Abstract
Purpose
Upregulation of receptor tyrosine kinase EphA2 has been found to be associated with a poor prognosis in many types of cancer and is considered an attractive therapeutic target. As yet, few efforts have been focused on its tumor suppressive activity triggered by its ligand, ephrinA1. Here, we aimed to determine the potential of ephrinA1 as an important player in melanoma metastasis.
Methods
Data from the Cancer Genome Atlas (TCGA) and the Cancer Cell Line Encyclopedia (CCLE) were analyzed to explore the expression and prognostic implications of EphA2 and ephrinA1 in melanoma. Western blotting, shRNA, colony formation and immunofluorescence assays, as well as two in vivo xenograft models (subcutaneous and metastatic) were used to evaluate the role of EphA2 in melanoma progression. Akt inhibition and ephrinA1-Fc were used to confirm the influence of Akt activation and ephrinA1 levels on the EphA2 effects. Immunohistochemistry (IHC) was performed on xenograft and patient melanoma tissues.
Results
We found that high levels of ephrinA1, but not EphA2, were negatively correlated with melanoma metastasis. The expression levels of EphA2 and ephrinA1 were not correlated. After EphA2 downregulation, colony forming abilities and lung metastatic growth were reduced in melanoma cell lines with a low ephrinA1 expression, but were increased in melanoma cell lines with a high ephrinA1 expression. EphA2-mediated colony formation in EphA2-high/ephrinA1-low cells was found to be Akt-dependent and to be inhibited by the addition of ephrinA1-Fc. IHC staining of primary melanoma specimens revealed that EphA2-high/ephrinA1-low patients exhibited poorer outcomes than EphA2-high/ephrinA1-high patients.
Conclusions
From our data we conclude that evaluation of ephrinA1 levels may be helpful for the application of EphA2-targeted therapies and for prognostic predictions in melanoma patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Cancer-associated death is largely caused by metastatic disease rather than by primary tumors. The incidence of melanoma is increasing worldwide, and the prognosis of patients with metastatic melanoma is grim [1, 2]. Therefore, the identification of molecular markers that are predictive of melanoma metastasis is important for the early application of therapeutic strategies when intervention is still possible. The Eph family of receptor tyrosine kinases (RTKs) plays diverse roles in normal development and disease. Based on sequence homology and binding preferences, the Eph receptors and their ephrin ligands are subdivided into class A and class B, which can transduce both forward signaling and reverse signaling in receptor-expressing cells and ligand-expressing cells, respectively [3]. The first-described ligand, ephrinA1, which can exist as a membrane-bound ligand on the cell surface or in a soluble, monomeric form, is able to induce phosphorylation and internalization of its receptors, especially EphA2. The function of ephrinA1 in angiogenesis and vascular permeability has been documented, whereas its role in tumor biology still needs to be elucidated [4]. EphA2 has been found to be frequently overexpressed in various cancer types, including melanoma, and has been extensively studied [3, 5,6,7]. Notably, Eph/ephrin signaling is complex and is involved in both the RAS–PI3K–Akt and RAS–MAPK pathways, as well as in cross-talk among signaling networks [8]. EphA2 is, for example, known to mediate pro- and antitumorigenic effects depending on its different modes of activation [9]: (1) In the ligand-dependent mode, EphA2 receptor tyrosine phosphorylation is induced by binding of ephrinA ligand, which leads to inhibition of cell migration/invasion and tumor growth [10, 11]. Thus, ephrin-induced Eph receptor signaling is generally regarded as antitumorigenic. (2) In the ligand-independent mode, EphA2 can promote cell migration and cancer growth through a mechanism that involves Ser897 phosphorylation due to serine/threonine kinases such as Akt [12]. Thus, EphA2 can also behave as an oncoprotein in the absence of ligand.
Increased EphA2 levels correlate with increased malignancy [13] and EphA2 upregulation has been found to be induced in breast cancer, lung cancer and melanoma cell lines through acquisition of resistance to trastuzumab, EGFR kinase inhibitors and the BRAF inhibitor vemurafenib, respectively [14,15,16]. Given its essential role in melanoma survival and its association with a worse prognosis among human malignancies [5, 17, 18], EphA2 serves as a potential candidate for predicting melanoma metastasis. However, the role of its ligand ephrinA1 was not fully resolved in these studies. To elucidate the role of EphA2 in malignant progression, it is necessary to simultaneously consider the status of its ligand. Based on the complexity of the Eph/ephrin signaling pathway, we aimed to determine the possibility of ephrinA1, together with EphA2, to serve as a promising biomarker for melanoma progression. In this report, we demonstrate that, unlike the mutually exclusive expression mode in human breast cancer [19], there is no correlation of expression between EphA2 and ephrinA1 in melanoma. High levels of ephrinA1, but not high EphA2, were found to be negatively correlated with metastasis. Moreover, we found that depletion of EphA2 attenuated the antitumorigenic effect of the EphA2/ephrinA1 signaling system and promoted metastasis both in vitro and in vivo.
2 Materials and methods
2.1 TCGA and CCLE data analysis
To explore the expression and prognostic implications of EphA2 and ephrinA1 (EFNA1) expression in primary human melanomas and melanoma cell lines, patient melanoma data were downloaded from the TCGA website. Among them, from 469 cutaneous melanoma patients and 80 uveal melanoma patients complete RNA sequencing data were available. Data of 62 melanoma cell lines were downloaded from the CCLE website. Normalized mRNA expression values of EphA2 and EFNA1 were extracted and analyzed.
2.2 Cell lines and culture conditions
Melanoma cell lines MUM-2B, MUM-2B, A375 and A875 were obtained from the Cell Resource Center, Peking Union Medical College, China. Melanoma cell lines HMCB, CHL-1, IGR-39 and SKMEL-31 were obtained from the Shanghai XuRan Biological Technology CO.LTD. The cell lines were maintained in Dulbecco’s Modified Eagle’s Medium (DMEM) or RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS, Invitrogen, Grand Island, New York, USA). The identity of the cell lines was verified by short tandem repeat (STR) analysis (Genewiz Inc.) in 2017. For shRNA experiments, at least four independent shRNA sequences were tested and the ones with the highest efficiency were used. For ephrinA1 ligand experiments, 1 µg/ml ephrinA1-Fc or IgG-Fc was added to the cell cultures for 15 min. For inhibition of Akt or RSK signaling, cell cultures were treated with 20 µM MK-2206 or 10 µM BI-D1870 for 1 h.
2.3 Antibodies and reagents
Antibodies directed against p-EphA2 (Ser-897; #6347 and Tyr-588; #12677), p-Akt (Ser-473; #9271), p-ERK (Thr-202/Tyr-204; #4370), p-RSK (p-90RSK-Ser380; #11989) and GFP (#2956) were purchased from Cell Signaling Technology (Danvers, MA, USA), against EphA2 (C-20; sc-924), ERK (C-9; sc-514302), ephrinA1 (A-5; sc-377362), RSK2 (E-1; sc-9986) and GAPDH (B-7; sc-5286) from Santa Cruz Biotechnology (Santa Cruz, CA., USA), against Akt (AF6259) from Affinity Biosciences (Cincinnati, OH, USA) and against Ki-67 (ZA-0502) form ZSGB-BIO (Beijing, China). Recombinant human ephrinA1-Fc was obtained from R&D Systems (MN, USA), MK-2206 from Selleckchem (TX, USA) and RSK inhibitor BI-D1870 from Enzo Life Sciences (Farmingdale, NY, USA).
2.4 EPHA2 gene silencing and transduction
Recombinant lentiviruses expressing shEphA2 (HSH004657; four clones) and a control plasmid (CSHCTR001-LVRU6GP) were constructed by GeneCopoeia Inc. (Guangzhou Science Park, Guangzhou, China). Transduction was performed using a Lenti-Pac HIV Expression Packaging Kit (HPK-LvTR-40, GeneCopoeia Inc) according to the manufacturer’s protocol. Cells were selected with puromycin and experiments were performed after selection. The transduction efficiency was confirmed via Western blot analysis. The No.3 shRNA sequence was chosen for subsequent studies.
2.5 Colony formation assay
For colony formation assays, melanoma cells were seeded at low density in six-well plates and the medium was changed every 3 days. Colonies were counted at day 14 after staining with 0.1% crystal violet.
2.6 2.6 Western blotting
Western blotting analyses were performed as previously described [20]. Whole-cell extracts were prepared by lysing the cells with RIPA lysis buffer supplemented with a proteinase inhibitor cocktail and phosphatase inhibitors (Sigma, Shanghai, China).
2.7 Immunofluorescence (IF) and immunohistochemistry (IHC)
IF and IHC were performed following standard protocols as previously described [20]. The staining intensities of EphA2 and ephrinA1 were indicated as low and high according to previously described criteria [21]. The Ki-67 proliferation index of the tumor cells was defined as the ratio of labeled cells to total cells in ten high-power fields.
2.8 In vivo tumor formation and lung metastasis
Male BALB/c nude mice at 4–6 weeks of age (Beijing HFK Bioscience Co. Ltd, Beijing, China) were used for all in vivo studies. To establish subcutaneous tumors, mice were injected with 5 × 106 MUM-2B-shcon/shEphA2 cells or A375-shcon/shEphA2 cells into the upper right flank region at 0.1 ml/mouse. Tumor formation/growth was monitored weekly using calipers, and tumor volumes (TV) were determined using the formula [TV (mm3) = π/ 6 × 0.5 × length × (width)2] [22]. To establish lung metastases, mice were injected with 2 × 105 (0.1 ml) MUM-2B-shcon/shEphA2 cells or A375-shcon/shEphA2 cells via the tail vein. Six mice were used for the subcutaneous category and twelve mice for the metastatic category. After 4 (MUM-2B) or 8 (A375) weeks, mice were sacrificed, after which the xenograft tumors and lungs were removed, weighed, and processed for histology and immunohistochemical analysis. All animal experimental protocols were approved by the Tianjin Medical University Institutional Animal Care committee.
2.9 Statistical analysis
Differences within each set of categorical data were determined using χ2 analysis. Differences in tumor volumes, lung weights and immunohistochemical staining intensities were determined using Student’s t-test with two-tailed p values. Prognostic significance was estimated by log-rank tests and plotted as Kaplan-Meier curves. To stratify patients into groups of low and high expression of genes with a maximum difference in survival, optimal cut-off points were determined using the maximally selected rank test from the R package MaxStat downloaded from CRAN (http://CRAN.project.org/package) [23, 24]. Cox regression (univariate and multivariate) analyses were used to assess the impact of patient baseline clinical characteristics and gene expression levels on overall survival. Analysis of gene expression data and other statistical analyses were performed in R. p < 0.05 was considered statistically significant. Biological experiments were repeated at least three times independently.
3 Results
3.1 EphrinA1 negatively correlates with metastasis and does not correlate with EphA2 levels in melanoma
Previously, it has been demonstrated that EphA2 protein expression is increased in malignant melanoma and predicts a poor survival. When we analyzed TCGA melanoma cancer data sets, we found that the mRNA level of ephrinA1 (EFNA1), but not EphA2, was negatively correlated with skin melanoma metastasis (Fig. 1a). The expression of these two genes was not significantly associated with metastasis in uveal melanoma (TCGA) and CCLE melanoma cell lines (Fig. S1a and S1b). Basic characteristics of the TCGA melanoma patients and correlations of clinicopathological features with EphA2 or EFNA1 expression are summarized in Tables S1 and S2. We found that EphA2 high patients had a higher Breslow thickness than did EphA2 low patients, whereas no other significant differences were found in terms of correlations of other clinicopathological factors with EphA2 or EFNA1 expression in skin or uveal melanomas. In addition, we explored the role of the EphA2/ephrinA1 ratio in patient prognosis via Kaplan-Meier survival analysis. When using the optimal cut-off values (ephrinA1: 16.42; EphA2: 17.68; EphA2/ephrinA1 ratio: 1.10), we observed a high association of ephrinA1, EphA2 and the EphA2/ephrinA1 ratio with overall survival (ephrinA1: p = 0.023; EphA2: p = 0.013; EphA2/ephrinA1: p = 0.001) (Fig. 1b and Table S3). Patients with high EphA2, low ephrinA1 and a high EphA2/ ephrinA1 ratio exhibited shorter survival durations than patients with low EphA2, high ephrinA1 and a low EphA2/ ephrinA1 ratio, respectively, in the TCGA skin melanoma dataset. The results were considered based on correlations of gene expression with two approved independent risk factors of melanoma: ephrinA1 with metastasis (p = 0.002) and EphA2 with Breslow thickness (p = 0.045) (Tables S1). After superposition of the effects of EphA2 and ephrinA1 on survival, the difference between survival curves of patients with high EphA2/ephrinA1 ratios and those with low ratios was larger than the difference between the curves of either gene alone. These results indicate that no or low expression of ephrinA1 is involved in a higher likelihood of a poor clinical outcome. If EphA2 levels are high, patients with melanoma may benefit from low levels of ephrinA1. This result is consistent with the ligand-dependent antitumorigenic signaling mode. Unlike the TCGA skin melanoma dataset, EphA2 or ephrinA1 expression levels were not related to patient survival in the TCGA uveal melanoma dataset (Fig. S1c and Table S4).
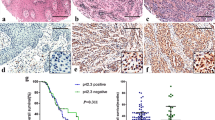
Expression of EFNA1 (ephrinA1) is associated with metastasis and overall survival of melanoma patients. (a) Levels of EphA2 and ephrinA1 compared between skin melanoma groups in TCGA with and without metastasis. (b) Overall Kaplan-Meier survival curves of skin melanoma (TCGA) patients compared according to ephrinA1 low or high status, EphA2 low or high status, and EphA2/ephrinA1 low or high ratio. (c) Correlation analysis between EphA2 and ephrinA1 in skin melanomas (TCGA), uveal melanomas (TCGA) and melanoma cell lines (CCLE)
Previous analyses of human breast cancer revealed that high levels of EphA2 expression coincided with low levels of ephrinA1 expression, which was also shown in our analysis of CCLE breast cancer cell lines (Fig. S2). However, the mRNA expression levels of EphA2 and ephrinA1 were not significantly correlated in a cohort of 469 skin melanomas and 80 uveal melanomas from the TCGA and 62 melanoma cell lines from the CCLE (Fig. 1c). We also carried out Cox proportional hazards analyses, and the results are shown in Tables S5 and S6 (TCGA skin and uveal melanoma patients, respectively). The prognostic value of ephrinA1 and EphA2 expression on overall survival was compared with that of usual prognostic factors, i.e., age, sex, Clark level, Breslow thickness, mitotic count, ulceration, stage and metastasis, which were included in univariate and multivariate analyses. Using univariate Cox analysis we found that all these factors, except sex, ephrinA1 and EphA2 expression, had prognostic value, and that the estimated EphA2/ephrinA1 risk ratio indicated that the risk of death in patients with high ratios was 5.1 times higher than that of those with low ratios (Tables S5). However, the EphA2/ephrinA1 ratio did not reach significance when tested together with other prognostic factors, indicating that it may not serve as an independent survival factor.
3.2 EphrinA1 expression status correlates with the effect of EphA2 on colony formation
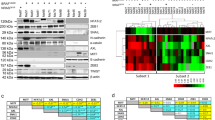
Although the expression levels of EphA2 and ephrinA1 were found to be inversely correlated in breast cancer [25], a tissue microarray analysis showed that strong cytoplasmic EphA2 and ephrinA1 staining were present in 16% of the melanomas tested [26]. To evaluate the expression patterns of EphA2 and ephrinA1 in the melanoma cell lines used in this study, we performed Western blotting and found that the expression levels of EphA2 and ephrinA1 were not directly or inversely correlated (Fig. 2a). To further investigate the effect of EphA2 and ephrinA1 expression on melanoma progression, we generated stable short hairpin RNA (shRNA) knockdown sublines from six independent human melanoma cell lines based on their expression of EphA2 and ephrinA1. Knockdown was most efficient with shRNA No. 3, which was used for subsequent experiments (Fig. 2b). To further establish the absence of an inverse correlation between EphA2 and ephrinA1 expression in melanoma, ephrinA1 levels were monitored by Western blotting in 6 cell lines after EphA2 knockdown. Consistent with the results presented in Fig. 1c, the ephrinA1 expression levels were not increased conversely to EphA2 knockdown. Next, we set out to assess the proliferation, cell cycle, apoptosis, invasion and colony formation characteristics of these melanoma cell lines (Fig. S3 and Fig. 2c). No significant differences were found, except in the colony formation assay, i.e., loss of EphA2 expression significantly reduced colony numbers in MUM-2B, IGR39 and SKMEL31 (high EphA2, low ephrinA1) cells, and increased colony numbers in A375, HMCB and CHL-1 (high EphA2, high ephrinA1) cells relative to those in the vector control cells (Fig. 2c). To rule out that cell death affected the efficiency of colonization, apoptosis staining was performed. No differences between the shEphA2 and control groups were found.
EphA2 knockdown impairs colony formation in human melanoma cell lines with low levels of ephrinA1. (a) EphA2 and ephrinA1 expression was measured in a panel of 8 melanoma cell lines as indicated. Melanoma cell lines with BRAF mutations are marked by one asterisk and with NRAS mutations by two asterisks. The melanoma cell lines were analyzed on the same gel but in consecutive lanes. (b) Representative Western blot (of three separate experiments) of EphA2 knockdown and ephrinA1 levels in melanoma cell lines (stably transfected with control shRNA or shRNA #3 against EphA2). Fold changes were determined by densitometry and normalized to control. (c) Impact of EphA2 knockdown on colony formation. The results represent the average of three replicates. The bars indicate the mean ± SD. * p < 0.05
To determine how high EphA2 expression affects melanoma cells with a high or low expression of ephrinA1, we studied the effect of EphA2 overexpression on colony formation of MUM-2B (low ephrinA1) and A375 (high ephrinA1) melanoma cells. To this end, both MUM-2B and A375 cells were infected with lentiviruses overexpressing full-length wild-type EphA2-GFP and con-GFP (control). Compared to the corresponding control groups, significantly higher levels of EphA2 were detected in both MUM-2B and A375 cells by Western blotting. Subsequent colony formation assays revealed that MUM-2B cells overexpressing EphA2 formed more colonies and that, by contrast, A375 cells overexpressing EphA2 formed fewer colonies (Fig. S4a). Since the ephrinA1 status is essential for colony formation, we also knocked down ephrinA1 in A375, HMCB and CHL-1 cells, after which the level of ephrinA1 was analyzed by Western blotting. Compared to the corresponding control groups, the level of ephrinA1 was reduced to various degrees. Cells transfected with shEFNA1 formed more colonies than those transfected with shcon (control), suggesting a role of ephrinA1 signaling in inhibiting tumorigenesis (Fig. S4b). Taken together, these results indicate that the effect of EphA2 on colony formation may depend on the expression status of both EphA2 and ephrinA1 in melanoma cells.
3.3 EphrinA1 expression status correlates with the effect of EphA2 on metastasis in vivo
To validate our in vitro findings in human melanoma cell line models, we tested the impact of EphA2 loss in an experimental metastatic mouse model of melanoma. To this end, MUM-2B-shEphA2 cells, A375-shEphA2 cells and their vector control cells were injected intravenously into BALB/c nude mice. At 4 (MUM-2B) or 8 (A375) weeks after injection, lungs were harvested, fixed and processed for histological analyses. Gross examination revealed numerous large lung surface metastases in mice injected with A375 cells transduced with shEphA2. Due to the limited numbers, small sizes and internal locations of metastatic foci, those derived from A375-shcon (control) cells were difficult to see macroscopically, but could be seen microscopically. In contrast, significantly fewer lung surface metastases were observed in mice injected with MUM-2B-shEphA2 cells compared to more lung metastases in mice injected with MUB-2B-shcon cells (Fig. 3a, b). Histological examination of hematoxylin/eosin-stained lung sections revealed numerous large pulmonary metastases in lungs from mice harboring MUM-2B-shcon and A375-shEphA2 tumors, while small metastases could be seen in lungs from mice harboring MUM-2B-shEphA2 or A375-shcon tumors (Fig. 3c). Comparison of tumor metastasis by lung weight confirmed these observations. As EphA2 and ephrinA1 were previously shown to be involved in tumor cell proliferation, we also determined the expression of Ki-67. Consistent with proliferation data in vitro (MTT assay, Fig. S3), no significant difference in Ki-67 expression was observed between tumors expressing shcon and shEphA2 (Fig. 3c), suggesting that proliferation of the tumor population may not be a determining factor in the incidence of metastasis in vivo.
Effect of EphA2 downregulation on metastasis in vivo. (a) Incidence of lung metastasis of mice injected with MUM-2B and A375 (shcon and shEphA2) cells. (b) Representative pictures of mouse lungs (left) and histograms (right) showing the average weights of tumor-bearing lungs. Lung surface metastases in mice injected with MUM-2B cells (white arrowheads) and A375 cells (black arrowheads). (c) H&E and Ki-67 staining of lung sections (scale bar, 100 µm) and analysis of Ki-67 proliferation index of MUM-2B and A375 xenograft tumors (right)
For tumor progression and metastasis, blood supply is required. It has been reported that ephrinA1 and EphA2 are involved in endothelial cell proliferation and migration [27]. To rule out the possibility that the effect of ephrinA1 on angiogenesis leads to different degrees of tumor growth in intravenous models of lung metastasis, we evaluated the presence of alveolar capillaries by staining for the vascular endothelial marker endomucin and analyzed the maximum distance between a tumor mass and the nearest blood vessel (Fig. S5). We found that both metastatic MUM-2B and A375 cells, with low and high levels of ephrinA1, respectively, mainly contained alveolar vessels but did not depend on new blood vessel formation, suggesting that these tumors inherently have sufficient supplies of blood.
3.4 EphA2 is not essential for tumor growth in a subcutaneous xenograft model
To study the impact of decreased EphA2 receptor levels on the growth of subcutaneous xenografts, MUM-2B-shEphA2 cells, A375-shEphA2 cells and their respective control cells were injected into BALB/c nude mice subcutaneously. A slightly higher incidence of tumor formation was observed for MUM-2B cells than for A375 cells, while there was no significant difference in tumor volume between the shEphA2 and control xenograft groups (Fig. 4a). Also, shEphA2-transduced melanoma cells behaved similarly to their parental cells in terms of Ki-67 expression (Fig. 4b). Taken together, based on the different results in metastatic and subcutaneous xenograft models, it seems that isolated melanoma cells are more responsive to loss of EphA2 expression than clusters of melanoma cells in terms of inhibition of survival and growth in vivo and in vitro. This may be due to different levels of cell-cell contacts at different cell densities and distinct signaling dynamics of single cells.
Effect of EphA2 downregulation on the growth of subcutaneous melanoma in vivo. BLAB/c nude mice were subcutaneously injected with 5 × 105 MUM-2B and A375 cells that stably expressed either shcon or shEphA2. (a) Incidence of tumor formation. Tumor sizes were monitored for 28 days and are expressed as the mean ± SD. (b) Representative pictures of H&E and Ki-67 staining of xenograft sections (scale bar, 100 µm) and analysis of Ki-67 proliferation index of MUM-2B and A375 xenograft tumors (right)
3.5 Colony formation via ligand-independent EphA2 signaling is Akt-dependent
Previous analyses revealed that ligand-independent phosphorylation of EphA2 on serine 897 by Akt contributes to tumor progression, and that in addition to being a downstream effector of Akt, EphA2 may also serve as an upstream negative regulator of Akt [9]. We found that both the levels of pS897-EphA2 and pS473-Akt were notably decreased in MUM-2B-shEphA2 cells, partially confirming that Akt may be influenced by EphA2 knockdown (Fig. 5a). In contrast, we found that pS473-Akt was largely unaffected in A375-shEphA2 cells. We speculate that this discrepancy may be due to the fact that the A375 cell line harbors a mutation in BRAF (MUM-2B does not), which regulates Akt independent of EphA2. To verify that EphA2 is regulated by Akt, we next set out to assess whether phosphorylated EphA2 could be detected in cells pretreated with an Akt inhibitor (MK-2206). We found that Akt inhibition significantly reduced the phosphorylation of EphA2 at S897, but not Y588 (Fig. 5b) in MUM-2B cells, suggesting phosphorylation of EphA2 at the Akt substrate site. In contrast, we found that both p-S897 and p-Y588 of EphA2 in A375 cells were unaffected by the Akt inhibitor, probably due to noncritical cross-talk between Akt and EphA2 in this cell line. Previous reports have shown that EphA2 can cause inhibition of Ras, a positive signal for proliferation [8]. To test whether the effect of EphA2 knockdown is caused by ERK, an effector of Ras-Raf-MAPK signaling, we assessed changes in ERK expression and its phosphorylation status and found that the levels of ERK and pERK were not affected (Fig. 5a). It has also been reported that RSK can phosphorylate EphA2 at Ser897 [12]. To test this, the above cells were treated with the RSK inhibitor BI-D1870s. We found that phosphorylation at Ser897 was not affected (Fig. 5b). To validate that ephrinA1 induces tyrosine phosphorylation of EphA2 and can inhibit tumor progression, exogenous ephrinA1-Fc ligand was added to the culture medium for 15 min. We found that the total levels of EphA2 and S897-EphA2 were reduced, while that of Y588-EphA2 was increased (Fig. 5c). In addition, we found that treatment of MUM-2B cells with ephrinA1-Fc caused a decrease in the number of colonies, and similar results were also obtained in A375 cells (Fig. 5d). To further establish the EphA2 kinase activation sites, we used immunofluorescence to detect the status of Y588-EphA2 and S897-EphA2 in the presence of ephrinA1-Fc or Fc. We found that the level of S897-EphA2 in MUM-2B cells was decreased with ephrinA1-Fc, while that of Y588-EphA2 in A375 cells was increased with ephrinA1-Fc (Fig. 5e). These results suggest that, consistent with previous studies, Akt phosphorylation of S897-EphA2 is inhibited by its ligand ephrinA1 and results in an anti-oncogenic effect on melanoma cells.
Colony formation via ligand-independent EphA2 signaling is Akt dependent. (a) Effect of EphA2 downregulation on the phosphorylation of EphA2, Akt and ERK. (b) Changes in EphA2 phosphorylation after pretreatment of cells with the Akt signaling inhibitor MK-2206 or the RSK inhibitor BI-D1870. (c) Cells were stimulated with ephrinA1-Fc or Fc, after which lysates were blotted for phosphorylation of EphA2 and Akt. (d) Colony formation of MUM-2B and A375 cells after treatment with ephrinA1-Fc for 15 min. (e) MUM-2B and A375 cells pretreated with ephrinA1-Fc or Fc for 15 min were stained with pS897-EphA2 or pTyr588-EphA2 (red) and DAPI (blue) for confocal microscopy analysis. Scale bars, 50 µm and 10 µm
3.6 EphA2-high/ephrinA1-low patients have a poor prognosis
It has been shown that aberrant expression of EphA2 and ephrinA1 is closely related to the metastasis and prognosis of several tumors. Therefore, we explored the role of the EphA2/ephrinA1 pathway in patient prognosis using primary melanoma tissue samples. Of the 91 samples tested, 26 (28.6%) were EphA2 positive and 28 (30.8%) were ephrinA1 positive (Fig. 6a). The ephrinA1 staining scores did not correlate with the EphA2 scores. As anticipated, EphA2-high/ephrinA1-low patients exhibited shorter survival durations than EphA2-high/ephrinA1-high patients (Fig. 6b). Consistent with the in vivo data, these data support the clinical relevance of EphA2 and ephrinA1 in melanoma.
Clinical relevance of EphA2 and ephrinA2 expression in human melanomas. (a) Ninety-one melanoma tissues were subjected to immunohistochemical staining for EphA2 and ephrinA1. Representative images of staining are shown. Scale bar, 100 µm. (b) Kaplan-Meier survival analysis of 91 melanoma patients according to the levels of EphA2 and ephrinA1 expression. The log-rank test was used to calculate p values
4 Discussion
The EphA2/ephrinA1 signaling system has been reported to affect tumorigenesis and is considered to be an attractive therapeutic target. EphA2 has been shown to have both pro- and anti-tumor properties, depending on its mode of activation. So far, however, the influence of ephrinA1 on EphA2 has remained under-investigated. Here, we found that the expression patterns of ephrinA1 and EphA2 were not directly or inversely correlated in melanoma. Moreover, ephrinA1 expression, but not EphA2 expression, was found to be associated with an increased risk of metastasis in melanoma patients. Downregulation of EphA2 eliminated the tumor suppressive activity of the EphA2/ephrinA1 system in cells with high levels of ephrinA1. EphA2-targeting therapies should, therefore, not be used before full evaluation of ephrinA1 expression. In addition, we found that ephrinA1 may be considered as a prognostic marker in melanoma.
Previously, it has been shown that the expression patterns of these two molecules, EphA2 and ephrinA1, are mutually exclusive [16, 19, 25, 28, 29]. Udayakumar et al. [5] reported EphA2 expression in a panel of 17 melanoma cell lines without mentioning that of ephrinA1, similarly, changes in ephrinA1 levels after upregulation of EphA2 expression were not referred to in the study by Paraiso et al. [5, 30]. We failed to find a correlation between the expression levels of ephrinA1 and EphA2 in the human melanoma tissues and cell lines tested. Given the complex regulatory networks involved, we speculate that there is no absolute positive or negative feedback mechanism regulating ephrinA1 and EphA2 expression in different malignances, or that such a mechanism may start to function in the process of tumor progression.
It has been reported that EphA2 promotes the migration and invasion of cancer cells, explaining the favorable prognosis of patients with a low EphA2 expression and the development of therapeutic strategies aimed at targeting EphA2 [31,32,33]. Inconsistent with these findings, David Kiewlich et al. observed no inhibition of tumor growth when the expression of EphA2 was reduced [34], whereas loss of EphA2 expression has been found to be associated with susceptibility to skin cancer in mice [13]. These latter data correspond to our results, which showed that downregulation of EphA2 expression may hamper the prevention of cancer development by suppressing the anti-oncogenic activities of the EphA2/ephrinA1 system in melanoma cells with high levels of ephrinA1 expression. Similarly, an expression pattern of high EphA2 and low ephrinA1 has been reported to be correlated with a poor prognosis in patients with glioma [28]. Both EphA2-high and ephrinA1-high statuses have been found to be related to well-differentiated non-small-cell lung carcinoma of early stage [35]. Indeed, the dual function of EphA2 has been shown to have ligand-independent oncogenic and ligand-dependent tumor suppressor roles [9]. Although EphA2 is a critical oncogenic protein regulating growth, apoptosis, invasion, metastasis and colony formation in various types of cancer, including melanoma, we failed to observe any effect of EphA2 on these capacities, except on colony formation. This may be due to differences in cellular contexts and/or treatments. The apparent paradoxical role of EphA2 is not only due to its mode of activation, but also to the presence of different phosphorylation sites and to interactions of EphA2 with other receptors. EphA2 may, for example, inhibit transendothelial migration of breast cancer cells depending on EphA2-Tyr772 phosphorylation [36], and phosphorylation of EphA2-Ser897 has been found to be associated with an increased transmigration of prostate cancer cells through the endothelium [37], indicating that metastasis may, at least in part, be governed by EphA2-associated tumor-endothelial cell interactions. Akt-dependent EphA2 activation has been found to require the eHsp90-LRP1 signaling axis for inducing glioblastoma cell invasion [38], whereas a functional association of sortilin/TrkA/EphA2 has been found to be essential for breast cancer promotion by proNGF [39]. In addition, molecular cross-talk between EphA2/EphrinA1/EGFR has been found to be associated with a poor response of colorectal cancer patients to cetuximab treatment [40]. These examples of EphA2 cross-talk with other cellular signaling networks emphasize the importance of considering specific interactions between receptors in various cellular contexts.
To validate the paradoxical function of EphA2 with or without stimulation of endogenous ephrinA1, we selected melanoma cell lines exhibiting high levels of EphA2 protein with high or low levels of ephrinA1. In addition, we evaluated the relationship of ephrinA1 with survival in the context of high EphA2 expression. Collectively, these results show that a high expression of ephrinA1 primes EphA2 anti-oncogenic activity. Nevertheless, we found that neither ephrinA1 nor EphA2 serves as independent risk factors for melanoma patient prognosis (Table S5 and S6), which is consistent with previous reports [28, 35]. These results indicate that the application of EphA2-targeting therapeutics is challenging, and that assessment of endogenous or environmental ephrinA1 may be necessary. In the past, various EphA2-targeting approaches have been attempted and clinically tested [41, 42], but some of them, such as those involving siRNAs, may attenuate EphA2-related tumor suppressor activities. Previously, the existence of a negative feedback loop between RAS/MAPK signaling and EphA2/ephrinA1 has been reported, whereas we found that the expression of EphA2 in a panel of 8 melanoma cell lines did not correlate with their RAS mutation status. Therefore, the level of EphA2 in cancer cells may be regulated by alternative pathways related to different genetic backgrounds.
As far as metastasis is concerned, metastatic colonization is regarded as the most inefficient and rate-limiting step of the invasion-metastasis cascade, and the outgrowth of colonized micro-metastases requires a high self-renewal capacity. We found that EphA2 only affects colony formation in vitro and lung metastasis in vivo and, in contrast to previous reports, no effects on biological processes such as migration [9], cell cycle progression [43] and apoptosis [32] were observed. These findings suggest that EphA2 may specifically regulate the survival and growth of melanoma cells used in the present study. Interestingly, although colony formation and lung metastasis were affected by loss of EphA2 expression, this loss did not appear to significantly affect cell proliferation in vitro or subcutaneous xenograft growth in vivo. Fang et al. found that the expression of ephrinA1 did not correlate with cell density [31], suggesting that ephrinA1 was not the primary reason for the differences observed in our study. Therefore, we suppose that in the condition where individual tumor cells lack cell-cell contact, EphA2 may be a key regulator of survival and proliferation. However, in the condition where clusters of tumor cells possess sufficient cell-cell cross-talk, EphA2 may not be a critical factor and other oncogenic signaling pathways may predominate. In other words, whereas downregulation of EphA2 inhibits tumor growth, metastatic tumors are more responsive to EphA2 than subcutaneously growing tumors. Thus, we consider it unadvisable to use EphA2-targeting therapies for combatting primary melanomas, but rather for suppressing their micro-metastases.
In summary, we have identified ephrinA1 as a potential prognostic marker in melanoma and confirmed that EphA2-targeting alone, without considering ephrinA1 expression, may not achieve the anticipated goal of tumor progression suppression. Further efforts are needed to clarify the mechanism by which ephrinA1/EphA2 signaling contributes to the regulation of melanoma tumor cell self-renewal, and more studies are needed to improve the specification of EphA2-targeting therapies by identifying potentially responsive attributes.
References
C.M. Balch, S.J. Soong, M.B. Atkins, A.C. Buzaid, N. Cascinelli, D.G. Coit, I.D. Fleming, J.E. Gershenwald, A. Houghton Jr., J.M. Kirkwood, K.M. McMasters, M.F. Mihm, D.L. Morton, D.S. Reintgen, M.I. Ross, A. Sober, J.A. Thompson, J.F. Thompson, An evidence-based staging system for cutaneous melanoma. CA Cancer J. Clin. 54(3), 131–149 (2004) quiz 182 – 134
S.P. Leong, J.E. Gershenwald, S.J. Soong, D. Schadendorf, A.A. Tarhini, S. Agarwala, A. Hauschild, C.W. Soon, A. Daud, M. Kashani-Sabet, Cutaneous melanoma: a model to study cancer metastasis. J Surg Oncol 103(6), 538–549; (2011)
E.B. Pasquale, Eph-ephrin bidirectional signaling in physiology and disease. Cell 133(1), 38–52 (2008)
T.T. Ma, L. Wang, J.L. Wang, Y.J. Liu, Y.C. Chen, H.J. He, Y. Song, Hypoxia-Induced cleavage of soluble ephrinA1 from cancer cells is mediated by MMP-2 and associates with angiogenesis in oral squamous cell carcinoma. OncoTargets Therapy 12, 8491–8499 (2019)
D. Udayakumar, G. Zhang, Z. Ji, C.N. Njauw, P. Mroz, H. Tsao, EphA2 is a critical oncogene in melanoma. Oncogene 30(50), 4921–4929 (2011)
E.B. Pasquale, Eph receptors and ephrins in cancer: bidirectional signalling and beyond. Nat. Rev. Cancer 10(3), 165–180 (2010)
M. Nakamoto, A.D. Bergemann, Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc. Res. Tech. 59(1), 58–67 (2002)
C.W. Menges, D.J. McCance, Constitutive activation of the Raf-MAPK pathway causes negative feedback inhibition of Ras-PI3K-AKT and cellular arrest through the EphA2 receptor. Oncogene 27(20), 2934–2940 (2008)
H. Miao, D.Q. Li, A. Mukherjee, H. Guo, A. Petty, J. Cutter, J.P. Basilion, J. Sedor, J. Wu, D. Danielpour, A.E. Sloan, M.L. Cohen, B. Wang, EphA2 mediates ligand-dependent inhibition and ligand-independent promotion of cell migration and invasion via a reciprocal regulatory loop with Akt. Cancer Cell 16(1), 9–20 (2009)
A. Barquilla, E.B. Pasquale, Eph receptors and ephrins: therapeutic opportunities. Annu. Rev. Pharmacol. Toxicol. 55, 465–487 (2015)
H. Miao, E. Burnett, M. Kinch, E. Simon, B. Wang, Activation of EphA2 kinase suppresses integrin function and causes focal-adhesion-kinase dephosphorylation. Nat. Cell Biol. 2(2), 62–69 (2000)
Zhou Y., Yamada N., Tanaka T., Hori T., Yokoyama S., Hayakawa Y., Yano S., Fukuoka J., Koizumi K., Saiki I., Sakurai H. Crucial roles of RSK in cell motility by catalysing serine phosphorylation of EphA2. Nat. Commun. 6:7679 (2015)
H. Guo, H. Miao, L. Gerber, J. Singh, M.F. Denning, A.C. Gilliam, B. Wang, Disruption of EphA2 receptor tyrosine kinase leads to increased susceptibility to carcinogenesis in mouse skin. Cancer Res. 66(14), 7050–7058 (2006)
B. Miao, Z. Ji, L. Tan, M. Taylor, J. Zhang, H.G. Choi, D.T. Frederick, R. Kumar, J.A. Wargo, K.T. Flaherty, N.S. Gray, H. Tsao, EPHA2 is a mediator of vemurafenib resistance and a novel therapeutic target in melanoma. Cancer Discov. 5(3), 274–287 (2015)
G. Zhuang, D.M. Brantley-Sieders, D. Vaught, J. Yu, L. Xie, S. Wells, D. Jackson, R. Muraoka-Cook, C. Arteaga, J. Chen, Elevation of receptor tyrosine kinase EphA2 mediates resistance to trastuzumab therapy. Cancer Res. 70(1), 299–308 (2010)
K.R. Amato, S. Wang, L. Tan, A.K. Hastings, W. Song, C.M. Lovly, C.B. Meador, F. Ye, P. Lu, J.M. Balko, D.C. Colvin, J.M. Cates, W. Pao, N.S. Gray, J. Chen, EPHA2 Blockade Overcomes Acquired Resistance to EGFR Kinase Inhibitors in Lung Cancer. Cancer Res. 76(2), 305–318 (2016)
D. Vaught, J. Chen, D.M. Brantley-Sieders, Regulation of mammary gland branching morphogenesis by EphA2 receptor tyrosine kinase. Mol. Biol. Cell 20(10), 2572–2581 (2009)
D.P. Zelinski, N.D. Zantek, J.C. Stewart, A.R. Irizarry, M.S. Kinch, EphA2 overexpression causes tumorigenesis of mammary epithelial cells. Cancer Res. 61(5), 2301–2306 (2001)
W. Song, Y. Hwang, V.M. Youngblood, R.S. Cook, J.M. Balko, J. Chen, D.M. Brantley-Sieders, Targeting EphA2 impairs cell cycle progression and growth of basal-like/triple-negative breast cancers. Oncogene 36(40), 5620–5630 (2017)
J. Mo, B. Sun, X. Zhao, Q. Gu, X. Dong, Z. Liu, Y. Ma, N. Zhao, Y. Liu, J. Chi, R. Sun, The in-vitro spheroid culture induces a more highly differentiated but tumorigenic population from melanoma cell lines. Melanoma Res. 23(4), 254–263 (2013)
L. Deng, J. Sun, X. Chen, L. Liu, D. Wu, Nek2 augments sorafenib resistance by regulating the ubiquitination and localization of beta-catenin in hepatocellular carcinoma. J. Exp. Clin. Cancer Res.: CR 38(1), 316 (2019)
T. Schatton, G.F. Murphy, N.Y. Frank, K. Yamaura, A.M. Waaga-Gasser, M. Gasser, Q. Zhan, S. Jordan, L.M. Duncan, C. Weishaupt, R.C. Fuhlbrigge, T.S. Kupper, M.H. Sayegh, M.H. Frank, Identification of cells initiating human melanomas. Nature 451(7176), 345–349 (2008)
L. Li, J. Chen, J. Wang, D. Cai, Prevalence and risk factors of diabetic peripheral neuropathy in Type 2 diabetes mellitus patients with overweight/obese in Guangdong province, China. Primary care diabetes 9(3), 191–195 (2015)
A. Kassambara, C. Gourzones-Dmitriev, S. Sahota, T. Reme, J. Moreaux, H. Goldschmidt, A. Constantinou, P. Pasero, D. Hose, B. Klein, A DNA repair pathway score predicts survival in human multiple myeloma: the potential for therapeutic strategy. Oncotarget 5(9), 2487–2498 (2014)
M. Macrae, R.M. Neve, P. Rodriguez-Viciana, C. Haqq, J. Yeh, C.R. Chen, J.W. Gray, F. McCormick, A conditional feedback loop regulates Ras activity through EphA2. Cancer Cell 8(2), 111–118 (2005)
O. Straume, L.A. Akslen, Importance of vascular phenotype by basic fibroblast growth factor, and influence of the angiogenic factors basic fibroblast growth factor/fibroblast growth factor receptor-1 and ephrin-A1/EphA2 on melanoma progression. Am. J. Pathol. 160(3), 1009–1019 (2002)
E. Wiedemann, S. Jellinghaus, G. Ende, A. Augstein, R. Sczech, B. Wielockx, S. Weinert, R.H. Strasser, D.M. Poitz, Regulation of endothelial migration and proliferation by ephrin-A1. Cell. Signal 29, 84–95 (2017)
X. Li, L. Wang, J.W. Gu, B. Li, W.P. Liu, Y.G. Wang, X. Zhang, H.N. Zhen, Z. Fei, Up-regulation of EphA2 and down-regulation of EphrinA1 are associated with the aggressive phenotype and poor prognosis of malignant glioma. Tumour biology: the journal of the International Society for Oncodevelopmental Biology and Medicine 31(5), 477–488 (2010)
J. Wykosky, W. Debinski, The EphA2 receptor and ephrinA1 ligand in solid tumors: function and therapeutic targeting. Mol. Cancer Res.: MCR 6(12), 1795–1806 (2008)
K.H. Paraiso, M. Das Thakur, B. Fang, J.M. Koomen, I.V. Fedorenko, J.K. John, H. Tsao, K.T. Flaherty, V.K. Sondak, J.L. Messina, E.B. Pasquale, A. Villagra, U.N. Rao, J.M. Kirkwood, F. Meier, S. Sloot, G.T. Gibney, D. Stuart, H. Tawbi, K.S. Smalley, Ligand-independent EPHA2 signaling drives the adoption of a targeted therapy-mediated metastatic melanoma phenotype. Cancer Discov. 5(3), 264–273 (2015)
W.B. Fang, D.M. Brantley-Sieders, M.A. Parker, A.D. Reith, J. Chen, A kinase-dependent role for EphA2 receptor in promoting tumor growth and metastasis. Oncogene 24(53), 7859–7868 (2005)
K.R. Amato, S. Wang, A.K. Hastings, V.M. Youngblood, P.R. Santapuram, H. Chen, J.M. Cates, D.C. Colvin, F. Ye, D.M. Brantley-Sieders, R.S. Cook, L. Tan, N.S. Gray, J. Chen, Genetic and pharmacologic inhibition of EPHA2 promotes apoptosis in NSCLC. J. Clin. Invest. 124(5), 2037–2049; (2014)
Y. Sheng, J. Wei, Y. Zhang, X. Gao, Z. Wang, J. Yang, S. Yan, Y. Zhu, Z. Zhang, D. Xu, C. Wang, Y. Zheng, Q. Dong, L. Qin, Mutated EPHA2 is a target for combating lymphatic metastasis in intrahepatic cholangiocarcinoma. Int. J. Cancer 144(10), 2440–2452 (2019)
D. Kiewlich, J. Zhang, C. Gross, W. Xia, B. Larsen, R.R. Cobb, S. Biroc, J.M. Gu, T. Sato, D.R. Light, T. Heitner, J. Willuda, D. Vogel, F. Monteclaro, A. Citkowicz, S.R. Roffler, D.A. Zajchowski, Anti-EphA2 antibodies decrease EphA2 protein levels in murine CT26 colorectal and human MDA-231 breast tumors but do not inhibit tumor growth. Neoplasia 8(1), 18–30 (2006)
M. Ishikawa, R. Miyahara, M. Sonobe, M. Horiuchi, T. Mennju, E. Nakayama, M. Kobayashi, R. Kikuchi, J. Kitamura, N. Imamura, C.L. Huang, H. Date, Higher expression of EphA2 and ephrin-A1 is related to favorable clinicopathological features in pathological stage I non-small cell lung carcinoma. Lung cancer 76(3), 431–438 (2012)
M. Locard-Paulet, L. Lim, G. Veluscek, K. McMahon, J. Sinclair, A. van Weverwijk, J.D. Worboys, Y. Yuan, C.M. Isacke, C. Jorgensen, Phosphoproteomic analysis of interacting tumor and endothelial cells identifies regulatory mechanisms of transendothelial migration. Sci. Signal. 9(414), ra15 (2016)
T. Tawadros, M.D. Brown, C.A. Hart, N.W. Clarke, Ligand-independent activation of EphA2 by arachidonic acid induces metastasis-like behaviour in prostate cancer cells. Brit. J. Cancer 107(10), 1737–1744 (2012)
U. Gopal, J.E. Bohonowych, C. Lema-Tome, A. Liu, E. Garrett-Mayer, B. Wang, J.S. Isaacs, A novel extracellular Hsp90 mediated co-receptor function for LRP1 regulates EphA2 dependent glioblastoma cell invasion. PloS one 6(3), e17649 (2011)
R. Leveque, C. Corbet, L. Aubert, M. Guilbert, C. Lagadec, E. Adriaenssens, J. Duval, P. Finetti, D. Birnbaum, N. Magne, V. Chopin, F. Bertucci, X. Le Bourhis, R.A. Toillon, ProNGF increases breast tumor aggressiveness through functional association of TrkA with EphA2. Cancer Lett. 449, 196–206 (2019)
M. De Robertis, L. Loiacono, C. Fusilli, M.L. Poeta, T. Mazza, M. Sanchez, L. Marchionni, E. Signori, G. Lamorte, A.L. Vescovi, J. Garcia-Foncillas, V.M. Fazio, Dysregulation of EGFR Pathway in EphA2 Cell Subpopulation Significantly Associates with Poor Prognosis in Colorectal Cancer. Clin Cancer Res 23(1), 159–170 (2017)
M. Tandon, S.V. Vemula, S.K. Mittal, Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert Opin. Ther. Targets 15(1), 31–51 (2011)
H.M. Kluger, A.Z. Dudek, C. McCann, J. Ritacco, N. Southard, L.B. Jilaveanu, A. Molinaro, M. Sznol, A phase 2 trial of dasatinib in advanced melanoma. Cancer 117(10), 2202–2208 (2011)
Y. Kaibori, Y. Saito, Y. Nakayama, EphA2 phosphorylation at Ser897 by the Cdk1/MEK/ERK/RSK pathway regulates M-phase progression via maintenance of cortical rigidity. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 33(4), 5334–5349 (2019)
Acknowledgements
This work was funded by the National Natural Science Foundation of China (No. 81572872).
Author information
Authors and Affiliations
Contributions
Baocun Sun and Jing Mo conceived and designed the study. Xiulan Zhao, Xueyi Dong, Tieju Liu, Nan Zhao, Danfang Zhang, Wei Wang and Yanhui Zhang performed the experiments. Xiulan Zhao, Jing Mo and Xueyi Dong performed cell culture, H&E and IHC. Jing Mo, Tieju Liu, Nan Zhao, Danfang Zhang and Wei Wang conducted Western blotting and animal experiments. Jing Mo and Yanhui Zhang performed immunofluorescence assays. Baocun Sun and Jing Mo interpreted and analyzed the data. Jing Mo wrote and Baocun Sun reviewed the manuscript. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Ethics approval and consent to participate
The experimental protocol was approved by the Tianjin Medical University Ethical Committee Board, Tianjin Medical University, Tianjin, China. Written informed consent to participate in the study was obtained from all individual participants or their guardians.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Mo, J., Zhao, X., Dong, X. et al. Effect of EphA2 knockdown on melanoma metastasis depends on intrinsic ephrinA1 level. Cell Oncol. 43, 655–667 (2020). https://doi.org/10.1007/s13402-020-00511-x
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13402-020-00511-x