Abstract
A novel heavy metal ion adsorbent, UiO-66-NH2@cellulose nanofiber membrane, was successfully synthesized using cellulose and polyvinyl alcohol as substrates and UiO-66-NH2 as the loading material. The adsorption performance of the composite membrane towards Cd(II) was investigated through adsorption experiments. The adsorption results showed that under the optimal adsorption conditions (temperature of 45 °C, adsorption time of 2 h, and Cd(II) solution pH of 0.89), the maximum adsorption capacity was 611.89 mg·g−1 with cellulose, UiO-66-NH2, and PVA added at 0.8 g, 0.15 g, and 1.2 g, respectively. Subsequently, the structure of the composite membrane was characterized using SEM, FT-IR, and XPS, and the adsorption mechanism was preliminarily explored by combining adsorption kinetics and isotherms. The results indicated the formation of a complex three-dimensional network structure during the crosslinking of cellulose and polyvinyl alcohol, with UiO-66-NH2 loaded on the surface and internal pores of the network structure. Furthermore, FT-IR and XPS spectra showed the formation of coordination bonds between the composite membrane and Cd(II) through -OH, -NH2, and Zr-O and the confirmation of a negative surface charge on the composite membrane through isoelectric point testing, indicating the significant role of electrostatic interactions in Cd(II) adsorption. Adsorption kinetics and isotherm results indicated that the adsorption process was primarily complex multilayer chemisorption. In summary, the adsorption of Cd(II) by UiO-66-NH2@cellulose nanofiber membrane involves coordination between metal ions and NO, as well as pore filling and electrostatic adsorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
With the continuous development of industrialization, environmental pollution issues have become increasingly prominent, among which heavy metal pollution is one of the most serious environmental issues, with Cd(II) ranking at the forefront of heavy metal pollution. A large amount of Cd is discharged into the environment through exhaust gases, wastewater, and waste residues, causing serious pollution. Cd pollution manifests in two forms: gaseous and aqueous. Gaseous pollution mainly originates from industrial exhaust gases, with cadmium spreading with the gases to the surroundings of factories and naturally settling, accumulating in the surrounding soil. Aqueous pollution is mainly caused by the discharge of wastewater from lead–zinc ore dressing and related industries (such as electroplating and alkaline batteries) into surface water or seeping into groundwater. With the accumulation of Cd in soil and water bodies, cadmium accumulates in organisms, entering the human body through the food chain, directly affecting human life [1]. When Cd enters the human body through the respiratory and digestive tracts, it forms cadmium-metallothionein complexes in the body, circulating throughout the body via the bloodstream and selectively accumulating in the kidneys and liver. Additionally, there is a certain accumulation in the spleen, pancreas, thyroid, testes, and hair. When the internal content is excessive, it can lead to bone disorders, liver damage, cardiovascular diseases, and sexual dysfunction and disrupt the mineral balance in the body [2]. Therefore, the environmental pollution problem caused by Cd urgently needs to be addressed.
At present, the methods of removing heavy metal ion pollutants mainly include precipitation method, electrochemical method, adsorption separation method, membrane separation method, ion exchange method, physical and chemical method and biological method (biological flocculation method, biological adsorption method, plant remediation method) [3,4,5,6,7,8]. Among them, the adsorption separation method is relatively simple, and there are many kinds of adsorbents, which is the most commonly used method. Common materials such as carbonaceous materials, metal organic framework materials, mineral materials, and biomass materials, for environmentally friendly biocompatible biodegradable, low toxicity and renewable heavy metal adsorption materials have become a research hotspot.
Metal–organic framework (MOF) is a crystal structure synthesized by organic linkers and inorganic metal ions or metal clusters. Due to its large surface area, high porosity, and good stability, it has been widely used in gas separation, energy conversion, catalysis, sensing, organic degradation, metal ion adsorption and biomedical applications [9,10,11]. UiO-66-NH2 is a metal–organic framework material developed with Zr4+ as the metal center and 2-aminoterephthalic acid as the ligand. It has strong thermal stability and chemical stability, and the porosity of UiO-66-NH2 is large. The introduction of amino groups will make it more excellent in some aspects [12,13,14] and easier to be functionalized. Therefore, the function of UiO-66-NH2 as an excellent heavy metal ion adsorbent in removing pollutants from aqueous solution has received extensive attention. Due to the crystallinity of the material itself, it mostly exists in the form of powder and is prone to agglomeration in water, and it is easy to cause pipeline blockage in the adsorption system [15], which is not conducive to recycling. Therefore, the combination of UiO-66-NH2 with appropriate substrate materials is an effective method to realize its functional recovery and reuse [16]. Zhang et al. [17] successfully fabricated a PEI/metal–organic framework@polyvinylidene fluoride (PEI/MOF@PVDF) composite membrane by depositing UiO-66-NH2 and cross-linking it with PVDF and PEI. The composite membrane demonstrated an effective treatment volume of 2322.23 L·m−2 for Cr (VI) through electrostatic attraction, while Cr(III) maintained a stable effluent concentration of 10.4 mg·L−1 via the Donnan effect. Furthermore, the effective treatment volume of the composite membrane remained above 91.83% after three cycles, making it an ideal adsorbent material.
Cellulose nanofiber is an essential component of biomass, being the most abundant natural polymer material on Earth. Its stable physicochemical properties, non-toxicity, biodegradability, and low cost make cellulose materials representative of economically and environmentally friendly materials. Additionally, due to its abundant hydroxyl groups on the surface, cellulose nanofiber is widely used in the field of heavy metal ion adsorption [18].
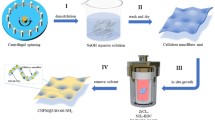
Therefore, to address the issues of pipeline blockage and difficult recovery of UiO-66-NH2 in aqueous environments, UiO-66-NH2 was loaded onto cellulose nanofiber membranes (UNC) using cellulose as the substrate and an ionic liquid ([AMIM]Cl) as the solvent via wet spinning. The optimal preparation conditions of the composite membrane were investigated by varying the amounts of cellulose, PVA, and UiO-66-NH2. The optimal adsorption conditions of UNC for Cd(II) were determined by altering the initial concentration and pH of the Cd(II) solution, as well as the adsorption temperature and time. The materials were characterized using SEM, FT-IR, XRD, and XPS to explore the microstructural changes during processing, and the adsorption mechanism was preliminarily elucidated by combining adsorption kinetics and isotherm studies. This method offers shorter adsorption time, lower usage, and reduced costs for treating Cd(II) wastewater, while also addressing issues of UiO-66-NH2 aggregation and pipeline blockage during adsorption, providing valuable insights for the application of MOF materials and Cd(II) wastewater treatment.
2 Experiment part
2.1 Experimental materials and instruments
Tables 1 and 2 are the materials and instruments required for the experiment.
2.2 Synthesis of the UiO-66-NH2
UiO-66-NH2 was synthesized by an improved solvothermal method [19]. Then, 0.4 g of 2-aminoterephthalic acid and 0.5 g of ZrCl4 were added to 30 mL of DMF and ultrasonically dissolved to form a solution. The solution was then poured into a hydrothermal reactor and reacted at 120 °C for 12 h. After that, the solution after the reaction was filtered, and the yellow solid was obtained by washing with DMF and methanol for many times. The yellow solid was dried in an oven at 60 ℃ for 12 h to get a yellow powder UiO-66-NH2.
2.3 Preparation of the UNC
A certain capacity of cellulose ( 0.4 g, 0.8 g, 1.2 g, 1.6 g, and 2.0 g) was dissolved in 15 g dimethyl sulfoxide and 4 g ionic liquid and stirred in a water bath at 85 °C for 4 h. Then, an appropriate capacity of UiO-66-NH2 (0.05 g, 0.1 g, 0.15 g, 0.2 g, and 0.25 g) was added to continue stirring for 1 h, and then, a certain capacity of PVA (0.6 g, 0.8 g, 1.0 g, 1.2 g, and 1.4 g) was added to stir for 1.5 h. The spinning solution was prepared and the primary filament was obtained. UNC was prepared by soaking in anhydrous ethanol for 2 h in coagulation bath and drying in a vacuum oven at 50 °C for 12 h.
2.4 Cd(II) adsorption experiments
50 mL Cd(II) solution with a certain concentration (600 mg·L−1, 700 mg·L−1, 800 mg·L−1, 900 mg·L−1, and 1000 mg·L−1) was added to the conical flask, and then, 0.05 g UNC was added. The solution was placed in a constant temperature water bath oscillation box at different temperatures (25 °C, 35 °C, 45 °C, 55 °C, and 65 °C). At different pH values (0.89, 2, 3, 4, and 5), adsorption time (1 h, 2 h, 3 h, 4 h, and 5 h). After the adsorption experiment, the concentration of Cd(II) in the water after adsorption was measured by ultraviolet spectrophotometer at 600 nm. Substituted into the formula (1), the adsorption capacity of UNC to Cd(II) was calculated.
Qe is the capacity of adsorption (mg·g−1), C0 is the Cd(II) concentration before adsorption (mg·L−1), Ce is the Cd(II) concentration after adsorption (mg·L−1), V is the volume of Cd(II) solution (L), and m is the mass of adsorbent (g).
2.5 Characterization
2.5.1 SEM characterization
The microstructure of the samples was analyzed by scanning electron microscopy. The conductive adhesive is attached to the sample, and an appropriate capacity of sample is taken on the conductive adhesive. The unfixed sample is cleaned, and then, the sample is dried and sprayed with gold. The acceleration current is 5 kV, and the appropriate magnification is observed.
2.5.2 FTIR characterization
The Fourier transform infrared spectrometer was used to analyze the infrared spectrum of the sample. The sample was subjected to KBr tableting, with a resolution of 4 cm−1 and a wavenumber range of 450 ~ 4000 cm−1. The ZnSe prism and 60° incident angle were used, and the number of scans was 160 times.
2.5.3 XRD characterization
The samples were analyzed by XRD. The samples were placed on a blank glass slide, compacted and sent to the sample chamber. The incident wavelength was 0.514 nm, the scanning range was 10–90°, the scanning speed was 4 (°)/min, and the scanning interval was 0.02°.
2.5.4 XPS characterization
The surface elements and their valence states of the samples were analyzed by X-ray photoelectron spectroscopy. The Al target Ka ray was used as the laser source, the photoelectron energy was 1486.6 eV, and the electron binding energy was corrected using the 284.6 eV of CIs as the reference. The characteristic peak error was ± 0.47 eV.
3 Result and discussion
3.1 Preparation experiments
Figure 1 shows images of UNC composite membranes with (a) different cellulose contents and (b) different UiO-66-NH2 contents.
3.1.1 Dosage of cellulose
When the dosage of PVA is 1.2 g and the dosage of UiO-66-NH2 is 0.15 g, the effect of the dosage of cellulose on the adsorption of Cd(II) by UNC is shown in Fig. 2a. From Fig. 2a, it can be seen that as the dosage of cellulose gradually increases, the adsorption capacity of UNC to Cd(II) increases first and then decreases. When the dosage of cellulose is 0.8 g, the adsorption capacity of UNC to Cd(II) reaches the maximum, which is 599.72 mg·g−1. This may be due to the fact that cellulose is used as the substrate material to accommodate UiO-66-NH2. When the dosage is insufficient, UiO-66-NH2 cannot be completely evenly distributed in it. It can be seen from Fig. 1a that when the dosage of cellulose is small, UiO-66-NH2 agglomerates and accumulates, and UNC shows dark yellow. With the increase of cellulose dosage, the color gradually becomes lighter, which indicates that UiO-66-NH2 is gradually uniformly dispersed in it. However, when the capacity of cellulose is too much, UiO-66-NH2 is too dispersed in it. The content of UiO-66-NH2 in UNC with the same mass decreased sharply, and the adsorption capacity decreased. Therefore, the optimum dosage of cellulose was 0.8 g.
3.1.2 Dosage of PVA
When the dosage of cellulose was 0.8 g and the dosage of UiO-66-NH2 was 0.15 g, the effect of PVA dosage on the adsorption of Cd(II) by UNC was shown in Fig. 2b. It can be seen from Fig. 2b that with the increase of PVA dosage, the adsorption capacity of UNC to Cd(II) increased first and then decreased. When the dosage of cellulose was 1.2 g, the adsorption capacity of UNC to Cd(II) reached the maximum, which was 609.11 mg·g−1. It can be seen from the SEM characterization results that the dissolved cellulose PVA was crosslinked to form a three-dimensional network structure [20], and UiO-66-NH2 was uniformly attached to the three-dimensional network structure. When the dosage of PVA is less, it cannot be well crosslinked with the dissolved cellulose, so that the adsorption capacity is low. When the dosage of PVA increased, it was too tightly crosslinked with cellulose, so that the UiO-66-NH2 was tightly wrapped, some adsorption sites could not be exposed, and the adsorption capacity decreased. Therefore, the optimum dosage of PVA is 1.2 g.
3.1.3 Dosage of UiO-66-NH2
When the dosage of PVA was 1.2 g and the dosage of cellulose was 0.8 g, the effect of the dosage of UiO-66-NH2 on the adsorption of Cd(II) by UNC was shown in Fig. 2c. It can be seen from Fig. 2c that with the increase of the dosage of UiO-66-NH2, the adsorption capacity of UNC to Cd(II) increased first and then decreased. When the dosage of cellulose was 0.15 g, the adsorption capacity of UNC to Cd(II) reached the maximum, which was 601.08 mg·g−1. From Fig. 1b, it can be seen that as the dosage of UiO-66-NH2 gradually increases, the color deepens from light yellow to dark yellow, indicating that the content of UiO-66-NH2 in UNC per unit weight gradually increases, and the number of adsorption sites and active functional groups [21] also increases, so the adsorption capacity gradually increases. However, when the content of UiO-66-NH2 is too high, it can be seen from the SEM photos that UiO-66-NH2 is agglomerated and accumulated in the three-dimensional network structure, which makes the adsorption sites gradually decrease and the adsorption capacity decrease. Therefore, the optimal dosage of UiO-66-NH2 is 0.15 g.
3.1.4 Preparation mechanism
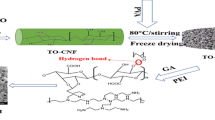
Figure 3 illustrates the formation process of the UNC composite membrane and the dissolution process of cellulose. Under the action of ionic liquids, the synergy of anions and cations disrupts the hydrogen bonds of cellulose, gradually dissolving cellulose and cross-linking with PVA to form a three-dimensional network structure. With the passage of time, the ionic liquid in the membrane begins to precipitate, and this precipitation process forms an excellent pore structure, which is conducive to the distribution of UiO-66-NH2 within it. The interaction between cellulose and PVA generates hydrogen bonds, which enhance the stability of the structure.
3.2 Adsorption experiment
3.2.1 Initial concentration of Cd(II) solution
When the adsorption time was 2 h, the adsorption temperature was 45 ℃, and the pH was 0.89, the effect of the initial concentration of Cd(II) solution on the adsorption of Cd(II) by UNC was shown in Fig. 4a. It can be seen from Fig. 4a that with the increase of Cd(II) solution concentration, the adsorption capacity of UNC to Cd(II) increased first and then decreased slightly. When the concentration of Cd(II) solution was 900 mg·L−1, the adsorption capacity of UNC to Cd(II) reached the maximum, which was 611.89 mg·g−1. In the early stage of adsorption, the diffusion rate of Cd(II) was accelerated due to the increase of concentration difference driving force, which increased the probability of Cd(II) capture by UNC surface active sites and internal voids [22]. When the concentration of Cd(II) continued to increase, the surface active sites and internal voids of UNC tended to be saturated, and the diffusion rate of Cd(II) slowed down due to the increase of concentration, so that the rate of Cd(II) reaching the surface of UNC slowed down. Therefore, the adsorption capacity decreased slightly. Consequently, when the initial concentration of Cd(II) solution is 900 mg·L−1, the adsorption effect is the best.
3.2.2 The pH value of the Cd(II) solution
When the initial concentration of Cd(II) solution was 900 mg·L−1, the adsorption temperature was 45 ℃, and the adsorption time was 2 h, the effect of pH value of Cd(II) solution on the adsorption capacity of Cd(II) adsorbed by UNC was shown in Fig. 4b. From Fig. 4b, it can be seen that the adsorption capacity gradually increases with the decrease of pH value. When pH = 0.89, the adsorption capacity of UNC is the largest, and the adsorption capacity is 606.41 mg·g−1. With the increase of pH, the -OH in the solution gradually increased, and Cd(II) and -OH formed a complex [23], which was not conducive to the adsorption of Cd(II) by UNC, so the adsorption capacity gradually decreased. This phenomenon can be explained by Pearson’s HSAB theory ( hard and soft acid–base theory). According to HSAB theory, Cd(II) is classified as a hard acid. Therefore, Cd(II) has a smaller ionic radius and a higher charge density, which can form strong ionic bonds with hard bases. -OH is classified as hard base. Therefore, in the case of high pH, with the increase of -OH in the solution, Cd(II) is more likely to react with -OH to form stable compounds, resulting in a decrease in adsorption capacity. When the solution is in low acidity, the content of H+ is high. Due to the high porosity and strong acid and alkali resistance of UNC, Cd(II) is more adsorbed by UNC. Therefore, pH = 0.89 is the best adsorption pH.
3.2.3 Adsorption temperature
When the adsorption time was 2 h, the initial concentration of Cd(II) solution was 900 mg·L−1, and the pH was 0.89, the effect of adsorption temperature on the adsorption capacity of UNC for Cd(II) was shown in Fig. 4c. From Fig. 4c, it can be seen that with the increase of adsorption temperature, the adsorption capacity of UNC for Cd(II) increased first and then decreased. When the adsorption temperature was 45 ℃, the adsorption capacity reached a maximum of 610.34 mg·g−1. When the temperature is low, the activity of Cd(II) is low, and it cannot fully contact with the adsorption sites and active sites of UNC [24]. When the temperature is too high, the adsorption capacity decreases sharply, which indicates that the temperature has a great influence on the adsorption of Cd(II) by UNC. The increase of temperature will make the internal structure of UNC loose, and the movement of Cd(II) will be intensified [25]. Some Cd(II) will be separated from UNC and the adsorption capacity will be reduced. Therefore, the adsorption effect is the best when the adsorption temperature is 45 ℃.
3.2.4 Adsorption time
When the initial concentration of Cd(II) solution is 900 mg·L−1, the adsorption temperature is 45 ℃, and the pH is 0.89, the effect of adsorption time on the adsorption capacity of UNC for Cd(II) is shown in Fig. 4d. From Fig. 4d, it can be seen that with the increase of adsorption time, the adsorption capacity of UNC for Cd(II) increases first and then decreases. When the adsorption time is 2 h, the adsorption capacity reaches a maximum of 607.37 mg·g−1. With the continuous growth of adsorption time, when the time reaches 2 h, the adsorption of UNC reaches saturation [26]. After the time continues to increase, the adsorption capacity begins to decrease, which is because the adsorption sites continue to decrease, and the electrostatic repulsion continues to increase [27]. In addition, the internal structure of UNC becomes looser due to the increase of adsorption time, resulting in some Cd(II) gradually falling off from UNC. Therefore, 2 h is the best adsorption time.
Through the exploration of the aforementioned four factors, UNC achieved an adsorption capacity of 611.89 mg·g−1 for Cd(II) within 2 h. To better understand the performance of UNC, the adsorption performance of this adsorbent is compared with other studies [28, 29]. Table 3 lists some adsorbents used by researchers, and by comprehensive comparison with the different adsorbents listed in Table 3, UNC demonstrates excellent Cd(II) adsorption capability.
3.3 Adsorption kinetics
The relationship between adsorption rate and adsorption dynamic equilibrium was analyzed by adsorption kinetics method. In order to further understand the adsorption mechanism of Cd(II) on UNC, the pseudo-first-order and pseudo-second-order kinetic models were used to fit the experimental data, which were Eqs. (2) and (3), respectively.
The pseudo-first-order kinetic model is represented as Eq. (2) [35]:
qt (min) the amount of drug adsorbed at moment t (mg·g−1), qe is the amount of drug adsorbed at equilibrium (mg·g−1), and k is the rate constant for the quasi-primary kinetic model (min−1).
The pseudo-second-order kinetic model is represented as Eq. (3):
v0 (mg·g−1·min−1) is the initial adsorption rate of the pseudo-second-order kinetic model (mg·g−1·min−1).
Figure 5 shows the pseudo-first-order (a) and pseudo-second-order (b) kinetic models of UNC adsorbing Cd(II). Table 4 is the pseudo-first-order and pseudo-second-order kinetic parameters of UNC adsorbing Cd(II). According to the parameters in the table, the linear correlation coefficients R2 of the pseudo-first-order and pseudo-second-order kinetic models of UNC adsorbing Cd(II) are 0.986 and 0.995, respectively, and the theoretical adsorption capacities are 553.110 mg·g−1 and 658.594 mg·g−1, respectively. The actual maximum adsorption capacity of UNC for Cd(II) is 611.89 mg·g−1, which has a good linear relationship with the pseudo-second-order kinetics. It can be seen that the adsorption process of Cd(II) by UNC is dominated by chemical adsorption and supplemented by physical adsorption.
3.4 Adsorption isotherms
Adsorption isotherms can further describe the adsorption process and adsorption mechanism. The adsorption mechanism of Cd(II) on UNC can be described more accurately by Langmuir and Freundlich isotherm models. They are Eqs. (4) and (5), respectively.
The Langmuir model is represented by Eq. (4) [36]:
qe is the equilibrium adsorption capacity of OTC (mg·g−1), qm is the theoretical maximum adsorption capacity (mg·g−1) when the surface is completely covered by OTC, and b is the Langmuir constant (g·mg−1).
The Freundlich model is represented by Eq. (5):
KF (mg1−1/n·L1/n·g−1) is Freundlich constant and 1/n is the adsorption intensity (monolayer adsorption (n < 1) and cooperative adsorption (n > 1)).
Figure 6 shows the Langmuir (a) and Freundlich (b) models of UNC adsorption of Cd(II), and Table 5 presents the Langmuir and Freundlich model parameters for UNC adsorption of Cd(II). According to the data in the table, the linear correlation coefficient (R2) of the Langmuir isotherm model for Cd(II) on UNC is 0.924, with a theoretical adsorption capacity of 1355.691 mg·g−1, whereas the actual adsorption capacity is 611.89 mg·g−1, indicating a significant deviation from the theoretical value. The Freundlich isotherm model exhibits a good linear correlation, with a coefficient (R2) of 0.992. Therefore, the adsorption process of Cd(II) on UNC is better described by the Freundlich isotherm model, indicating a multilayer adsorption mechanism. Combined with the characterization analysis in Section 3.6.2, it is evident that due to the abundant -OH and -NH2 groups on the surface and within the pores of UNC, Cd(II) can form coordination bonds with -OH and -NH2, leading to its adsorption on both the surface and internal pores of UNC [37]. Additionally, zeta potential experiments revealed that UNC exhibited the maximum negative charge at pH = 0.89, corresponding to the maximum adsorption capacity. At this pH, Cd(II) was also adsorbed via electrostatic interaction, indicating that the adsorption process of Cd(II) by UNC involves multilayer adsorption including both physical and chemical adsorption.
3.5 Characterization results and analysis
3.5.1 SEM analysis
Figure 7 (a: 5 μm, b: 8 μm) is the morphology of UiO-66-NH2 at 5 μm and 8 μm. Figure 7c–e is the SEM photos of UNC (c) with UiO-66-NH2 dosage of 0.25 g, UNC (d) before adsorption of Cd(II) and UNC (e) after adsorption of Cd(II). As can be seen from Fig. 7a, b, the SEM image of UiO-66-NH2 is consistent with the literature description [38]. Due to its inherent agglomeration, it cannot be evenly dispersed, which reduces the number of adsorption sites and active functional groups, and cannot effectively exert its high specific surface area advantage. Figure 7c shows UNC with an added capacity of 0.25 g of UiO-66-NH2. From the figure, it can be seen that 0.25 g of UiO-66-NH2 is agglomerated on the surface of UNC and is too densely distributed. This not only reduces its own number of adsorption sites and active functional groups but also reduces the adsorption sites of cellulose, which seriously affects the adsorption capacity of UNC for Cd(II). Figure 7d is the UNC before adsorption. It can be seen from UNC (d) that UiO-66-NH2 is loaded in the three-dimensional pores and is evenly distributed. Figure 7e is the UNC after adsorption of Cd(II). SEM photos show that there are still a small number of narrow pores in UNC, but the surface is smooth and flat, which indicates that Cd(II) is successfully adsorbed.
3.5.2 FT-IR analysis
Figure 8 is the FT-IR spectra of UiO-66-NH2 (a), cellulose (b), UNC (c) before Cd(II) adsorption, and UNC (d) after Cd(II) adsorption. From the FT-IR spectra of UiO-66-NH2 (a), it can be seen that the adsorption band of 1380–1600 cm−1 is the characteristic adsorption band of UiO-66-NH2. Because the ligand of UiO-66-NH2 is 2-aminoterephthalic acid, it contains aromatic carboxylic acids. Therefore, the peak at 1434 cm−1 is the C–C stretching vibration peak, and the peaks at 1382 cm−1 and 1573 cm−1 are symmetric and asymmetric C-O stretching bonds, respectively, from aromatic and carboxylic acid groups. In addition, the C-N stretching vibration peak can be observed at 1255 cm−1, the peak at 1656 cm−1 is the bending vibration peak of N–H, and the characteristic peaks at 661 cm−1 and 767 cm−1 can be attributed to the Zr-O stretching vibration. These characteristic peaks proved that UiO-66-NH2 was successfully prepared [39, 40]. From the FT-IR spectra of cellulose (b), it can be seen that the stretching vibration peak of O–H is at 3100–3460 cm−1, and the absorption peak of C-H is at 2906 cm−1. However, after the dissolution of cellulose into UNC, the absorption peak at 2906 cm−1 is weakened, which is due to the destruction of the crystal structure of cellulose during dissolution. As evidenced by the FT-IR spectrum of UNC (c) post-adsorption, the O–H stretching vibration peak at 3460 cm−1 weakens following the adsorption of Cd(II), a phenomenon attributed to the reaction between Cd(II) and -OH. The N–H bending vibration peak shifts to a lower wavenumber (1625 cm−1), indicative of a chelation reaction between Cd(II) and -NH2. Concurrently, Zr-O shifts from a higher to a lower value, underscoring its pivotal role in the adsorption process. These findings are in harmony with the XPS characterization results. As revealed by the FT-IR spectrum of UNC (d) prior to adsorption, the C-H bending vibration peak at 2906 cm−1 in cellulose shifts to a higher wavenumber (3126 cm−1). This shift is attributed to the increase in hydrogen bonds during the crosslinking of cellulose with PVA. A characteristic peak of Zr-O emerges at 767 cm−1, and the stretching vibration peaks at 1400 cm−1, 1625 cm−1, and 3460 cm−1 are markedly enhanced. These observations provide compelling evidence that UiO-66-NH2 has been successfully loaded onto the cellulose.
3.5.3 XRD analysis
Figure 9 shows the XRD patterns of UiO-66-NH2 (a), cellulose (b) and UNC (c). From the XRD pattern of UiO-66-NH2 (a), it can be seen that the synthesized UiO-66-NH2 has a sharp diffraction peak, which indicates that it has excellent crystallinity. UiO-66-NH2 has obvious crystal plane characteristic diffraction peaks at 2θ = 6.8°, 8.1° and 25.4°, and its characteristic diffraction peaks are consistent with those reported in the literature [41], which proves that UiO-66-NH2 is successfully synthesized. From the XRD patterns of cellulose (b), it can be seen that the (101), (002), and (004) crystal planes of cellulose appeared at 2θ = 15.5°, 22.5°, and 34.5°, respectively, which belonged to the characteristics of cellulose crystal type I [42]. According to the XRD spectrum of UNC (c), the characteristic diffraction peak of the crystal plane of the composite film shifted from 2θ = 15.5 to 19.7°, which was due to the deposition of UiO-66-NH2 in the three-dimensional network structure formed by cellulose and PVA during the blending of UiO-66-NH2 and cellulose. At the same time, the diffraction peak of regenerated cellulose was weakened, which was due to the crosslinking reaction between cellulose and PVA during the blending process.
3.5.4 XPS analysis
Figure 10 shows the XPS spectra of the UNC before and after Cd(II) adsorption. It can be seen from Fig. 10 that the functional groups corresponding to O at 532.13 eV and 533.23 eV in UNC before adsorption are C = O and Zr-O, respectively. After adsorption, C = O and Zr-O shifted (532.13 eV → 531.08 eV and 533.23 eV → 532.03 eV), which may be due to the generation of hydrogen bonds and the participation of Zr-O in the adsorption process. The functional groups corresponding to N at 399.78 eV and 401.53 eV in UNC before adsorption were N–C and-NH2, respectively [15]. After adsorption, N–C and-NH2 shifted (399.73 → 398.43 eV and 401.53 → 400.33 eV), which was due to the chelation reaction between-NH2 and Cd(II), which was consistent with the results of FT-IR analysis. In the pristine UNC, the functional groups corresponding to the C peaks at 284.18 eV, 285.08 eV, and 288.18 eV are C–C, C-O, and O = C-O, respectively. Upon adsorption, a shift is observed in the C-O peak (from 285.08 to 285.53 eV). This shift is likely attributed to the partial transfer of electron cloud density from the oxygen atom in the C-O group to Cd(II), resulting in the formation of a coordination bond during the adsorption process. The functional groups corresponding to Zr at 184.83 eV and 182.48 eV before adsorption were Zr-O and Zr-Zr, respectively. After adsorption, both Zr-O and Zr-Zr shifted (184.83 eV → 183.78 eV and 182.48 eV → 181.48 eV), indicating that it was involved in the adsorption of Cd(II). The characteristic peaks of Cd appeared at 404.83 eV and 411.03 eV [43, 44], indicating that the successful adsorption of Cd(II) by UNC was consistent with the analysis results of SEM.
3.6 Adsorption mechanism
Figure 11 depicts the adsorption mechanism of Cd(II) on the UNC composite membrane. Combined with the analysis results of FT-IR and XPS spectra, it is evident that after Cd(II) adsorption, the FT-IR spectrum of UNC shows a weakening of the stretching vibration peak of O–H at 3460 cm−1, and the bending vibration peak of N–H shifts to a lower wavenumber at 1625 cm−1. The XPS spectrum displays a shift of the peaks of -NH2 and Zr-O to lower wavenumbers (401.53 → 400.33 eV and 184.83 eV → 183.78 eV). Thus, O–H, -NH2, and Zr-O in UNC all participate in the adsorption process of Cd(II). According to relevant literature, O–H and -NH2 form coordination bonds with Cd(II), while Zr-O forms a Zr-O-Cd coordination bond [45, 46]. Therefore, the chemical adsorption of Cd(II) on UNC is primarily due to coordination effects. SEM images show that the UNC composite membrane possesses a complex porous structure. During the dissolution and regeneration process, cellulose crosslinks with PVA, forming a three-dimensional network structure. Within the porous network structure, UiO-66-NH2 is distributed, and Cd(II) is adsorbed inside the material through the pore structure [47]. Through isoelectric point experiments, it is found that the UNC composite membrane exhibits the maximum negative charge at pH = 0.89, coinciding with the maximum adsorption capacity. Thus, the physical adsorption of Cd(II) on the UNC composite membrane comprises both electrostatic adsorption and pore filling processes [48]. In summary, the adsorption mechanism of Cd(II) on the UNC composite membrane involves coordination effects, pore filling, and electrostatic interactions.
3.7 Cyclic adsorption
Figure 12 shows five cycles of desorption experiments using 4% HNO3 as the desorbing agent. From the graph, it can be observed that after the first cycle, the adsorption capacity decreased from 611.89 to 580.23 mg g−1, resulting in a loss rate of 5%. However, from the second cycle onwards, the reduction in loss rate was approximately 19%, 29%, 37%, and 38%, respectively. The adsorption capacity for the fifth cycle was 381.36 mg g−1. Due to the predominantly chemical adsorption of UNC, it is difficult to remove during the desorption process, resulting in the inability of chemical adsorption sites to adsorb again. Moreover, 4% HNO3 during the desorption process may to some extent damage the membrane material, causing the detachment of UiO-66-NH2 embedded on the surface and internally. Therefore, the cycling adsorption results show that after five cycles, the adsorption performance of UNC decreased to 381.36 mg g−1. However, compared to other types of adsorbents listed in the paper, it still exhibits good adsorption efficiency.
4 Conclusion
In summary, the UNC composite membrane was successfully prepared, with UiO-66-NH2 effectively loaded onto the cellulose nanofilm. Subsequent adsorption experiments revealed that the composite membrane exhibited an adsorption capacity of 611.89 mg·g−1 for Cd(II), which is superior to other adsorbents listed in the literature. SEM analysis showed that the synthesized UNC composite membrane possessed a complex three-dimensional network structure, originating from the cross-linking of regenerated cellulose and PVA, with UiO-66-NH2 observed loaded on the surface and within the pores of the composite membrane. FT-IR and XPS analysis of the UNC composite membrane before and after adsorption confirmed that the coordination between Cd(II) and -OH, -NH2, and Zr-O was the main driving force for chemical adsorption. Furthermore, combining adsorption kinetics and isotherms further elucidated that the adsorption of Cd(II) by the UNC composite membrane was a complex multilayered adsorption dominated by chemical adsorption with physical adsorption as a supplementary process. Overall, the adsorption mechanism of the UNC composite membrane for Cd(II) involves the coordination between metal ions and N, O, and pore filling and electrostatic interactions. The UNC composite membrane requires short treatment time, low dosage, and low cost for Cd(II) wastewater treatment, and it also addresses the problem of UiO-66-NH2 aggregation and pipeline blockage during adsorption. As a membrane material, it has the potential for cyclic adsorption, making it an ideal heavy metal adsorbent.
Data availability
Data in all figures are from the experimental results of this experiment.
References
Li YH, Ding J, Luan Z et al (2003) Competitive adsorption of Pb2+, Cu2+ and Cd2+ ions from aqueous solutions by multiwalled carbon nanotubes[J]. Carbon 41(14):2787–2792. https://doi.org/10.1016/S0008-6223(03)00392-0
Waisberg M, Joseph P, Hale B et al (2003) Molecular and cellular mechanisms of cadmium carcinogenesis[J]. Toxicology 192(2–3):95–117. https://doi.org/10.1016/S0300-483X(03)00305-6
Wang C, Zheng T, Luo R et al (2018) In situ growth of ZIF-8 on PAN fibrous filters for highly efficient U (VI) removal[J]. ACS Appl Mater Interfaces 10(28):24164–24171. https://doi.org/10.1021/acsami.8b07826
Hossain MS, Shenashen MA, Awual ME et al (2024) Benign separation, adsorption, and recovery of rare-earth Yb (III) ions with specific ligand-based composite adsorbent[J]. Process Saf Environ Prot 185:367–374. https://doi.org/10.1016/j.psep.2024.03.026
Awual ME, Salman MS, Hasan MM et al (2024) Ligand imprinted composite adsorbent for effective Ni (II) ion monitoring and removal from contaminated water[J]. J Ind Eng Chem 131:585–592. https://doi.org/10.1016/j.jiec.2023.10.062
Sheikh MC, Hasan MM, Hasan MN et al (2023) Toxic cadmium (II) monitoring and removal from aqueous solution using ligand-based facial composite adsorbent[J]. J Mol Liq 389:122854. https://doi.org/10.1016/j.molliq.2023.122854
Rasee AI, Awual E, Rehan AI et al (2023) Efficient separation, adsorption, and recovery of Samarium (III) ions using novel ligand-based composite adsorbent[J]. Surf Interfaces 41:103276. https://doi.org/10.1016/j.surfin.2023.103276
Hasan MN, Salman MS, Islam A et al (2021) Sustainable composite sensor material for optical cadmium (II) monitoring and capturing from wastewater[J]. Microchem J 161:105800. https://doi.org/10.1016/j.microc.2020.105800
Dias EM, Petit C (2015) Towards the use of metal–organic frameworks for water reuse: a review of the recent advances in the field of organic pollutants removal and degradation and the next steps in the field[J]. J Mater Chem A 3(45):22484–22506. https://doi.org/10.1039/C5TA05440K
Kumar P, Bansal V, Kim KH et al (2018) Metal-organic frameworks (MOFs) as futuristic options for wastewater treatment[J]. J Ind Eng Chem 62:130–145. https://doi.org/10.1016/j.jiec.2017.12.051
Kumar P, Pournara A, Kim KH et al (2017) Metal-organic frameworks: challenges and opportunities for ion-exchange/sorption applications[J]. Prog Mater Sci 86:25–74. https://doi.org/10.1016/j.pmatsci.2017.01.002
Chen H, Shen C, Chen X et al (2021) High-sensitivity optical fiber hydrogen sensor based on the metal organic frameworks of UiO-66-NH2[J]. Opt Lett 46(21):5405–5408. https://doi.org/10.1364/OL.443930
Luu CL, Van Nguyen TT, Nguyen T et al (2015) Synthesis, characterization and adsorption ability of UiO-66-NH2[J]. Adv Natl Sci: Nanosci Nanotechnol 6(2):025004. https://doi.org/10.1088/2043-6262/6/2/025004
Sarker M, Song JY, Jhung SH (2018) Carboxylic-acid-functionalized UiO-66-NH2: a promising adsorbent for both aqueous-and non-aqueous-phase adsorptions[J]. Chem Eng J 331:124–131. https://doi.org/10.1016/j.cej.2017.08.017
Fang X, Wu S, Wu Y et al (2020) High-efficiency adsorption of norfloxacin using octahedral UIO-66-NH2 nanomaterials: dynamics, thermodynamics, and mechanisms[J]. Appl Surf Sci 518:146226. https://doi.org/10.1016/j.apsusc.2020.146226
Tian S, Yi Z, Chen J et al (2023) In situ growth of UiO-66-NH2 in wood-derived cellulose for iodine adsorption[J]. J Hazard Mater 443:130236. https://doi.org/10.1016/j.jhazmat.2022.130236
Zhang Y, Xu X, Yue C et al (2021) Insight into the efficient co-removal of Cr (VI) and Cr (III) by positively charged UiO-66-NH2 decorated ultrafiltration membrane[J]. Chem Eng J 404:126546. https://doi.org/10.1016/j.cej.2020.126546
Cheng R, Chen Y, Jiang P et al (2023) Deeply removal of trace Cd2+ from water by bacterial cellulose membrane loaded with nanoscale zerovalent iron: practical application and mechanism[J]. Chem Eng J 468:143668. https://doi.org/10.1016/j.cej.2023.143668
Chen Q, He Q, Lv M et al (2015) Selective adsorption of cationic dyes by UiO-66-NH2[J]. Appl Surf Sci 327:77–85. https://doi.org/10.1016/j.apsusc.2014.11.103
Kumar A, Negi YS, Bhardwaj NK et al (2013) Synthesis and characterization of cellulose nanocrystals/PVA based bionanocomposite[J]. Adv Mater Lett 4(8):626–631. https://doi.org/10.5185/amlett.2012.12482
Li X, Tang Y, Xuan Z et al (2007) Study on the preparation of orange peel cellulose adsorbents and biosorption of Cd2+ from aqueous solution[J]. Sep Purif Technol 55(1):69–75. https://doi.org/10.1016/j.seppur.2006.10.025
Hokkanen S, Repo E, Westholm LJ et al (2014) Adsorption of Ni2+, Cd2+, PO43− and NO3− from aqueous solutions by nanostructured microfibrillated cellulose modified with carbonated hydroxyapatite[J]. Chem Eng J 252:64–74. https://doi.org/10.1016/j.cej.2014.04.101
Zhang L, Cai J, Zhou J et al (2005) Adsorption of Cd2+ and Cu2+ on ion-exchange beads from cellulose/alginic acid blend[J]. Sep Sci Technol 39(5):1203–1219. https://doi.org/10.1081/SS-120028579
Awual MR, Hasan MM (2015) Fine-tuning mesoporous adsorbent for simultaneous ultra-trace palladium (II) detection, separation and recovery[J]. J Ind Eng Chem 21:507–515. https://doi.org/10.1016/j.jiec.2014.03.013
Zhang Z, Wang T, Zhang H et al (2021) Adsorption of Pb (II) and Cd (II) by magnetic activated carbon and its mechanism[J]. Sci Total Environ 757:143910. https://doi.org/10.1016/j.scitotenv.2020.143910
Awual MR, Hasan MM, Islam A et al (2019) Offering an innovative composited material for effective lead (II) monitoring and removal from polluted water[J]. J Clean Prod 231:214–223. https://doi.org/10.1016/j.jclepro.2019.05.125
Awual MR (2017) Novel nanocomposite materials for efficient and selective mercury ions capturing from wastewater[J]. Chem Eng J 307:456–465. https://doi.org/10.1016/j.cej.2016.08.108
Ibrahim BM, Fakhre NA (2019) Crown ether modification of starch for adsorption of heavy metals from synthetic wastewater[J]. Int J Biol Macromol 123:70–80. https://doi.org/10.1016/j.ijbiomac.2018.11.058
Fakhre NA, Ibrahim BM (2018) The use of new chemically modified cellulose for heavy metal ion adsorption[J]. J Hazard Mater 343:324–331. https://doi.org/10.1016/j.jhazmat.2017.08.043
Hamoudi SA, Khelifa N, Nouri L et al (2023) Removal of Pb2+ and Cd2+ by adsorption onto Y zeolite and its selectivity of retention in an actual contaminated effluent[J]. Colloid Polym Sci 301(6):631–645. https://doi.org/10.1007/s00396-023-05089-y
Wu P, Wu W, Li S et al (2009) Removal of Cd2+ from aqueous solution by adsorption using Fe-montmorillonite[J]. J Hazard Mater 169(1–3):824–830. https://doi.org/10.1016/j.jhazmat.2009.04.022
Chen Y, Li M, Li Y et al (2021) Hydroxyapatite modified sludge-based biochar for the adsorption of Cu2+ and Cd2+: adsorption behavior and mechanisms[J]. Biores Technol 321:124413. https://doi.org/10.1016/j.biortech.2020.124413
Li D, Cui H, Cheng Y et al (2021) Chemical aging of hydrochar improves the Cd2+ adsorption capacity from aqueous solution[J]. Environ Pollut 287:117562. https://doi.org/10.1016/j.envpol.2021.117562
Dai W, Xu M, Zhao Z et al (2021) Characteristics and quantification of mechanisms of Cd2+ adsorption by biochars derived from three different plant-based biomass[J]. Arab J Chem 14(5):103119. https://doi.org/10.1016/j.arabjc.2021.103119
Sharef HY, Jalal AF, Ibrahim BM et al (2023) New ion-imprinted polymer for selective removal of Cu2+ ion in aqueous solution using extracted Aloe vera leaves as a monomer[J]. Int J Biol Macromol 239:124318. https://doi.org/10.1016/j.ijbiomac.2023.124318
Ibrahim BM, Fakhre NA, Jalhoom MG et al (2022) Removal of lead ions from aqueous solutions by modified cellulose[J]. Environ Technol 1–13. https://doi.org/10.1080/09593330.2022.2056086
Shahat A, Kubra KT, El-marghany A (2023) Equilibrium, thermodynamic and kinetic modeling of triclosan adsorption on mesoporous carbon nanosphere: optimization using Box-Behnken design[J]. J Mol Liq 383:122166. https://doi.org/10.1016/j.molliq.2023.122166
Chen J, Zhang B, Dang X et al (2020) A nanocomposite consisting of etched multiwalled carbon nanotubes, amino-modified metal-organic framework UiO-66 and polyaniline for preconcentration of polycyclic aromatic hydrocarbons prior to their determination by HPLC[J]. Microchim Acta 187:1–9. https://doi.org/10.1007/s00604-019-3997-1
Fang Y, Zhang L, Zhao Q et al (2019) Highly selective visible-light photocatalytic benzene hydroxylation to phenol using a new heterogeneous photocatalyst UiO-66-NH 2-SA-V[J]. Catal Lett 149:2408–2414. https://doi.org/10.1007/s10562-019-02842-3
Zhang X, Zhang Y, Wang T et al (2019) A thin film nanocomposite membrane with pre-immobilized UiO-66-NH2 toward enhanced nanofiltration performance[J]. RSC Adv 9(43):24802–24810. https://doi.org/10.1039/C9RA04714J
Shen L, Wu W, Liang R et al (2013) Highly dispersed palladium nanoparticles anchored on UiO-66(NH2) metal-organic framework as a reusable and dual functional visible-light-driven hotocatalyst[J]. Nanoscale 5(19):9374–9382. https://doi.org/10.1039/C3NR03153E
Lu J, Wang T, Drzal LT (2008) Preparation and properties of microfibrillated cellulose polyvinyl alcohol composite materials[J]. Compos A Appl Sci Manuf 39(5):738–746. https://doi.org/10.1016/j.compositesa.2008.02.003
Xie F, Guo J, Wang H et al (2022) Construction of ZnO/CdS/Ag composite photocatalyst and its catalytic and antibacterial performance[J]. Chinese J Mater Res 37(1):10–20. https://doi.org/10.11901/1005.3093.2021.586
Du Z, Yang D, Cao Q et al (2023) Recent advances in catalytic synthesis of 2, 5-furandimethanol from 5-hydroxymethylfurfural and carbohydrates[J]. Bioresources Bioprocess 10(1):1–17. https://doi.org/10.1186/s40643-023-00676-x
Awual MR, Hasan MM, Khaleque MA et al (2016) Treatment of copper (II) containing wastewater by a newly developed ligand based facial conjugate materials[J]. Chem Eng J 288:368–376. https://doi.org/10.1016/j.cej.2015.11.108
Waliullah RM, Rehan AI, Awual ME et al (2023) Optimization of toxic dye removal from contaminated water using chitosan-grafted novel nanocomposite adsorbent[J]. J Mol Liq 388:122763. https://doi.org/10.1016/j.molliq.2023.122763
Hasan MN, Shenashen MA, Hasan MM et al (2021) Assessing of cesium removal from wastewater using functionalized wood cellulosic adsorbent[J]. Chemosphere 270:128668. https://doi.org/10.1016/j.chemosphere.2020.128668
Awual MR (2019) A facile composite material for enhanced cadmium (II) ion capturing from wastewater[J]. J Environ Chem Eng 7(5):103378. https://doi.org/10.1016/j.jece.2019.103378
Acknowledgements
The authors are sincerely appreciative of School of Materials Science and Art and Design, Inner Mongolia Agricultural University and the authors whose research publications were used for this review work.
Funding
This study was supported by the Inner Mongolia Autonomous Region Science and Technology Department Plan Project (2019GG018), the Natural Science Foundation of Inner Mongolia (2021MS02024), and the Inner Mongolia Autonomous Region Science and Technology Department Project (2021GG0213).
Author information
Authors and Affiliations
Contributions
Hao He: investigation, validation, and writing—original draft. Bai-yun Zhao: methodology and conceptualization. Xiao-kai Liu: methodology and conceptualization. Li-juan Zhou: investigation and writing—review. Xuan Zhao: investigation and writing—review. Chen-xu Wang: investigation and writing—review. Li Wang: conceptualization, writing-review and editing, and supervision.
Corresponding author
Ethics declarations
Ethics approval
This study is in compliance with ethical standards.
Consent to participate
All authors agreed with the content.
Consent for publication
All authors agreed with the content.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
He, H., Liu, Xk., Zhao, By. et al. Preparation of UiO-66-NH2@cellulose nanofiber composite film and its adsorption performance study towards Cd(II). Biomass Conv. Bioref. (2024). https://doi.org/10.1007/s13399-024-05859-w
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-024-05859-w