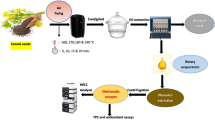
Abstract
The baru almond stands out as almond rich in proteins, lipids, fibers, bioactive compounds, and antioxidant potential. This study aimed to optimize the extraction of fixed oil from baru seed (Dipteryx alata), performed by the hydraulic press and Soxhlet using eco-friendly solvents (ethanol, 2-methyl tetrahydrofuran (2-METHF), and d-limonene), using preliminary treatment with ultrasound and microwaves, as well as carrying out the chemical and functional oil and evaluate its stability during 60 days of storage at 25 °C. Evaluating the combinations of extraction methods and solvents, ethanol was the solvent that presented the best results. The ultrasound method as a pretreatment in 30 min, followed by hydraulic pressing, was the one that presented the best results in oil yield and quality. Therefore, this method was adopted for the other stages of this study. Regarding the chemical composition of the oil, it was rich in unsaturated fatty acids, the main fatty acid being oleic acid at 45.90%, followed by linoleic acid at 25.96%. Regarding oxidative stability, the oil remained stable during 30 days of storage at 25 °C. During the days of storage, there were changes in the peroxide index (of 0.15 for 4.32 meq Kg−1), in the composition of polyunsaturated fatty acids (of 26.04 for 25.03%), and a reduction in the total tocopherols (of 10.94 for 7.43 mg 100 g−1) present in the oil. Finally, ultrasound has shown promise in extracting baru oil. Still, special methods are needed to protect the extracted oils from pro-oxidative factors.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Vegetable oils are important sources of lipids, antioxidants, and bioactive compounds, which are bioavailable for human consumption [1, 2]. These oils are generally obtained from fruits or almonds through different extraction processes. Among the most common compounds in vegetable oils are fatty acids, hydrocarbons, tocopherols, phenolic compounds, terpenes, and phytosterols. The presence and quantity of these substances are directly related to the quality, nutritional, and functional values of these oils, which may vary depending on the species, the climatic conditions of cultivation, the oil extraction system, and the refining processes applied [3]. Consumers are concerned about the quality of edible oils in food preparation and consumption. Therefore, quality, stability, and nutritional characteristics are vital factors for using oils in the food industry. Oil extraction from seeds and almonds mainly uses pressing or solvent extraction as conventional methods [4]. However, limitations associated with these vegetable oil extraction methods include long extraction times, environmental hazards, health hazards, high solvent and energy consumption, and possible changes in the characteristics of the vegetable oil. Thus, new sustainable extraction techniques and ecological solvents for oil extraction, which are efficient and provide quality products without toxic chemicals, must be developed, studied, and applied [5].
The concept of green extraction originates from green chemistry, whose principles aim to innovate all aspects of a solid–liquid extraction process through using renewable plant resources and ecological solvents, co-product production instead of waste, and processing safer and more controllable. The main objective of ecological solvents is to develop an environmentally friendly process with the simultaneous reduction or elimination of pollutants, guaranteeing the safety and well-being of the handler. The vast majority are derived from renewable sources of natural or agricultural waste (biomass) [6].
Among the ecological solvents, we can highlight ethanol, 2-methyl tetrahydrofuran (2-METHF), and d-limonene. Ethanol is an environmentally friendly solvent, readily available, produced on a large scale, and its use is permitted in the food industry. Its advantages include operational safety, low toxicity, high purity, and biodegradability [7]. It is not new about the use of ethanol to obtain oil from the most diverse vegetable matrices, for example, Potrich et al. [8] present ethanol as a replacement for hexane in the extraction of soybean oil, aiming at a technical–economic-environmental analysis. According to Amarante et al. [9], ethanol proved favorable to castor bean oil extraction. Several other authors present extraction with ethanol as a viable and green alternative compared to traditional methods using organic solvents such as hexane [10,11,12,13]. 2-METHF has wide applications in organic chemistry, including the pharmaceutical industry. It has low toxicity and high biodegradability [14]. And finally, d-limonene is one of the main by-products of the citrus fruit industry. This monoterpene molecule plays an important role in flavors and fragrances and is an industry’s cleaning and degreasing agent [15].
As with ecological solvents, using preliminary treatment to improve oil recovery in extraction has gained attention. In this sense, we can highlight microwave and ultrasound preliminary treatment. Microwave heating occurs due to changes in cell structure caused by electromagnetic waves. Based on this assumption, the high yields observed in extractions with techniques involving ultrasound may be related to the heat and mass gradients that act in synergism since the heat dissipates the volume from within the irradiated medium, thus guaranteeing a higher extraction yield, caused by disruption of the cell wall [16]. Ultrasound is a safe technique that produces high-frequency ultrasonic waves, which can promote cavitation phenomena and increase the transfer of energy and mass in a liquid medium, leading to the rupture of plant cell walls, favoring the penetration of the solvent, and improving the release of intracellular compounds. Therefore, ultrasound-based food processing may represent an alternative to improve the bioavailability of bioactive compounds and a substantial increase in the process yield since using ultrasound as a preliminary treatment associated with other oil extraction methods has been showing a promising alternative [17, 18].
In this sense of sustainable extraction, we can highlight the baru (Dipteryx alata), an oilseed species native to the Brazilian Cerrado. Each tree is, on average, 12 m high and can produce between 1000 and 3000 fruits, whose diameter varies from 3 to 7 cm, weighing between 26 and 40 g and having a single seed of approximately 1.17 g. Its seeds are roasted and used as ingredients in gastronomy due to their high nutritional value, containing 38.2 g 100 g−1 of lipids and 23.9 g 100 g−1 of protein [19, 20]. In addition, almonds have higher levels of total phenolic compounds than many other almonds consumed in Brazil, such as pine nuts, macadamia nuts, Brazil nuts, cashew nuts, hazelnuts, and peanuts [21]. Thus, the objective of this work was to optimize the extraction of oil from the baru seed, carried out by the hydraulic press and Soxhlet (using environmentally friendly solvents), using sustainable preliminary treatment with ultrasound and microwaves, as well as to characterize the extracted oil and evaluate its oxidative stability during storage for 60 days at 25 °C.
2 Materials and methods
2.1 Chemicals and plant material
Folin-Ciocalteu reagent, potassium sulfate, potassium persulfate, petroleum ether, aluminum chloride, sodium molybdate, sodium carbonate, sodium hydroxide, potassium hydroxide sodium phosphate, ethanol, acetone, ascorbic acid, gallic acid, glacial acetic acid, and Supelco FAME Mix C4-C24 standards were obtained from Sigma Aldrich® (São Paulo-Brazil). All chemical reagents were analytical grade.
Samples of baru (Dipteryx alata Vogel) were purchased from local businesses in Palmas, in Tocantins, Brazil. The samples came from the city of Pirenópolis, State of Goiás, Brazil (15° 50′ 22.6″ S 48° 57′ 56.4″ W), in September 2021. Both regions are part of the Cerrado biome. Only ripe fruits in good condition were collected. After collection, the fruits were kept in 100 ppm chlorinated water for 15 min for sanitization. The different parts of the fruits were packed in low-density polyethylene bags, protected from light. Then, the samples were stored at − 18 ± 2 °C in a domestic refrigerator until further analysis.
2.2 Preliminary testing of solvents and pretreatments
Preliminary tests were carried out to define the best ecological solvents and the best pre-treatment for baru oil extraction. The effects of using ethanol, 2-methyltetrahydrofuran (2-METHF), and d-limonene solvents were evaluated. Extraction with hexane was also performed for comparative purposes. The oil content of the seeds was determined in triplicate in a Soxhlet apparatus; using the mentioned solvents in the seed-solvent ratio of 1:5 (w/v), for 6 h, at a temperature according to the boiling point of each solvent, ethanol (~ 79 °C), 2-METHF (~ 80 °C), d-limonene (~ 175 °C), and n-hexane (~ 70 °C), for preliminary tests of sustainable techniques related to pre-treatment, ultrasound, and microwaves were used. For the ultrasound test, 250 g of baru seeds were used, submerged in distilled water (25 °C), and inserted in an ultrasonic bath, with a frequency of 40 kHz, for 15 and 30 min. The seeds were dried in an air circulation oven at 60 °C for 48 h and sent for Soxhlet oil extraction. For microwave-assisted extraction, 250 g of seeds were heated in a microwave at 200 W, varying the time between 5 and 10 min. After heating, the seeds were sent for extraction.
In response to using environmentally friendly pre-treatments, the following analyses were carried out: total yield, peroxide value, and acidity of the oils. The peroxide value and acidity were determined according to the protocols standardized by the AOCS (2009). The peroxide value was determined according to the Cd 8b-90 method and acidity according to the Cd 3d-63 method. The yield of each extraction was determined according to Eq. 1.
2.3 Optimized baru oil extraction
The extraction conditions evaluated in this study are described in Table 1. Different extraction methods were analyzed through preliminary tests. The ecological solvent that presented the best results for the yield, peroxide index, and acidity responses was ethanol, which will be used in the other stages of this study.
Two pre-treatments were tested, ultrasound and microwave, at two different times, times of 15 and 30 min for ultrasound and 5 and 10 min for microwave. For the pre-treatment with ultrasound, 250 g of whole seeds were submerged in distilled water (25 °C) and inserted in the ultrasonic bath, with a frequency of 40 kHz, at 15 and 30 min. The seeds were then dried in an oven at 60 °C for 48 h and sent for extraction with a press and Soxhlet. For microwave-assisted extraction, 250 g of whole seeds were heated in a microwave at 200 W, varying the time between 5 and 10 min. After heating, the seeds were sent for extraction with a press and Soxhlet. For mechanical extraction by hydraulic pressing, a 30-t press was used. Two hundred fifty grams of samples were weighed, placed in a stainless-steel cylinder, and pressed up to 10 t. For oil extraction using ethanol, about 30 g of seeds were packed in cellulose cartridges and placed under reflux, keeping the flow constant for 6 h. After extraction, ethanol was eliminated in a rotary evaporator, and the remaining content was dried in an oven until reaching constant weight. After the extraction, the crude oil was weighed, stored in amber bottles, and stored in a freezer at − 18 °C until the analysis time. To determine the best condition for extracting baru oil among the proposals, the response variables used were also the extraction yields and the peroxide and acidity index analyses. Finally, the extraction method that presented the best result was characterized and sent for chemical, physical, identity, and quality analysis.
2.4 Quality assessment of baru oil
The method that presented the best results, according to the analyzed parameters, was the UP30 method (30-min ultrasound pre-treatment + extraction with a hydraulic press). The baru oil extracted by the UP30 method was characterized by analyzing the fatty acid profile, refractive index, iodine index, saponification index, unsaponifiable matter, atherogenicity and thrombogenicity index, phenolic compounds, tocopherols, and carotenoids totals.
2.4.1 Fatty acids
The fatty acid profile of pressed baru oils was determined by gas chromatography described in the methodology proposed by Hartman & Lago [22]. A gas chromatography system coupled to a Shimadzu GC-FID flame ionization detector (model CG − 17A) was used, equipped with a fused silica OV-fused capillary column (30 m × 0.25 mm, 0.25 μm) with nitrogen and synthetic air and hydrogen as ignition gas. The oils were submitted to saponification and esterification processes with potassium hydroxide in methanol (0.1 mol L−1) and hydrochloric acid in methanol (0.12 mol L−1) converting them into fatty acid methyl esters (FAMES), being subsequently extracted with hexane and injected with a volume of 1 μL in the GC. The chromatographic conditions used were initial column temperature equal to 40 °C for 5 min, increased at a rate of 10 °C min−1 until the temperature of 140 °C, remaining 15 min, until the final column temperature of 240 °C with heating of 4 °C min−1, remaining for 30 min. The carrier gas used was ultrapure nitrogen with a flow of 1 mL min−1, and injector and detector temperatures were 260 °C. The fatty acids were identified based on the retention time of the sample peaks and compared with the peaks of the respective standards (Supelco FAME, Sigma-Aldrich®). Then, quantification was performed by normalizing the area and expressing it as a percentage.
2.4.2 Identity and quality standards
The acidity value (Ca 3d-63) and peroxide value (Cd 8–53). The refractive index was performed according to the Cc 7–25 method. The iodine value was calculated using the Cd 1c-85 and expressed in g I2 100 g−1. The saponification index was defined by the amount in milligrams of potassium hydroxide necessary to saponify 1 g of oil, being calculated according to the Cd 3–25 method, and expressed in mg KOH g−1. The saponification index was defined by the amount in milligrams of potassium hydroxide necessary to saponify 1 g of oil, being calculated according to the Cd 3–25 method, and expressed in mg KOH g−1. The unsaponifiable matter corresponds to the total amount of substances dissolved in oils and fats, which are insoluble in an aqueous solution after saponification with alkali. It was determined according to the Ca 6b-53 method, expressed in g 100 g−1. The oxidative stability was measured by the Rancimat method (METRODATA—Stabnet, version 1.1 full), where the time corresponded to the inflection point in the oxidation curve. In this method, a constant airflow (20 L h−1) passes through the reaction container containing 5 g of oil at a constant temperature of 110 °C. An abrupt increase in electrical conductivity characterizes the change over time, thus indicating the period required for detecting secondary oxidation products. Oxidative stability followed the Cd 12b-92 method. All methods performed on baru oil are described and standardized by the American Oil Chemists Society [23].
To determine the nutritional quality of lipids, the compositions of fatty acids were used, by calculating the atherogenicity (AI) and thrombogenicity (TI) indexes [24], according to the following equations:
where C12:0, C14:0, C16:0, and C18:0 are relative percentage masses of lauric, myristic, palmitic, and stearic acids, respectively; MUFA is the relative percentage mass of monounsaturated fatty acids; FA ω6 and FA ω3 are the relative percentage mass of omega-3 fatty acids and omega-6 fatty acids, respectively.
2.4.3 Total phenolic compounds (TPC) and total carotenoids (TC)
The extracts for making the phenolic compounds were prepared using 2 g of baru seed and 2 g of baru oil and homogenized with 20 mL of ethanol. After 30 min, the extract was filtered through filter paper, and the total volume increased to 50 mL with the addition of ethanol. The extracts’ total phenolic compounds (TPC) were determined using the Folin–Ciocalteau method, according to the methodology described by Singleton & Rossi [25]. For the oxidation reaction, 0.2 mL of Folin-Ciocalteu (2.0 N) was added, followed by 2 mL of ultrapure water and 0.1 mL of the obtained extracts. After 5 min, 1 mL of 10% sodium carbonate (Na2CO3) was added to the complex. The mixture was allowed to stand in the dark at room temperature for 60 min. Absorbance was measured at 725 nm in a spectrophotometer. The content of total phenolic compounds was determined from the standard curve of gallic acid in the range of 40 to 200 mg L−1. The results were expressed in the gallic acid equivalent per 100 g sample (mg GAE 100 g−1). Phenolic compounds were analyzed in the oil extracted by the UP30 method and in the natura baru seed.
Carotenoid extraction was performed according to Rodriguez-Amaya [26] at a controlled temperature (20 °C) and reduced lighting. First, 5 g was weighed and macerated with 30 mL of acetone in a porcelain mortar. The mixture was then added to a separatory funnel containing 50 mL of petroleum ether and filtered three times in a row until the residue was colorless. Next, 50 mL of acetone was added to the extract, followed by carefully adding distilled water (300 mL). After phase separation, the lower phase consisting of water and acetone was discarded. Then, the ether phase was washed five times with distilled water to remove the acetone. The extracts obtained were used to read the absorbance in a spectrophotometer at 450 nm (Rayleigh, UV − 1800). Results were expressed in mg 100 g−1. Total carotenoid analyses were carried out in the oil extracted by the UP30 method and in the natura baru seed.
2.4.4 Tocopherols
The profile of tocopherols was determined according to the Ce 8–89 method of the AOCS [23] (2009), expressed in mg 100 g−1. In addition, the tocopherol profile was analyzed in the oil extracted by the UP30 method and in the natura baru seed. For the baru oil extraction process, 1 g of the sample was dissolved in n-hexane. The almond extraction process in natura was based on the alkaline saponification method proposed by Zhu et al. [27]. Briefly, 0.25 g of the in natura samples were mixed with 2.5 mL of ethanol and 0.25 mL of 80% potassium hydroxide solution and vigorously stirred. After shaking, the samples were incubated in a water bath at 70 °C for 30 min, with periodic shaking every 10 min. Then, the tubes were cooled in ice water for 5 min, and 1.5 mL of water and 2.5 mL of n-hexane were added, which were vortexed for 30 s and then centrifuged at 1000 × g at 20 °C for 10 min. The n-hexane layer was transferred to vials. Then, 1 mL of hexane was added to each vial for HPLC analysis. Tocopherols were analyzed using an Agilent liquid chromatography (HPLC) system (model 7890 A) coupled to a fluorescence detector (model LC 305, Lab Alliance − California − USA), using excitation wavelengths of 294 nm and emission of 326 nm; a pump (Radpump III model, Lab Alliance) and a LiChrospher SI 60 capillary column (250 × 4 mm × 5 μm) were used. The isocratic elution of the mobile phase consisted of n-hexane:ethyl acetate:acetic acid (97.6:1.8:06, v/v/v), with a flow rate of 1.5 mL min−1 and volume of 250 μL injection. Quantification was performed with external standardization with tocopherol standard 613,424-SET (Calbiochem-Merck). Tocopherol standards (mixture of α-, β-, γ-, and δ-tocopherols) were used for confirmation and quantification purposes.
2.5 Stability during storage
To evaluate the stability of baru oil extracted by the method that presented the best results (UP30), the oil obtained was stored in an amber glass bottle and inertized in a nitrogen atmosphere. After the inertization process, the oils were held without the incidence of light in a BOD-type incubator for 60 days at a controlled and stable temperature of 25 °C. During this period, analyses were performed every 15 days (totaling five analysis times). The response variables of the UP30 oil analyzed during the storage period were identity and quality analyses (peroxide, acidity, and iodine index), tocopherol profile, and fatty acid profile.
2.6 Statistical analysis
Statistical analysis was performed using Statistica 10.0 software (StatSoft Inc., Tulsa, OK, USA). The experimental design of the extraction methods was carried out in a 2 × 2 × 2 factorial scheme, with pre-treatments (ultrasound and microwave), times (15 and 30 min for ultrasound and 5 and 10 min for microwave) as variables, and the extraction methods (press and Soxhlet), performed in triplicate. The results of the different extraction methods were evaluated by comparing the means, using the Tukey test at 5% (p < 0.05) to evaluate the statistical difference between the tested methods. In addition, the stability results were evaluated by comparing the means, using the Tukey test at a significance level of 5% (p < 0.05), through analysis of variance and regression analysis to explain the changes due to the significant effect of time.
3 Results and discussion
3.1 Preliminary tests
The effect of different solvents and sustainable techniques on the yield response variables and peroxide and acidity indexes in the extraction of baru oil are shown in Table 2. The analysis of the mean values (Table 2) for the different solvents applied showed that there were significant differences (p < 0.05) for the parameter total yield of extractions. D-limonene showed a higher yield than the others (42.80%), followed by 2-METHF (18.76%), ethanol (16.85%), and n-hexane (15.80%). However, we can observe no significant differences between the yield of oil extracted with ethanol and n-hexane when evaluating more concisely. Furthermore, according to Kozłowska et al. [28], oils extracted with polar solvents, such as ethanol, can cause extraction of polar materials (phospholipids) in addition to neutral triacylglycerols and, therefore, can provide higher oil yields, and act as critical structural components of cell membranes and organelles, where they operate as signaling molecules.
Another noteworthy point is the high yield obtained when using a d-limonene solvent. This result is mainly due to the problematic separation between the solvent and the extracted oil after the extraction time. D-limonene is completely miscible in oils and slightly polar [29]. Due to the fact that d-limonene is miscible in oils, its evaporation is difficult; thus, the extracted oil remains with a significant amount of residual solvent. This fact can be observed by the characteristic smell of the d-limonene solvent in the final oil after its evaporation. The 2-METHF solvent showed promising results for obtaining oil from baru seeds, with its extraction yield (18.76%) being slightly higher than that obtained with ethanol (16.85%). However, the peroxide index of the oil extracted with 2-METHF showed values considered high (48.17 meq Kg−1), which is above that recommended by the Codex Alimentaruis [30], where the maximum acceptable levels for the peroxide index are 10 meq Kg−1 and 15 meq Kg−1, for refined oils and cold-pressed and unrefined oils, respectively. Based on the assumptions of the ecological solvents tested, ethanol had the lowest yield but the best quality solvent, with peroxide and acidity values within the standards standardized by the Codex Alimentaruis [30]. Because it is an economically viable solvent, easily acquired, with its use allowed in the food industry, and can be applied on an industrial scale or even by small producers who process baru, ethanol was chosen to continue the research and application in other extraction methods.
The effect of different pre-treatments (ultrasound and microwave) on the yield, peroxide value, and acidity of fixed baru oil was also verified. Pre-treatment with sustainable techniques precedes the extraction stage via Soxhlet with ethanol, a solvent defined through preliminary tests as previously reported. Evaluating the mean values (Table 2) for the ultrasound pretreatment, it is observed that the yield was not affected by the sustainable technique applied (p < 0.05). However, there were significant differences (p < 0.05) between the different pre-treatment times applied for the peroxide and acidity response variables. The value of peroxide and acidity indicates the quality or state of conservation of oils and fats [31]. The pre-treatment, which precedes the extraction process, has the primary objective of weakening the cell structure that acts as a barrier to the release of oil.
Microwave and ultrasound are very versatile and efficient extraction tools, and both can be used as a pre-treatment due to their advantages, such as improving cell penetration of the solvent, intensification of mass transfer, cell disruption, as well as maintaining the qualities of the extracted oil [32,33,34]. Evaluating the results of the preliminary tests for the application of pre-treatment in the extraction of fixed baru oil, it is observed that the ultrasound and microwave pre-treatment did not interfere with the performance of the tested parameters but were effective in reducing the peroxide oxidation of the analyzed samples, resulting in more excellent oxidative stability. Thus, applying pre-treatment becomes feasible for the extraction of fixed baru oil.
3.2 Evaluation of the best conditions for extracting baru fixed oil
Table 3 presents the ANOVA results for fixed baru oil extraction and the possible interactions between the variables. For the yield parameter, it was possible to notice that there was a significant interaction only in the isolated variables pre-treatment and time. On the other hand, for the parameters of peroxide and acidity, all factors studied had a significant effect, except for the interaction between pre-treatment/time and pre-treatment/time/extraction technique.
Evaluating the yield in relation to the pre-treatment, the oil samples that used ultrasound as a pre-treatment showed a yield of 16.43%, with a yield 4.6% higher than the samples that used microwaves as pre-treatment (15.71%), demonstrating a significant difference (p < 0.05) between both. Numerically, the difference in yield was relatively small; however, this difference, when taken to an industrial scale, in which tons of oil are extracted in large proportions, can generate a significant impact on the final yield of production. Evaluating the peroxide index, it is observed that the analysis of variance (Table 3) did not indicate a significant influence between the pre-treatment × time interaction; however, when a detailed analysis is carried out, a difference is found (p < 0.05) between the times used for the ultrasound pretreatment. Table 4 presents the peroxide and acidity index averages, evaluating the pre-treatment and extraction methods in time.
Table 4 shows that when using ultrasound as pretreatment, the time of 30 min (1.95 meq Kg−1) showed lower peroxide results compared to the time of 15 min (3.27 meq Kg−1) regardless of the extraction method used. When microwaves were used as a pretreatment, there was no significant difference between peroxide values, regardless of the extraction method (p < 0.05). Evaluating the peroxide index by the type of extraction over time, it is observed that when the extraction was performed by hydraulic press, regardless of the pre-treatment used, no peroxide index was identified in the samples. Low peroxide values indicate low lipid oxidation in the fixed baru oil samples. For the samples using a solvent in the extraction, there was an increase in the peroxide value, which can be attributed to the heating of the solvent, which is necessary for Soxhlet extraction and evaporation of the residual solvent, causing possible degradation in the sample. In this sense, both for acidity and for peroxides, the absence of significant differences (p < 0.05) between the pre-treatment × time interaction in the analysis of variance was observed. However, when conducting a detailed analysis, significant differences were found between the samples (p < 0.05) between the ultrasound and microwave pretreatment times.
Increasing the ultrasound pretreatment time from 15 to 30 min and the microwave pretreatment time from 5 to 10 min increased the acidity value. This detection, in acidity values, is due to triglyceride hydrolysis reactions, which can occur with increasing time in which the sample was exposed to pre-treatment [35]. Evaluating the averages for the type of extraction over time, it is observed that when the extraction was performed using a hydraulic press, regardless of the pre-treatment used and at all different times, the acidity values were lower than when comparing the extracted samples using ecological solvents (ethanol), a fact that can be attributed to the variation in the temperature required for extraction with solvents, since this parameter is associated with the thermal susceptibility of the triglycerides and the increase in the free fatty acid content of the extracted oil [36]. In this sense, the proposed treatment UP30 (30-min ultrasound pre-treatment + extraction with a hydraulic press) presented the best characteristics according to the analyzed parameters. Thus, this method will be used for the other analyzes of this study.
3.3 Fatty acids
The fatty acid profile and composition of baru oil are shown in Table 5. The fatty acid composition of baru oil indicated the presence of five saturated fatty acids (C16:0, C18:0, C20:0, C22:0, and C24:0), four monounsaturated fatty acids (C18:1, C20:1, C22:1, and C24:1), and two polyunsaturated fatty acids (C18:2, C18:3). The main fatty acid identified as oleic acid C18:1 (45.90%) followed by linoleic acid C18:2 (25.96%). The content of monounsaturated fatty acids (AGM) (48.91%) was higher than the content of polyunsaturated fatty acids (AGP) (26.04%) and saturated fatty acids (AGS) (20.28%). This profile compares to the fatty acid profile reported for the gurguéia nut (Dipteryx lacunifera Ducke), where the authors reported low content of saturated fatty acids (20.66%) and high contents of mono and polyunsaturated fatty acids (75.56%) [37].
A significant concentration of AGM and AGP stands out, emphasizing the fatty acid linoleic (ω6), which presented a concentration of 25.96%. Humans do not synthesize this polyunsaturated fatty acid. Therefore, it must be provided through foods containing them, vegetable oils being the main source of this essential fatty acid. Thus, baru oil becomes a promising source for obtaining this compound [38, 39]. The reduced content of saturated fatty acids (20.28%), in relation to the high average content of monounsaturated and polyunsaturated fatty acids (74.95%) in baru seed oil, maybe the main characteristic attributed to the use of baru oil to reduce the risk of cardiovascular diseases [40]. Due to the fatty acid profile and predominance of unsaturated lipids, baru oil seems suitable for human consumption. In addition, unsaturated fatty acids have often been associated with the modulation of energy metabolism, acting directly in preventing diseases associated with hepatic and vascular lipotoxicity [41].
3.4 Identity and quality standards of baru oil
The baru oil extracted by the UP30 treatment showed refractive index values of 1.46. This parameter indicates the general degree of unsaturation of the oil and is often used as a criterion for detecting the purity value of extracted oils [36, 42]. The iodine value measures the total number of double bonds (unsaturation) in the oil sample [43]. The iodine value was 88.68 g I2 100 g−1. The value of the saponification index for the baru oil extracted in this study was 180.41 mg KOH g−1. In food terms, the higher the saponification index, the better the oil for food [44]. Nevertheless, the saponification index defines approximately the average size of the carbonic chain of the fatty acids present in the composition of the lipid sample. The lower the average molecular weight of the fatty acids, the higher the value of the saponification index. Therefore, unsaturated fatty acids have a lower molecular weight than saturated fatty acids [45].
The unsaponifiable matter content of baru oil found in this study was 1.49%. The unsaponifiable matter of vegetable oils consists of minor compounds, such as tocopherols, phytosterols, phenolic compounds, and carotenoids, which have antioxidant activity and protect the oil from oxidation [39]. Baru oil showed relatively low oxidative stability (2.37 h at 120 °C). Oxidative stability is the time required to reach the point where one or both of the oxidative parameters, including peroxide value, is suddenly increased after going through an incremental process and causes an unpleasant taste and smell in the oil or extracted product [36]. The longer the induction time, the more stable the oil remains. The low stability of baru oil may be related to the high content of linoleic acid (C 18:2) (Table 5), a compound more prone to oxidation [46]. The refractive index, iodine index, and saponification index are related to the amount of unsaturated fatty acids in the sample. Baru oil is rich in unsaturated fatty acids. Therefore, the abovementioned levels match the unsaturated fatty acid content of baru oil (74.95%). Edible vegetable oils, especially those with a high content of unsaturated fatty acids, have beneficial health effects, such as lowering cholesterol and preventing atherosclerosis [47].
The atherogenicity index (AI) and the thrombogenicity index (TI) are lipid health indices that take into account the effects of saturated and unsaturated fatty acids on the development of coronary heart disease [24]. The atherogenicity and thrombogenicity indices for the extracted baru oil were 0.08 and 0.28, respectively. The results found in this study show low AI and TI values, which characterizes a positive potential of baru oil, given that lower AI and TI values (close to zero) are favorable, as they represent a higher content of antiatherogenic fatty acids and therefore can prevent coronary heart disease. Antiatherogenic lipids inhibit plaque aggregation and decrease the levels of esterified fatty acids, cholesterol, and phospholipids, preventing the appearance of micro and macro coronary diseases [48].
3.5 Bioactive compounds and tocopherol profile
Table 6 presents the values of phenolic compounds, total carotenoids, and tocopherols for the baru oil extracted with the UP30 treatment and for the baru seed in natura. It is observed that all the values of the analyzed parameters (Table 6) present significant differences (p < 0.05) when comparing the oil and the seed in natura. It is observed that the value of phenolic compounds in the seed is higher in relation to the extracted oil. The value of phenolic compounds found in baru oil after extraction was 4.02 mg EAG 100 g−1. This difference between the values of phenolic compounds in the seed about the extracted baru oil may be related to the extraction method used. Pineli et al. [49] also observed a reduction in the content of phenolic compounds after the production of partially defatted baru flour and reported that this loss of phenolic compounds might be related to the unit operations involved in oil extraction and flour processing. Heating during pressing and the distribution of compounds between the oil and the cake can be the leading causes of the loss of phenolic compounds. For carotenoids, behavior similar to phenolic compounds was identified. There was a decrease in the value of carotenoids in the extracted oil in relation to the seed. No carotenoids were identified in the extracted baru oil; a relatively low value of 0.21 mg 100 g−1 was identified in the seed. Thus, the extraction of baru oil using a hydraulic press cannot be considered a viable alternative for processes aimed at obtaining lipid extracts rich in phenolic compounds and flavonoids.
Table 6 shows the values of tocopherols in baru oil and in natura seed. To analyze tocopherols, the opposite of what was reported for phenolic and carotenoid compounds was observed. The extraction of baru oil provided higher levels of tocopherols than the in natura seed, with 10.94 mg, 100 g−1, and 2.60 mg 100 g−1, respectively. This increase in tocopherols in the seed for baru oil is possibly due to the application of ultrasound pretreatment to the seed before extraction. In their study, Liu et al. [18] reported an increase in total tocopherols after applying pre-treatment with ultrasound for oil extraction from Iberis amara seed. According to Sicaire et al. [15], the implosions are caused by applying ultrasound fragments or breaking the surfaces of the solid matrix, increasing mass transfer, and accelerating diffusion. This way, the oil extraction process with ultrasound and hydraulic press positively transferred tocopherols from the seed to the baru oil.
3.6 Stability during storage
Storage stability was performed for 60 days at 25 °C and evaluated using several response variables. Table 7 presents the main variations between the proposed analyses during the shelf life of the fixed baru oil.
The data referring to the peroxide content (Table 7) shows that the storage time (days) exerted a significant influence (p ≤ 0.05) on the quality parameters of baru oil. It is possible to observe a gradual increase in the peroxide index identified after the 45th day, starting the degradation of the fatty acids present in the baru oil. Peroxide is the primary parameter in evaluating the oxidation of the lipid content of different products; generally, when unsaturated fatty acids are present in large amounts, the oil is more prone to oxidation [36]. A significant increase in the peroxide value was observed from the 30th day (0.15 meq Kg−1) to the 45th day (2.98 meq Kg−1). A new increase was registered from the 45th day to the 60th day, with a value of 4.32 meq Kg−1. The recorded values are within the standards (15 meq Kg−1) of the Codex Alimentarius [30] defined for the peroxide index; with the passing of the days of storage, a gradual increase in the peroxide value is observed. This increase during storage is due to the oxidation and degradation of unsaturated fatty acids due to oxidation reactions identified after the 45th day, with baru oil being rich in unsaturated fatty acids (74.95%), as per Table 5.
The averages for the acidity index in the fixed baru oil (Table 7), stored for 60 days, we can observe that the storage time had a significant influence (p ≤ 0.05) on the acidity values. The results demonstrate a gradual decrease over the storage days, ranging from 1.32 mg KOH g−1 (1st day) to 0.82 mg KOH g−1 (60th day). All acidity results, measured over 60 days, are within the Codex Alimentarius [30] standards (4.0 mg KOH g−1) defined for acidity value. The increase in an oil’s acidity index indicates the breakdown of triacylglycerols and the degradation of the product since the higher the oil’s acidity, the higher its concentration of free fatty acids [50]. Therefore, the results obtained in this work for the acidity index indicate a satisfactory decrease since it corresponds to low levels of free fatty acids present in the oil. Regarding the iodine index (Table 7), it is possible to verify the decline in the iodine value from 88.68 I2 100 g−1 (day 1) to 86.56 I2 100 g−1 (day 60), indicating the influence of time (p ≤ 0.05) on the product. Generally, a decrease in the iodine index is expected due to the reduction in the content of polyunsaturated fatty acids, provided by oxidation, dependent on time [49].
The storage time exerted a significant influence (p ≤ 0.05) in relation to the parameter of total tocopherols. The behavior observed in Table 7 indicates a decrease in the values of tocopherols in fixed baru oil, from 10.94 mg 100 g−1 (1st day) to 7.43 mg 100 g−1 after 60 days of storage, this being. The same behavior was verified when analyzing the tocopherols individually (α-tocopherol, β-tocopherol, γ-tocopherol, and δ-tocopherol). Tocopherols are natural antioxidants found in many vegetable oils. However, the decrease of tocopherols in the oil during storage is possibly linked to oxidative reactions and cell damage [51], as observed by the onset of oxidative degradation reactions detected by the presence of peroxides after 45 days (Table 7).
The ANOVA and linear regression results were analyzed for the total amount of saturated, monounsaturated, and polyunsaturated fatty acids. The values presented indicate that the storage time significantly influenced (p ≤ 0.05) the values of fatty acids in baru oil. Data referring to saturated fatty acids (Table 7) show that storage time had a significant influence (p ≤ 0.05) on its value. Therefore, it is possible to observe a slight increase in AGS after 60 days, going from 20.28% (1st day) to 21.06% (60th day). Table 7 also presents the results for monounsaturated fatty acids in fixed baru oil. The results show a gradual decrease over the days of storage with values of 48.91% (1st day) and 48.85% (60th day), showing a significant difference (p ≤ 0.05), with the same behavior observed in polyunsaturated fatty acids, with a decrease of 26.04% (1st day) and 25.30% (60th day). In general, and evaluating the results of fatty acids, it is observed that the amount of unsaturated fatty acids (mono and poly) decreased at the same time that saturated fatty acids increased. This is due to the degradation and oxidation of unsaturated fatty acid chains that are degraded by oxidation processes with O2, resulting in the degradation of all fat chains (saturated and unsaturated) [52]. Unsaturated fatty acids are unstable and susceptible to oxidation even at room temperature [53]. The most significant proportion in relation to the decrease of unsaturated fatty acids occurred in AGP. Bonds in polyunsaturated fatty acids are more reactive than a double bond in a monounsaturated chain during lipid oxidation [54].
During storage or handling, the utmost care must be taken to avoid oil contamination with oxygen. Oil can normally absorb 2% oxygen when stored in contact with air. If any significant amount of this absorbed air reacts with the oil, its quality will deteriorate [55]. The concentration of dissolved oxygen in the oil is important in the oxidative process, with the solubility of oxygen being greater in virgin oil than in refined oil, which, theoretically, would make it more susceptible [56]. Thus, the correlation of the results of fatty acids is consistent with the other results presented. The oxidation of baru oil, extracted by the UP30 method, began after the 45th day, thus resulting in higher peroxide indices, loss of essential compounds such as tocopherols, and the breaking of mono and polyunsaturated fatty acid bonds, with an increase in the amount of saturated fatty acids.
4 Conclusions
Baru seed is rich in lipids and has shown promise for fixed oil extraction. Evaluating the extraction conditions with different solvents, it was concluded that ethanol was the ecological solvent that presented the lowest yield; it provided one best quality oil, with peroxide and acidity values within the established by law. The test carried out with the pre-treatment with ultrasound and microwaves, at different times, proved to be essential for the extraction of fixed baru oil, with the application of the pre-treatment being optimistic about the peroxide and acidity quality indicators. Among the different methods of extraction tested for the extraction of fixed oil from the baru seed, the method using ultrasound as pre-treatment, in the time of 30 min extracted with a hydraulic press, was the method chosen to carry out the characterization.
The characterization of baru oil indicates that baru oil has a higher composition of unsaturated chains, which contribute to reducing the risk of obesity and cardiovascular disease. The characterization analyses of the fixed baru oil extracted by the UP30 method demonstrated that the cold pressing process using ultrasound as a pre-treatment did not interfere with the characteristics of the baru oil and can be a viable technology to replace the traditional process of hot solvent oil extraction such as Soxhlet. The oxidative stability of baru oil, extracted by the UP30 method, was considered relatively low, and its storage stability at 25 °C showed the beginning of oxidation after 45 days of storage. During storage, the decomposition of baru oil extracted by the UP30 method requires unique methods to protect it from pro-oxidative factors, such as oxygen, temperature, and light, not being considered a stable oil at a temperature of 25 °C.
Data availability
The article material presents all data relevant to this study.
References
Herculano LS, Lukasievicz GVB, Sehn E, Torquato AS, Belançon MP, Savi E, Kimura NM, Malacarne LC, Baesso ML, Astrath NGC (2021) The correlation of physicochemical properties of edible vegetable oils by chemometric analysis of spectroscopic data. Spectrochim Acta A: Mol Biomol Spectrosc 245:118877. https://doi.org/10.1016/j.saa.2020.118877
Morais RA, Teixeira GL, Ferreira SRS, Cifuentes A, Block JM (2022) Nutritional composition and bioactive compounds of native Brazilian fruits of the Arecaceae family and its potential applications for health promotion. Nutrients 14:4009. https://doi.org/10.3390/nu14194009
El Khetabi A, Lahlali R, Ezrari S, Radouane N, Nadia L, Banani H, Barka EA (2022) Role of plant extracts and essential oils in fighting against postharvest fruit pathogens and extending fruit shelf life: a review. Trends Food Sci Technol 120:402–417. https://doi.org/10.1016/j.tifs.2022.01.009
Olagunju AI, Adelakun OS, Olawoyin MS (2022) The effect of rice bran extract on the quality indices, physicochemical properties and oxidative stability of soybean oil blended with various oils. Measurement: Food 6:100032. https://doi.org/10.1016/j.meafoo.2022.100032
Perumal AB, Huang L, Nambiar RB, He Y, Li X, Sellamuthu PS (2022) Application of essential oils in packaging films for the preservation of fruits and vegetables: a review. Food Chem 375:131810. https://doi.org/10.1016/j.foodchem.2021.131810
Oliveira ÉR, Silva RF, Santos PR, Queiroz F (2019) Potential of alternative solvents to extract biologically active compounds from green coffee beans and its residue from the oil industry. Food Bioprod Process 115:47–58. https://doi.org/10.1016/j.fbp.2019.02.005
Santos KA, Silva EA, Silva C (2021) Ultrasound-assisted extraction of favela (Cnidoscolus quercifolius) seed oil using ethanol as a solvent. J Food Process Preserv 45:e15497. https://doi.org/10.1111/jfpp.15497
Potrich E, Miyoshi SC, Machado PF, Furlan FF, Ribeiro MP, Tardioli PW, Giordano RC (2020) Replacing hexane by ethanol for soybean oil extraction: modeling, simulation, and techno-economic-environmental analysis. J Clean Prod 244:118660. https://doi.org/10.1016/j.jclepro.2019.118660
Amarante RC, Oliveira PM, Schwantes FK, Morón-Villarreyes JA (2014) Oil extraction from castor cake using ethanol: kinetics and thermodynamics. Ind Eng Chem Res 53:6824–6829. https://doi.org/10.1021/ie500508n
Baümler ER, Carrín ME, Carelli AA (2016) Extraction of sunflower oil using ethanol as solvent. J Food Eng 178:190–197. https://doi.org/10.1016/j.jfoodeng.2016.01.020
Comerlatto A, Voll FA, Daga AL, Fontana É (2021) Mass transfer in soybean oil extraction using ethanol/isopropyl alcohol mixtures. Int J Heat Mass Transfer 165:120630. https://doi.org/10.1016/j.ijheatmasstransfer.2020.120630
Stevanato N, Silva C (2019) Radish seed oil: ultrasound-assisted extraction using ethanol as solvent and assessment of its potential for ester production. Ind Crops Prod 132:283–291. https://doi.org/10.1016/j.indcrop.2019.02.032
Ferreira MC, Gonçalves D, Bessa LC, Rodrigues CE, Meirelles AJ, Batista EA (2022) Soybean oil extraction with ethanol from multiple-batch assays to reproduce a continuous, countercurrent, and multistage equipment. Chem Eng Process-Process Intensific 170:108659. https://doi.org/10.1016/j.cep.2021.108659
Tarazanov SV, Lukyanova VA, Ilin DY, Dorofeeva OV, Druzhinina AI, Pimenova SM (2022) Enthalpy of formation of 2-methyltetrahydrofuran: experimental and computational study. J Chem Thermodyn 165:106651. https://doi.org/10.1016/j.jct.2021.106651
Sicaire AG, Vian MA, Fine F, Carré P, Tostain S, Chemat F (2016) Ultrasound induced green solvent extraction of oil from oleaginous seeds. Ultrasond Sonochem 31:319–329. https://doi.org/10.1016/j.ultsonch.2016.01.011
Rodrigues SN, Mishra S, Sahu JK, Naik SN (2022) Effect of ultrasound-assisted extraction on efficiency, antioxidant activity, and physicochemical properties of sea buckthorn (Hippophae salicipholia) seed oil. LWT - Food Sci Technol 153:112386. https://doi.org/10.1016/j.lwt.2021.112386
Souza CLM, Lemos MCM, Sanches EA, Silva LS, Bezerra JA, Aguiar JPL, Campelo PH (2020) Improvement of the bioaccessibility of bioactive compounds from Amazon fruits treated using high energy ultrasound. Ultrason Sonochem 67:105148. https://doi.org/10.1016/j.ultsonch.2020.105148
Liu HM, Yao YG, Ma YX, Wang XD (2020) Ultrasound-assisted desolventizing of fragrant oil from red pepper seed by subcritical propane extraction. Ultrason Sonochem 63:104943. https://doi.org/10.1016/j.ultsonch.2019.104943
Moraes C, Anjos JL, Maruno M, Alonso A, Filho PR (2018) Development of lamellar gel phase emulsion containing baru oil (Dipteryx alata Vog.) as a prospective delivery system for cutaneous application. Asian J Pharm Sci 13:183–190. https://doi.org/10.1016/j.ajps.2017.09.003
Silva JS, Ferreira NBS, Asquieri ER, Damiani C, Asquieri EMDAR (2020) Chemical monitoring of baru (Dipteryx alata Vog.) pulp fermented beverage. Food Sci Technol 41:155–162. https://doi.org/10.1590/fst.14420
Pineli LLO, Carvalho MV, Aguiar LA, Oliveira GT, Celestino SMC, Assunção RBB, Chiarello MD (2015) Use of baru (Brazilian almond) waste from physical extraction of oil to produce flour and cookies. LWT Food Sci Technol 60:50–55. https://doi.org/10.1016/j.lwt.2014.09.035
Hartman L, Lago RC (1973) Rapid preparation of fatty acid methyl esters from lipids. Lab Pract 22:475–476
AOCS (2009) Official methods and recommended practices of the American Oil Chemist’ Society, 6th. AOCS, Champaign
Ulbricht TLV, Southgate DAT (1991) Coronary heart disease: seven dietary factors. Lancet 338:985–992. https://doi.org/10.1016/0140-6736(91)91846-M
Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. American Journal of Enology and Viticulture 16: 144–158. Available: https://www.ajevonline.org/content/16/3/144.short. Access: 14 march. 2023
Rodriguez-Amaya DB (2001) A guide to carotenoid analysis in foods; ILSI Press: Washington, DC, USA. Available: https://pdf.usaid.gov/pdf_docs/pnacq929.pdf. Access: 11 January. 2023
Zhu Y, Wilkinson KL, Wirthensohn MG (2015) Lipophilic antioxidant content of almonds (Prunus dulcis): a regional and varietal study. J Food Compos Anal 39:120–127. https://doi.org/10.1016/j.jfca.2014.12.003
Kozłowska M, Gruczyńska E, Ścibisz I, Rudzińska M (2016) Fatty acids and sterols composition, and antioxidant activity of oils extracted from plant seeds. Food Chem 213:450–456. https://doi.org/10.1016/j.foodchem.2016.06.102
Siddiqui SA, Pahmeyer MJ, Assadpour E, Jafari SM (2022) Extraction and purification of d-limonene from orange peel wastes: recent advances. Ind Crops Prod 177:114484. https://doi.org/10.1016/j.indcrop.2021.114484
Codex Alimentarius (2010) Fats, oils and related products. Food Agricult. Organization, Rome
Vicente J, Carvalho MG, Rojas EEG (2015) Fatty acids profile of Sacha Inchi oil and blends by 1H NMR and GC-FID. Food Chem 181:215–221. https://doi.org/10.1016/j.foodchem.2015.02.092
Hu B, Li Y, Song J, Li H, Zhou Q, Li C, Zhang Z, Liu Y, Liu A, Zhang Q, Liu S, Luo Q (2020) Oil extraction from tiger nut (Cyperus esculentus L.) using the combination of microwave-ultrasonic assisted aqueous enzymatic method - design, optimization and quality evaluation. J Chromatogr A 1:1627. https://doi.org/10.1016/j.chroma.2020.461380
Jesus SS, Filho RM (2020) Recent advances in lipid extraction using green solvents. Renew Sustain Energy Rev 133:110289. https://doi.org/10.1016/j.rser.2020.110289
Panadare DC, Rathod VK (2020) Process intensification of three phase partition for extraction of custard apple seed oil using microwave pretreatment. Chem Eng Process-Process Intensif 157:108095. https://doi.org/10.1016/j.cep.2020.108095
Rigane G, Yahyaoui A, Acar A, Mnif S, Salem RB, Arslan D (2020) Change in some quality parameters and oxidative stability of olive oils with regard to ultrasound pretreatment, depitting and water addition. Biotechnol Reports 26:4–9. https://doi.org/10.1016/j.btre.2020.e00442
Bakhshabadi H, Mirzaei H, Ghodsvali A, Jafari SM, Ziaiifar AM, Farzaneh V (2017) The effect of microwave pretreatment on some physico-chemical properties and bioactivity of Black cumin seeds’ oil. Ind Crops Prod 97:1–9. https://doi.org/10.1016/j.indcrop.2016.12.005
Polmann G, Teixeira GL, Santos PH, Rivera GÁ, Ibañez E, Cifuentes A, Block JM (2023) Chemical characterization of gurguéia nut (Dipteryx lacunifera Ducke) and press cake oil obtained by hydraulic pressing and supercritical extraction. Biomass Convers Biorefin 1:1–16. https://doi.org/10.1007/s13399-023-04042-x
Paucar LOC, Tobón JFO, Johner JCF, Meireles MAA (2021) A comparative and economic study of the extraction of oil from Baru (Dipteryx alata) seeds by supercritical CO2 with and without mechanical pressing. Heliyon 7:e05971. https://doi.org/10.1016/j.heliyon.2021.e05971
Ferreira MJA, Mota MFS, Mariano RGB, Freitas SP (2021) Evaluation of liquid-liquid extraction to reducing the acidity index of the tucuma (Astrocaryum vulgare Mart.) pulp oil. Sep Purif Technol 257:117894. https://doi.org/10.1016/j.seppur.2020.117894
Fetzer DL, Cruz PN, Hamerski F, Corazza ML (2018) Extraction of baru (Dipteryx alata vogel) seed oil using compressed solvents technology. J Supercrit Fluids 137:23–33. https://doi.org/10.1016/j.supflu.2018.03.004
Reis MÁ, Novaes RD, Baggio SR, Luiz A, Viana M, Cesar B, Salles C, Maris S, Rodrigues MR, Borges F, Paula DA (2018) Hepatoprotective and antioxidant activities of oil from Baru almonds (Dipteryx alata Vog.) in a preclinical model of lipotoxicity and dyslipidemia. Evid-Based Complement Alternat Med 11:8376081. https://doi.org/10.1155/2018/8376081
Serra JL, Rodrigues AMC, Freitas RA, Meirelles AJA, Darnet SH, Silva LHM (2019) Alternative sources of oils and fats from Amazonian plants: fatty acids, methyl tocols, total carotenoids and chemical composition. Food Res Int 116:12–19. https://doi.org/10.1016/j.foodres.2018.12.028
Kogawa NRDA, Arruda EJ, Micheletti AC, Matos MDFC, Oliveira LCS, Lima DP, Carvalho NCP, Oliveira PD, Cunha MDC, Ojeda M, Beatriz A (2015) Synthesis, characterization, thermal behavior, and biological activity of ozonides from vegetable oils. RSC Adv 5:65427–65436. https://doi.org/10.1039/c5ra02798e
Brasil RV, Cavallieri ÂLF, Costa ALM, Gonçalves MÁB (2011) Caracterização física e química do óleo de pequi exposto a diferentes condições de armazenamento. Jornal Da Ciência - SBPC 14. Available in <http://www.sbpcnet.org.br/livro/63ra/conpeex/pibic/trabalhos/renata_v.pdf>
Ponte FAF, Rodrigues JS, Malveira JQ, Filho JASR, Albuquerque MCG (2017) Avaliação físico-química dos óleos de babaçu (Orbignya speciosa) e coco (Cocos nucifera) com elevado índice de acidez e dos ácidos graxos (C6 a C16). Scientia Plenan13: 1–8. https://doi.org/10.14808/sci.plena.2017.085301
Figueiredo PS, Martins TN, Ravaglia LM, Alcantara GB, Guimarães RDCA, Freitas KDC, Hiane PA (2022) Linseed, baru, and coconut oils: NMR-based metabolomics, leukocyte infiltration potential in vivo, and their oil characterization. Are there still controversies? Nutrients 14:1161. https://doi.org/10.3390/nu14061161
Marques FG, Neto JRO, Cunha LC, Paula JR, Bara MTF (2015) Identification of terpenes and phytosterols in Dipteryx alata (baru) oil seeds obtained through pressing. Rev Bras 25:522–525. https://doi.org/10.1016/j.bjp.2015.07.019
Bezerra CV, Manoel A, Oliveira PD, Albuquerque D, Helena L (2017) Technological properties of amazonian oils and fats and their applications in the food industry. Food Chem 221:1466–1473. https://doi.org/10.1016/j.foodchem.2016.11.004
Pineli L, Oliveira G, Mendonça M, Borgo L, Freire É, Celestino S, Botelho R (2015) Tracing chemical and sensory characteristics of baru oil during storage under nitrogen. LWT-Food Sci Technol 62:976–982. https://doi.org/10.1016/j.lwt.2015.02.015
Polette CMV, Ramos PR, Gonçalves CB, Oliveira AL (2021) Determination of free fatty acids in crude vegetable oil samples obtained by high-pressure processes. Food Chem X 12:100166. https://doi.org/10.1016/j.fochx.2021.100166
Naebi M, Torbati M, Damirchi SA, Siabi S, Savage GP (2022) Changes in physicochemical properties of cold press extracted oil from Balangu (Lallemantia peltata) seeds during storage. J Food Compos Anal 107:104358. https://doi.org/10.1016/j.jfca.2021.104358
Alarcon RT, Gaglieri C, Lamb KJ, North M, Bannach G (2020) Spectroscopic characterization and thermal behavior of baru nut and macaw palm vegetable oils and their epoxidized derivatives. Ind Crops Prod 154:112585. https://doi.org/10.1016/j.indcrop.2020.112585
Gupta M (2017) Practical guide to vegetable oil processing, 2nd edn. Academic Press and AOCS Press, Lynnwood. https://doi.org/10.1016/B978-1-63067-050-4.00018-0
Ishak I, Hussain N, Coorey R, Ghani MA (2021) Optimization and characterization of chia seed (Salvia hispanica L.) oil extraction using supercritical carbon dioxide. J CO2 Utiliz 45:101430. https://doi.org/10.1016/j.jcou.2020.101430
Santos CS, Cunha SC, Casal S (2017) Deep or air frying? A comparative study with different vegetable oils. Eur J Lipid Sci Technol 119:1600375. https://doi.org/10.1002/ejlt.201600375
Musakhanian J, Rodier JD, Dave M (2022) Oxidative stability in lipid formulations: a review of the mechanisms, drivers, and inhibitors of oxidation. AAPS PharmSciTech 23:151. https://doi.org/10.1208/s12249-022-02282-0
Funding
This work was partially funded by the Coordination for the Improvement of Higher Education Personnel—Brazil (CAPES)—Financial Code 001. G.A.S. Martins received funding with the CAPES/Brazil no.: 88881.200497/2018–01, PROCAD-AM 1707/2018.
Author information
Authors and Affiliations
Contributions
Greice Folis Dagostin Santinoni: conceptualization, methodology, validation, formal analysis, original draft, investigation; Rômulo Alves Morais: methodology, validation, original draft, investigation, review and editing; Gabriela Fonsêca Leal: methodology, validation, formal analysis; Vinícius Soares dos Reis: methodology, validation, formal analysis; Glêndara Aparecida de Souza Martins: formal analysis, conceptualization, resources, supervision; Clarissa Damiani: conceptualization, resources, supervision, project administration, review and editing.
Corresponding authors
Ethics declarations
Ethical approval
Not applicable.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Santinoni, G.F.D., Morais, R.A., Leal, G.F. et al. Agroindustrial valorization of baru almond oil (Dipteryx alata) through sustainable techniques: a study on nutritional quality, oxidative stability, fatty acid, and tocopherol profile. Biomass Conv. Bioref. (2023). https://doi.org/10.1007/s13399-023-04578-y
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13399-023-04578-y