@Au magnetic microspheres as recyclable substrates for surface-enhanced Raman scattering. Nanoscale 6:5971–5979. doi:10.1039/c4nr00975d
Xu ZC, Hou YL, Sun SH (2007) Magnetic core/shell Fe3O4/Au and Fe3O4/Au/Ag nanoparticles with tunable plasmonic properties. J Am Chem Soc 129:8698–8699. doi:10.1021/ja073057v
Zhang MF, Zhao AW, Wang DP, Sun HH (2015) Hierarchically assembled NiCo@SiO2@Ag magnetic core-shell microspheres as highly efficient and recyclable 3D SERS substrates. Analyst 140:440–448. doi:10.1039/c4an01275e
Wang J, Song DQ, Zhang H, Zhang J, Zhang HQ, Zhou H, Sun Y (2013) Studies of Fe3O4/Ag/Au composites for immunoassay based on surface plasmon resonance biosensor. Colloids Surf B: Biointerfaces 1:165–170. doi:10.1016/ j.colsurfb.2012.08.040
Jiang WQ, Zhou YF, Zhang YL, Xuan SH, Gong XL (2012) Superparamagnetic Ag@Fe3O4 core–shell nanospheres: fabrication, characterization and application as reusable nanocatalysts. Dalton Trans 41:4594–4601. doi:10.1039/c2dt12307j
Zhu MY, Diao GW (2011) Magnetically recyclable Pd nanoparticles immobilized on magnetic Fe3O4@C nanocomposites: preparation, characterization, and their catalytic activity toward Suzuki and Heck coupling reactions. J Phys Chem C 115:24743–24749. doi:10.1021/jp206116e
Liu SH, Lu F, Liu Y, Jiang LP, Zhu JJ (2013) Synthesis, characterization, and electrochemical applications of multifunctional Fe3O4@C-Au nanocomposites. J Nanopart Res 15:1331–1339. doi:10.1007/ s11051-012-1331-5
Acknowledgments
The authors gratefully acknowledge the support for this research from the Key Disciplines of Applied Chemistry of Zhejiang Province, Taizhou University, and the National Natural Science Foundation of China under Grant No. 21403150.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, M., Chen, S., Jia, W. et al. Facile synthesis of Ag@Fe3O4@C-Au core-shell microspheres for surface-enhanced Raman scattering. Gold Bull 49, 103–109 (2016). https://doi.org/10.1007/s13404-016-0185-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13404-016-0185-5