Abstract
In this study, Scenedesmus sp. ASK22’s growth, lipid productivity and nutrient removal capacity were examined in indoor bench-scale and outdoor pilot-scale experiments using different culture media. The possibility of producing biodiesel from Scenedesmus sp. ASK22 was explored, and the physicochemical parameters of the biodiesel produced were compared to those of Indian petro-diesel. The findings revealed that in an indoor bench-scale culture, the maximal biomass concentration can approach 3.44 g L−1, when compared to the outdoor pilot-scale (2.09 g L−1), using nutrients supplemented simulated dairy effluent (NSDE) as a culture medium. Moreover, as compared to the BG11 medium, the cost of NSDE medium was ~3–4.7-fold lower. Maximum N-NO3−1, P-PO4−3 and chemical oxygen demand (COD) removal efficiency obtained in indoor culture conditions was 99.19%, 95.78% and 95.00%, respectively, compared to that of 95.10%, 84.87% and 92.50%, respectively, for outdoor conditions using NSDE as Scenedesmus sp. ASK22 culture medium. C16/C18 methyl esters make up most of the Scenedesmus sp. ASK22–derived biodiesel. Finally, the qualities of the produced biodiesel and blends were within the approved biodiesel standard specification, showing that Scenedesmus sp. ASK22 culture in dairy effluent has a lot of potential for scaling up for high-quality biodiesel production and dairy effluent treatment. The results demonstrated the potential of Scenedesmus sp. ASK22 to become feedstock of an integrated wastewater treatment and superior quality biodiesel production.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Algae biomass has the potential to become a major biofuel crop in the future, but lower production costs are required to make the product financially viable. Microalgae biofuel has as an alternative energy source, as microalgae cells contain carbohydrate (30–60%) and lipid (10–70%) that can be used for bioethanol and biodiesel production, respectively [1,2,3,4]. Additional research and development of algal production systems using wastewater as a nutrient source is required to establish a commercially viable algae biofuel. The wastewater is considered a sustainable and suitable medium for microalgae cultivation [5,6,7,8]. Using wastewater for microalgal cultivation serves the dual purpose of supplying nutrients and minimizing the requirements for fresh water along with wastewater treatment [6,7,8]. In this context, the Indian dairy industry generates a significant amount of nutrient-rich wastewater. Milk consumption and the number of dairy industries in India have expanded in recent years; as a result, dairy wastewater generation in India is increasing accordingly [8,9,10]. The dairy industry, like others, is struggling to keep up with rising wastewater handling and disposal expenses. Existing traditional water treatment systems, on the other hand, are frequently overloaded beyond design flows, resulting in inadequate operation and an undue financial drain that impacts the company’s bottom line [9, 11]. Microalgae are exceptionally useful to reduce the amounts of inorganic and organic ingredients in dairy wastewater as they can use both wastewater contaminants for their growth [6]. Many research groups use different microalgae for treating dairy wastewater to accomplish both wastewater treatment and biomass production [2, 6,7,8,9,10,11,12,13,14,15,16,17,18].
The wastewater is considered a sustainable and suitable medium for microalgae cultivation [5,6,7,8]. Using wastewater for microalgal cultivation serves the dual purpose of supplying nutrients and minimizing the requirements for freshwater along with wastewater treatment [2, 8, 9]. Microalgae culture in wastewater is becoming more popular. The majority of this research is done in a lab setting with artificial light, skilled technicians and carefully monitored growing conditions. Furthermore, the transfer of outcomes from laboratory study to outdoor production requires more research [2, 8,9,10,11,12,13]. The literatures on the combination of nutrient removal and microalgae cultivation in raw dairy wastewater (RDW) in outdoor pond level (without sterilizing or disinfecting) are scarce. Moreover, very few reports were available and they used pre-treated RDW viz. supplementation of nutrients and initial pH adjustment, which enhance not only the microalgal growth but also nutrient remediation too. This is particularly essential because the development of cost-effective production techniques is critical for industrial-scale production of microalgae-based biofuel feedstock [19, 20]. In this study, authors assess the scale-up potential of Scenedesmus sp. ASK22 cultivation in RDW for cost-effective production system to facilitating industrial-scale production of microalgae biomass for algal-based biodiesel and wastewater treatment. Finally, for cost-effective microalgae-based biodiesel commercialization, it is required to determine the difference in growth characteristics, nutrient removal capability between indoor bench-scale and outdoor pilot-scale cultures. Moreover, it is also important to reveal the properties of biodiesel derived from microalgal biomass. However, there is currently a lack of information from the literature.
The objective of this study is to is to evaluate the feasibility of simultaneous treatment of DWW and production microalgae-based biodiesel at an outdoor pilot scale using various culture media (viz. standard BG11, simulated dairy wastewater (SDE) and nutrient-supplemented simulated dairy effluent (NSDE)), site without disinfection, chemical pre-treatment, extra carbon source addition or pH control, make a distinction in microalgae growth characteristics and contaminant removal efficacy between indoor bench-scale and outdoor pilot-scale cultivations, elucidate the relative proportion fatty acid profile of Scenedesmus sp. ASK22 cultivated in different media (standard blue green algae medium (BG11), SDE and NSDE) grown under outdoor condition and also reveal the fuel properties of the Scenedesmus sp. ASK22 derived biodiesel.
2 Materials and methods
2.1 Microalgae strain, maintenance, cultivation and characterization of different media
The eukaryotic unicellular green algae Scenedesmus sp. ASK22 (isolated from dairy effluent treatment plant Allahabad) was used during this study [8]. The microalgae were maintained in sterilized BG11 medium in 1-L Erlenmeyer flask [21]. The culture conditions of Scenedesmus sp. ASK22 were as follows: continuous illumination ~ 55 μmol m−2 s−1 (light intensity) and 28 ± 2 °C (temperature) in recurrent stirring to prevent the settling of culture [2, 8, 9]. The exponentially grown culture inoculated into four indoor bench-scale air lift reactors (iALR, 3L working volume) containing three different sterile media viz. BG11, SDE and NSDE and a control experiment run short of inoculation. The standard BG11 medium composed of (g L−1) NaNO3 (1.5), MgSO4·7H2O (0.075), K2HPO4 (0.04), CaCl2·2H2O (0.036), Na2CO3 (0.02), C6H8O7 (0.006), C6H8FeNO7 (0.006) and EDTA (0.001) and micronutrient solution (1 mL L−1 that consists of (g L−1) H3BO3 2.86, MnCl2·H2O 1.81, ZnSO4·7H2O 0.222, CuSO4·5H2O 0.079, Na2MoO4·2H2O 0.39 and Co(NO3)2·6H2O 0.049). The SDE and NSDE were prepared as per Pandey et al. [2]. The culture medium’s pH was set to 7.18 ± 02 at the start of each experiment [2]. The initial physicochemical characteristics of BG11, SDE and NSDE were taken as per our previous study Pandey et al. [2] as shown in Table 1. After inoculation, the Scenedesmus sp. ASK22 culture broth was continuously blown with air via the bottom of the iPBRs (Fig. 1A) and the temperature (28 ± 2 °C) and illumination (55 μmol m−2 s−1) were kept constant [2, 8, 9].
Schematic diagram of A indoor photobioreactor pilot-scale (iPBR) and B outdoor pond photobioreactor (not drawn to scale) used for Scenedesmus sp. ASK22 cultivation. Key: 1, air; 2, air pump; 3, air filter; 4, sampling port; 5, valve; 6, air sparger; 7, white fluorescent tubes; 8, sunlight; 9, air filter; 10, air pump
For outdoor cultivation, the seed culture was prepared and transferred to outdoor pilot-scale open ponds in Prayagraj (UP) India. The experiments were performed during Autumn. The open pond contained 300 L (working volume) of different microalgal culture media (BG11, SDE and NSDE). The inoculum size (~0.208 g L−1) was kept constants for all outdoor treatment. All the trials were carried out in triplicate, one after the other.
2.2 Scenedesmus sp. ASK22 growth analysis
The dry cell weight (DCW) of microalgae is shown to have correlated with optical density (OD) of an algal cell suspension recorded at a wavelength of 680 nm, which would be correlated with chlorophyll adsorption [2]. A linear relationship between DCW and OD680, as shown in Eqs. (1–3), was used to determine Scenedesmus sp. ASK22 DCW in BG11, SDE and NSDE, respectively. The following equation was used to calculate the growth rate (μ, day−1), doubling time (Td, day) and biomass productivity (BP) (Eqs. (4–6)) [8, 22].
where Xt is the DCW at time t (day); X0 is the DCW at time 0 (day); ∆t is the time interval (day) and BP is the biomass productivity (mg L−1 day−1).
2.3 Determination of physiochemical characterization and nutrient remediation
Twenty millilitres of Scenedesmus sp. ASK22 suspension was withdrawn from each iPBRs and open ponds for the determination of residual COD (chemical oxygen demand), N-NO3−1 (nitrogen) and TP (phosphorus) as per experiment design. The algal suspension was first centrifuged at 5000 rpm for 10 min, and the filtrates were kept for future analysis. Finally, COD, N-NO3−1 and TP concentrations of the supernatant were measured as per standard analytical procedure [23]. Nutrient’s removal rate (mg L−1 day−1) and nutrients removal percentage were calculated using Eqs. (7–8), respectively [8].
where N0, Nt and ∆t are the initial nutrients load at time 0 (day), nutrients load at time t (day) and time interval (day), respectively.
2.4 Lipid content and FAME analysis
Scenedesmus sp. ASK22 cells were collected and freeze dried at the end of the experiment after centrifugation (10,000 rpm, 10 min), for further characterizations. A modified technique based on Pandey et al. was used to calculate total lipid content. In a clean screw-top glass tube, freeze-dried algae biomass (100 mg) was weighed, and a 1:2 chloroform-methanol (v/v) combination (10 mL) was added [8]. The tube was incubated overnight at 27 °C with 100 rpm shaking after being ultrasonicated for 1 h. The extraction mixture was sonicated again for 30 minutes the next day with an additional aliquot of chloroform (2 mL) added. The algal biomass leftover residues were removed by passing the suspension through glass fibre filter. To separate the chloroform and aqueous methanol layers, the filtrate was transferred to another clean screw-top glass tube containing 2 mL of water. Following centrifugation, a clear biphasic system was built, and the lower lipid containing organic layer was carefully removed, washed with NaCl solution (5 mL, 5% w/w), evaporated in a drying oven at 50 °C and the lipid gravimetrically measured. The following equations were used to compute the lipid content, lipid yield and lipid productivity (Eqs. 9–11).
Fatty acid compositional analysis was performed in two steps, including the preparation of fatty acid methyl esters (FAMEs) and gas chromatography-mass spectrometry (GCMS) analysis. The FAMEs were made using acidic transesterification technique as described by Pandey et al. [9]. The dried Scenedesmus sp. ASK22 (0.1 g) were placed in a 25-mL screw-top glass vial with a mixture of CH3OH, Conc. H2SO4 and CHCl3 (4.25:0.75:5; 10 mL), and tube was incubated at 90 °C in water bath for 2 h. The chloroform layer containing FAMEs was carefully collected when the reaction was completed and sent to GCMS analysis. The GC was equipped with a flame ionization detector and a 5% (phenyl)-methylpolysiloxane column (30 m × 0.25 mm × 0.25 μm) [2, 8, 9]. The temperature of oven was set to 100 °C for 3 min., then gradually increased to 200 °C at a pace of 4 °C per min. Finally, the temperature was raised to 250 °C at a rate of 3 °C per min for 5 min.
2.5 Biodiesel characterization and FTIR spectroscopy
The biodiesel derived from Scenedesmus sp. ASK 22 oil transesterification was examined using established methods of analysis for petroleum products, ASTM standard methodologies, to estimate its fuel characteristics. The blends of petro-diesel with various percent (v/v) of derived biodiesel were generated, and their physicochemical characteristics values were compared to biodiesel standards in Europe and the USA (EN-14214 and ASTM D6751-12) [24,25,26]. Fourier transform infrared spectroscopy (FTIR) was used to determine biodiesel content in reaction mixture to monitor transesterification reaction and biodiesel-petro diesel blends, respectively. All the samples’ spectra were measured in the 400–4000-cm−1 region, with a scan resolution of 4 cm−1 and a scan rate of 16 cm min−1 [24].
2.6 Statistical analysis
All the experiments in this study were done twice, and the average results were given. EXCEL and Origin® 8.5 for windows were used to generate the results.
2.7 Comparison of media costs
The costs of the media NSDE for growth for cost-benefit were compared with the costs of the original BG11 calculated for a volume of 1 m3. In addition, the cost of producing 1 kg of dry Scenedesmus sp. ASK22 biomass was estimated from the cost of the medium and the average dry weight of each treatment. Prices were obtained from local suppliers/vendors.
3 Results and discussion
3.1 Scenedesmus sp. ASK22 biomass and lipid productivity
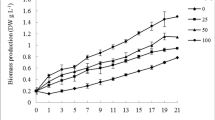
Although the main focus in the field of using wastewater for microalgae culture is to evaluate the viability of microalgae cultivated in wastewater for inorganic nutrient removal, as a sustainable process, it is undoubtedly important to consider utilisation of the produced biomass after wastewater treatment. The changes in biomass concentration throughout the culture period (12 days) are illustrated in Fig. 2. It is easy to see that the Scenedesmus sp. ASK22 adapts quickly in the indoor as compared to outdoor culture conditions with the three different tested culture media (BG11 (control), SDE, NSDE)). After 1 day of sustained growth, the biomass of Scenedesmus sp. ASK22 increased dramatically, and this value was maintained throughout the culture period. Finally, the maximum biomass concentration reached 3.44 g L−1 and 1.93 g L−1 for NSDE and SDE, which were 3.91- and 2.19-fold higher as compared to control medium, respectively, under indoor culture conditions (Table 2). The remarkable initial 2-day lag phase and prolonged steady growth were observed for SDE and BG11 under outdoor pilot-scale cultivation condition, whereas Scenedesmus sp. ASK22 showed continued steady growth in the NSDE medium. The maximum biomass yield achieved for NSDE, SDE and BG11 media under outdoor pilot-scale culture reached 2.09 g L−1, 1.32 g L−1 and 0.58 g L−1, respectively, after 12 days of batch cultivation. The Scenedesmus sp. ASK22 showed 3.60- and 2.28-fold higher growth in NSDE and SDE, respectively, as compared to control mediums (BG11) under outdoors. Similar fold increase in growth was also observed in indoor cultivation conditions. The controlled indoor environment can be contributed to the increased biomass concentration (from 0.88 to 3.44 g L−1) and growth rate (varies from 0.22 to 0.34 day−1) as compared to the outdoor cultivation conditions. The outdoor cultivation of microalgae for nutrient removal from wastewater may be affected by a number of abiotic and biotic parameters, including algal species, nutrients, location, season, temperature and irradiance, amount of rain and/or wind and turbidity. The various growth parameters must be controlled in order to maximise algal production. Nutrients can be regulated by introducing wastewater into the culture as a medium. Light intensity, temperature and evaporation, on the other hand, are affected by illuminance, region and season and hence cannot be controlled during outdoor cultivation. The temperature (15 to 31 °C) and light intensity (123 to 1125 μmol m−2 s−1) vary greatly in outdoors environment (Fig 3). The lipid content and lipid productivity of Scenedesmus sp. ASK22 are observed in Table 2 under various culture media (BG11, SDE and NSDE). The lipid content varies from 24.86 to 36.12 (% wt.) and 22.20 to 32.75 (% wt.) under indoor bench scale and outdoor pilot scale, respectively, using different culture media. The lipid accumulation and yield was maximum in NSDE medium as compared to standard BG11 and SDE under controlled indoor and outdoor conditions. These findings contradict each other, indicating that increased nitrogen in DE can result in higher lipid content and lipid production in microalgae. It is not unexpected, however, because the optimal condition range of N-NO3−1 and P-PO4−3 of response patterns for lipid production and lipid productivity is the same as the response pattern for biomass production and biomass productivity. This might suggest that biomass production and productivity are the most important factors influencing lipid production and productivity. However, the lipid production is also affected by the microalgal species utilised. Khan et al. evaluated the feasibility of a newly isolated cyanobacterium Trichocoleus desertorum in low-cost medium under controlled light, pH and nutrients, which achieved a maximum biomass and lipid productivity of 0.86 g L−1 day−1 and 0.256 g L−1 day−1, respectively [27]. Shahid et al. studied the impact of wastewater cultivation on biosynthesis of high-value metabolites by newly isolated blue green algae and demonstrated the biomass accumulated at 26–36% carbohydrates, 15–28% proteins, 38–43% lipids and 6.3–9.5% phycobilins [28]. Among all these tested culture mediums, Scenedesmus sp. ASK22 showed greater adaptability to NSDE as compared to others (SDE and BG11), with both culture conditions (indoors and outdoors). These results are comparable to the previously reported literature as shown in Table 3 [2, 8, 9, 13,14,15,16,17]. However, further optimization of indoor and outdoor culture conditions could significantly enhance biomass and lipid yield.
3.2 Pollutant (NO 3 −1 , TP and COD) stripping
Carbon, nitrogen and phosphorous are three essential nutrients required for microalgal biomass production. In order to use wastewater as a growth medium for Scenedesmus sp. ASK22 growth, it should possess suitable nutrient profile as shown in Table 1. Microalgal cultivation requires light energy and nutrients, mostly composed of carbon (organic/inorganic), nitrogen (NH4+, NO2− and NO3−), phosphorus (PO43− and P2O74−), macronutrients and micronutrients [9]. Nutrients play a major role in biomass enrichment by facilitating cell growth, maintenance and synthesis of different kinds of reserve metabolites. Microalgae mostly uptake soluble inorganic carbon, and this may change the pH of culture media used [33]. Carbon dioxide (CO2) first gets absorbed by aqueous media, where it forms carbonic acid (H2CO3), which dissociates into bicarbonates and hydrogen (H+1) ions. These bicarbonates are utilized by microalgae for their growth and lipid biosynthesis. Moreover, the bicarbonate ions dissociate into –CO3−2 and H+1 and, during the cultivation, the concentration of bicarbonates decreases, whereas carbonate ions increase which further combines with water to form hydroxide (–OH−1) ions. These –OH−1 slowly increase pH of the culture media [33]. During nitrification process, ammonium (–NH4+1) converts into nitrates (–NO3−1) and produces proton (H+) and microalgae consume oxygen released during nitrification, for their metabolic activity. Nitrogen plays an important role in lipid accumulation in microalgae, and nitrogen starvation has been found the only feasible and economically viable techniques to enhance lipid productivity [18]. Phosphorus is used to support the production of nucleic acids, lipid and proteins. The phosphates (PO43− and P2O74−) get integrated in to energy input organic compound (NADPH/ATP) via phosphorylation.
The variation of nitrate (N-NO3−1), total phosphorus (TP) and COD concentration in BG11, SDE and NSDE for 12 days of batch cultivation (indoor and outdoor conditions) are shown in Fig. 4A–C, respectively. From Fig. 4A, it is observed that the nitrate removal was continuous up to 4 days for all mediums (BG11, SDE and NSDE) indoors and remediate more than 96% nitrate. Afterwards, nitrate concentration does not decrease significantly, whereas under outdoor culture conditions, the nitrate remediation rate was slower and prolonged up to 6th day in NSDE and SDE media and 8th day in BG11 medium. Scenedesmus sp. ASK22 removed more than >99% nitrate at the end of day 12 in indoor culture conditions for all treatment (Table 4; Fig. 4B) which is 4–14% higher as compared to outdoor pilot-scale cultivation. The maximum nitrate removal rate (22.73 mg L−1 day−1) was observed indoors for NSDE and was remarkably close to NSDE treatment outdoors (21.73 mg L−1 day−1). The initial TP concentration and cultivation conditions greatly influenced the TP removal rate and efficiency. The maximum TP removal was observed in controlled indoors for NSDE (95.78%, 11.84 mg L−1 day−1) followed by BG11 (93.46%, 5.05 mg L−1 day−1) and SDE (81.00%, 5.59 mg L−1 day−1) (Table 4), whereas, in outdoor open pond cultivation, the maximum TP removal efficiency was found in NSDE medium (84.87%, 10.5 mg L−1 day−1) followed by SDE (71.46 %, 4.94 mg L−1 day−1) and BG11 (67.79 %, 3.66 mg L−1 day−1) (Table 2). High TP removal efficiency in wastewater may be due to the presence of organic carbon, which enhances the microalgal growth in wastewater mixotrophically as compared to control medium (BG11). Fig. 4B shows the variation in TP concentration during the 12-day batch cultivation indoors and outdoors. The sharp reduction (%) in TP was observed in the initial 4 days in indoor cultivation for all treatment. Afterwards, there was no or less TP remediation observed, whereas in outdoor cultivation conditions, the TP removal rate was slower and initial fast reduction was observed in initial 6 days. Similarly, for COD removal efficiency, the Scenedesmus sp. ASK22 performed better under controlled indoor conditions as compared to outdoor conditions. Scenedesmus sp. ASK22 showed comparably better adaptability in both indoor and outdoor culture conditions (Fig. 4C; Table 4). In indoor photo-bioreactors, the final pH exceeded ~11, whereas in outdoor cultivation conditions, the final pH reached ~8 to 9, during Scenedesmus sp. ASK22 cultivation in different media (data not shown), which may be due to algal photosynthesis as a result of CO2 uptake and the absence of pH control. The pH of the culture media is determined by photosynthetic activity, algal respiration, alkalinity and ionic composition of the wastewater, as well as the activity and kind of metabolisms (autotrophic, heterotrophic or mixotrophic) [27]. Similar kind of results was also observed by Shahid et al. During algal cultivation in urban wastewater, its pH was used to adjust from pH 8.0 to 11, offering contamination-free cultivation and flotation-based easy harvesting [28]. The results revealed that the phyco-remediation rate significantly depends on the culture composition, pH of the culture media and nutrient (NO3−1 and PO4−3) concentration.
3.3 FAME composition and FTIR analysis
FAMEs are the primary component of biodiesel, and their chemical structure has a significant impact on the qualities of biodiesel. Thus, it is critical to identify the unique profiles of fatty acids. Fig. 5 illustrates the relative proportion FAME of Scenedesmus sp. ASK22 cultivated in different media (BG11 (control), SDE and NSDE) grown under outdoor condition. FAMEs are the primary component of biodiesel, and their chemical makeup influences the qualities of the fuel. The fatty acids C8:0 (octanoic acid), C12:0 (dodecanoic acid), C14:0 (myristic acid), C16:0 (palmitic acid), C16:1 (methyl palmitoleate (cis-9)), C17:0 (margaric acid), C17:1 (methyl heptadecanoate(cis-10)), C18:0 (stearic acid), C18:1 (vaccenic acid), C18:3 (γ-linolenic acid), C20:0 (arachidic acid), C20:5 (eicosapentaenoic acid), C22:0 (behenic acid), C22:1 (erucic acid) and C24:0 (lignoceric acid) observed, in considerable amount. Scenedesmus sp. ASK22 grown in outdoor condition has similar FAME profile. Oleic acid (44.4–49.7% wt.) was found to be the prominent fatty acid followed by palmitic acid (19.5–23.7% wt.), linoleic acid (5.8–7.1% wt.), stearic acid (5.8-6.8% wt.), palmitoleic acid (3.5–4.3% wt.) and linolenic acid (3.2–3.6%), along with other fatty acids that ranged from 0.1% to 1.8% wt. The total saturated fatty acid was ~38% wt. in culture grown in BG11 medium and followed by culture grown in SDE and NSDE (~34 % wt.). [34]. The monounsaturated fatty acid (MUFA) accounts ~55 % wt. followed by ~52 wt. and ~50% wt., in Scenedesmus sp. ASK21 biomass grown in NSDE, SDE and BG11, respectively. In addition, the Scenedesmus sp. ASK22 biomass also contain more than 10% wt. of polyunsaturated fatty acids (PUFA) viz. C16:3, C18:2, C18:3 and C20:5. The FTIR spectra of diesel, biodiesel and biodiesel blends are shown in Figure 6. The aliphatic hydrocarbon is the main component of petro-diesel, whose chemical structure is like the long hydrocarbon chains of biodiesel. They all have bending type vibrations in the low energy and frequency ranges of the spectrum (550–900 cm−1), and they are all double bonded (=C–H). They are the unsaturated bonds in fatty acids methyl esters in biodiesel, such as methyl oleate and methyl linoleate. The methylene functional group in biodiesel is indicated by the specific group at 721.79 cm−1, which was overlapped by >C–H groups. It demonstrates that biodiesel is made up of long-chain aliphatic molecules. The peaks in the spectrum between 1015 and 1169 cm−1 suggest stretching vibrations of C=O, C–O–C and –OCH3. Furthermore, the existence of C=O groups in the biodiesel is related to the features peak at wavenumber 1741.18 cm−1, which is the strongest in the spectrum. Asymmetric and symmetric stretching vibrations of alkane (C–H) groups are indicated by the peaks at 2854.65 and 2923.89 cm−1, respectively. They could be methyl (–CH3) or methylene groups in the biodiesel’s ester chains. Complete trans-esterification is indicated in biodiesel by the absence of a peak greater than 3000 cm−1 corresponding to the –OH of carboxylic acid. Biodiesel has multiple overlapping peaks in the region of 1000–1300 cm−1 and 1741.18 cm−1, which are not found in diesel oil. There are many studies that use FTIR to analyse the composition of biodiesel. The research was carried out by Shohaimi and Marodzi [35]. It highlighted that the ester portion exists at the peak in biodiesel with a range of 1725 to 1700 cm−1. The ester groups will show the absorption peak between 1125 and 1095 cm−1, according to Farooq et al. [36], who obtained the peak at 1118.45 cm−1 and 1015.18 cm−1. The obtained range value is very identical, with maxima of 1117.65 cm−1 and 1030.06 cm−1. As a result, it is possible to deduce that the biodiesel developed in this investigation contains an ester component. Aside from that, biodiesel absorption peaks are usually seen around 3008.91 cm−1, 2925.76 cm−1, 2855 cm−1, 1743.54 cm−1, 1461.48 cm−1, 1360.75 cm−1, 1171.41 cm−1 and 722.75 cm−1. Most of the values provided by Qiu et al. [37] are nearly identical to the peak found in our investigation. After the transesterification process, the methyl peak of O–CH3 reduced [35,36,37]. The transesterification process in this work exhibits the same phenomenon, with the absorption peak for O–CH3 in biodiesel produced being lower than in unreacted oil. In contrast, according to Goli and Sahu [38], the peak for methylene (O–CH2) and methyl (–CH3) decreases as the transesterification process progresses. The transesterification process in our work exhibits the same phenomenon, with the absorption peak for O–CH3 in biodiesel. As a result, this pattern of peaks confirms the presence of the triglyceride transesterification process into biodiesel fuel [35, 36]. The values, or shape, of the FTIR spectra absorption for conventional diesel are considerably different from the shape of the FTIR spectra absorption for biodiesel generated, as shown in Fig. 6. This could be since the basic materials used to make biodiesel are vastly different from those used to make regular diesel. Diesel was produced by distilling crude heavy oil, which is a non-edible oil, at specific temperatures; in contrast, biodiesel was produced naturally from the major source, which is oil and fats from plants, which can be considered as edible oil.
3.4 The physicochemical properties of the microalgal biodiesel and their blends
The biodiesel developed was assessed for its physicochemical characteristics compared to Indian petro-diesel standard, American standards of biodiesel blends (ASTM D7467) and European standard of Biodiesel (EN14214). Table 5 illustrates the properties of microalgae biodiesel (B100) and its blend (20% and 5% (biodiesel/petro-diesel; v/v). The data indicates that the density value follows increasing trends with blending (density increases with increasing volume percentage of biodiesel). A significant property is the density at 15 °C since it affects the fuel's atomization capacity [24, 39]. The density of microalgal biodiesel blends was 868.5 kg m−3 (B100), 845.75 kg m−3 (B20) and 863.47 kg m−3 (B5) as compared to that of Indian petro-diesel (815–845 kg m−3) and biodiesel. The density of the biodiesel produced in the experiment was more or less than that of IS 15607, ASTM D6751-12, EN 14214 and biodiesel produced by algae consortium and Spirulina platensis [40, 41]. In an interesting study, the team of Islam et. al. reported that the density of algal biodiesel varies from 810 to 910 kg m−3 [42]. That may be due the difference in microalga strain, culture medium and FAME composition [43]. The higher the carbon chain length of the methyl esters, the higher is the density of the biodiesel, which will decrease with the increasing number of double bonds. In this study, the FAME composition revealed the presence of C14:0, C16:0, C18:0 and C18:1 and C18:3 was detected in biodiesel derived from Scenedesmus sp. ASK22 cultivated in NSDE under outdoor conditions (Fig. 5). Similarly, the density of biodiesel was derived from Chlorella protothecoides (836 kg m−3), Ankistrodesmus falcatus (820 kg m−3), Ankistrodesmus fusiformis (820 kg m−3), Scenedesmus obliquus (830 kg m−3) and Botryococcus terribilis (820 kg m−3), which is remarkably less than Scenedesmus sp. ASK22 biodiesel and their blends (845–869 kg m−3) [42,43,44,45]. The kinematic viscosity of microalgal biodiesel and biodiesel blends varies from 3.25 to 4.27 cSt which meet the required value for biodiesel, which must be between 1.9 and 6.0 cSt. That suggested superior injection and atomization efficiency of the microalgal biodiesel and offered better protection and lubrication for the moving part of an Engine [41, 46]. The calorific value (CV) of biodiesel and blends varies from 43.17 to 44.85 MJ kg−1 which is higher than that of the minimum value (32.9 MJ kg−1) suggested by international standard (EN14214) for biodiesel and another popular biodiesel like palm and Jatropha [41]. The CV of any fuel plays an important role as higher CV indicates higher power generation to run an engine [41]. The cetane number (CN) diesel fuel cetane number is a relative measure of the delay in ignition (ID) time [47]. The lower the CN, the higher the ID time and vice versa. The microalgal diesel and its diesel blend were characterized by higher CN (56–63), This will lead to improved combustion efficiency and better engine efficiency. The rise in saturated FAMEs and the length of the chain increases the CN of biodiesels [47]. The flash point (FLP) and fire point (FP) of produced biodiesel and blends vary from 135 to 158 °C and 146 to 170 °C, respectively, which are comparably higher than that recommended by Indian petro-diesel fuel (35–66°), biodiesel blends (>52 °C) and European standard of biodiesel (>101°C). The higher FLP and FP of produced biodiesel and blends reduce the chance of unexpected fire hazard. The cold flow characteristics including pour point (PP; −11 °C) and cloud point (CP; −3 °C) of produced biodiesel have lower values from the permissible limit. This indicates better cold flow characteristics which are suited for cold conditions. The carbon residue of the produced biodiesel and blends varies from 0.012 to 0.027% wt. which is remarkably close to the recommended value of carbon residue (<0.05%) [47]. All the microalgae biodiesel properties were measured, and its blend is appropriate in compliance with the permitted requirements set by Indian petro-diesel and international blend standards and can be used in an unmodified CI engine. So, it can be ranked as realistic fuel and as a sustainable alternative to petro-diesel.
3.5 Comparison of media costs
The Scenedesmus sp. ASK22 biomass yield and cost (kg m−3 and USD kg−1, respectively) obtained in both media BG11 and NSDE were 0.88 (1.92) and 3.44 (0.41), respectively, under controlled PBR, whereas 0.58 (2.42) and 2.09 (0.81), respectively, under outdoor pond scale. The cost analysis showed that the replacement of the standard BG11 medium with the NSDE (Pandey et al. 2019) represented an average cost reduction of Scenedesmus sp. ASK22 dry biomass 4.5- and 2.98-fold grown in iPBR and outdoor pond scale, respectively. Nevertheless, the simultaneous reduction of freshwater demand, nutrient sequestration from DE and fix atm. CO2 (6.2 kg and 3.8 kg under indoor and outdoor conditions, respectively) as well during their cultivation in the NSDE medium. This makes the production of Scenedesmus sp. ASK22 biomass more viable, especially for applications that require low-cost biomass, such as feed for aquaculture and biodiesel production.
4 Conclusions and future prospects
In both indoor bench-scale and outdoor pilot-scale environments, this study clearly reveals the differences in integrating nutrients removal from different growing mediums (BG11, SDE and NSDE) and biodiesel generation. NSDE found to be a promising growth medium as compared to SDE and BG11, which can significantly decline freshwater dependency. Scenedesmus sp. ASK22 is regarded as a promising strain capable of producing better biomass and lipid yields as well as superior nutrient remediation. These findings show that DE (SDE and NSDE) can be employed as a growth medium for long-term biofuel feedstock production in both controlled indoor and outdoor pilot size facilities. Scenedesmus sp. ASK22 biodiesel fuel qualities were found to be in good compliance with ASTM D6751 and EN1424 requirements in this investigation. As a result, it may be acceptable and appropriate for diesel engines. The created mix also has good qualities that are equivalent to Indian petro-diesel. This analysis validated the production of ester by finding it at absorption peaks of 1745.27 cm−1, 1117.65 cm−1 and 1030.06 cm−1. The cost ($ kg−1) of Scenedesmus sp. ASK22 biomass grown in NSDE was 2.98–4.5-fold lower as compared to commercially available BG11 medium. Outdoor results were promising, though growth was lower than at bench scale due to uncontrolled meteorological conditions. Thus, the results from the present work showed improved production of Scenedesmus sp. ASK22 for commercial scale and reducing the medium costs as well without prejudicing the biomass quality. However, additional research for performing an algae-based wastewater treatment process is still required to address future challenges such as technical and economic feasibility on a large outdoor scale, optimization of hydraulic retention time, contamination control and harvesting of algae biomass and evaluation of the potential challenges for the loss of algal biomass productivity.
References
Stephens E, Ross IL, Mussgnug JH, Wagner LD, Borowitzka MA, Posten C, Kruse O, Hankamer B (2010) Future prospects of microalgal biofuel production systems. Trends Plant Sci 15:554–564. https://doi.org/10.1016/j.tplants.2010.06.003
Pandey A, Srivastava S, Kumar S. (2019) Sequential optimization of essential nutrients addition in simulated dairy effluent for improved Scenedesmus sp. ASK22 growth, lipid production and nutrients removal. Biomass Bioenergy 128:105319 https://doi.org/10.1016/j.biombioe.2019.105319
Mondal M, Goswami S, Ghosh A, Oinam G, Tiwari ON, Das P, Gayen K, Mandal MK, Halder GN (2017) Production of biodiesel from microalgae through biological carbon capture: a review. 3 Biotech 7(2): 1–21. https://doi.org/10.1007/s13205-017-0727-4
Milano J, Ong HC, Masjuki HH, Chong WT, Lam MK, Loh PK, Vellayan V (2016) Microalgae biofuels as an alternative to fossil fuel for power generation. Renew Sustain Energy Rev 58:180–197. https://doi.org/10.1016/j.rser.2015.12.150
Baldev E, Mubarakali D, Saravanakumar K, Arutselvan C, Alharbi NS, Alharbi SA, Sivasubramanian V, Thajuddin N (2018) Unveiling algal cultivation using raceway ponds for biodiesel production and its quality assessment. Renew Energy 123:486–498. https://doi.org/10.1016/j.renene.2018.02.032
Shahid A, Malik S, Zhu H, Xu J, Nawaz MZ, Nawaz S, Asraful Alam Md, Mehmood MA (2020) Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci Total Environ 704:135303 https://doi.org/10.1016/j.scitotenv.2019.135303
Gupta S, Pawar SB, Pandey RA (2019) Current practices and challenges in using microalgae for treatment of nutrient rich wastewater from agro-based industries. Sci Total Environ 687:1107–1126. https://doi.org/10.1016/j.scitotenv.2019.06.115
Pandey A, Srivastava S, Kumar S (2019) Isolation, screening, and comprehensive characterization of candidate microalgae for biofuel feedstock production and dairy effluent treatment: a sustainable approach. Bioresour Technol 293:121998. https://doi.org/10.1016/j.biortech.2019.121998
Pandey A, Srivastava S, Kumar S (2020) Development and cost-benefit analysis of a novel process for biofuel production from microalgae using pre-treated high-strength fresh cheese whey wastewater. Environmental Science and Pollution Research, 27(19):23963–23980. https://doi.org/10.1007/s11356-020-08535-4
Daneshvar E, Zarrinmehr MJ, Hashtjin AM, Farhadian O, Bhatnagar A (2018) Versatile applications of freshwater and marine water microalgae in dairy wastewater treatment, lipid extraction and tetracycline biosorption. Bioresour Technol 268:523–530. https://doi.org/10.1016/j.biortech.2018.08.032
Carvalho F, Prazeres AR, Rivas J (2013) Cheese whey wastewater: characterization and treatment. Sci Total Environ 445–446:385–396. https://doi.org/10.1016/j.scitotenv.2012.12.038
Asadi P, Rad HA, Qaderi F (2019) Comparison of Chlorella vulgaris and Chlorella sorokiniana pa.91 in post treatment of dairy wastewater treatment plant effluents. Environ Sci Pollut Res Int 26:29473–29489 https://doi.org/10.1007/s11356-019-06051-8
Chokshi K, Pancha I, Ghosh A, Mishra S (2016) Microalgal biomass generation by phycoremediation of dairy industry wastewater: an integrated approach towards sustainable biofuel production. Bioresour Technol 221:455–460. https://doi.org/10.1016/j.biortech.2016.09.070
Ummalyma SB, Sukumaran RK (2014) Cultivation of microalgae in dairy effluent for oil production and removal of organic pollution load. Bioresour Technol 165:295–301. https://doi.org/10.1016/j.biortech.2014.03.028
Choi H-J (2016) Dairy wastewater treatment using microalgae for potential biodiesel application. Environ Eng Res 21:393–400. https://doi.org/10.4491/eer.2015.151
Chowdhury R, Freire F (2015) Bioenergy production from algae using dairy manure as a nutrient source: Life cycle energy and greenhouse gas emission analysis. Appl Energy 154:1112–1121. https://doi.org/10.1016/j.apenergy.2015.05.045
Hena S, Znad H, Heong KT, Judd S (2018) Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res 128:267–277. https://doi.org/10.1016/j.watres.2017.10.057
Pandey A, Srivastava S, Kumar S (2019) Phyco-remediation of dairy effluents and biomass valorization: a sustainable approach, in: S.K. Gupta, F. Bux (Eds.), Application of Microalgae in Wastewater Treatment: Volume 2: Biorefinery Approaches of Wastewater Treatment, Springer International Publishing, Cham, 195–213 https://doi.org/10.1007/978-3-030-13909-4_9
Torri C, Samorì C, Adamiano A, Fabbri D, Faraloni C, Torzillo G (2011) Preliminary investigation on the production of fuels and biochar from Chlamydomonas reinhardtii biomass residue after bio-hydrogen production. Bioresour Technol 102:8707–8713. https://doi.org/10.1016/j.biortech.2011.01.064
Lu W, Wang Z, Wang X, Yuan Z (2015) Cultivation of Chlorella sp. using raw dairy wastewater for nutrient removal and biodiesel production: characteristics comparison of indoor bench-scale and outdoor pilot-scale cultures. Bioresour Technol 192:382–388. https://doi.org/10.1016/j.biortech.2015.05.094
Stanier RY, Deruelles J, Rippka R, Herdman M, Waterbury JB (1979) Generic assignments, strain histories and properties of pure cultures of cyanobacteria. Microbiology 111:1–61. https://doi.org/10.1099/00221287-111-1-1
Mishra S, Mohanty K (2019) Comprehensive characterization of microalgal isolates and lipid-extracted biomass as zero-waste bioenergy feedstock: an integrated bioremediation and biorefinery approach. Bioresour Technol 273:177–184. https://doi.org/10.1016/j.biortech.2018.11.012
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Water environment Federation, Washington DC
Mostafa SSM, El-Gendy NSh (2017) Evaluation of fuel properties for microalgae Spirulina platensis bio-diesel and its blends with Egyptian petro-diesel. Arab J Chem 10:S2040–S2050. https://doi.org/10.1016/j.arabjc.2013.07.034
ASTM (2002) Standard specification for biodiesel fuel (B100) blend stock for distillate fuels. American Society for Testing and Materials, D6751-02
ACEA, 2009. “Biodiesel guidelines”, European Automobile Manufacturers Association, Brussels, Belgium, March 2009, http://www.acea.be/uploads/publications/20090423_B100_Guideline.pdf
Khan F, Malik S, Shahid A, Siddiqui AJ, Musharraf SG, Zhu H, Alkhattabi NA, Gull M, Mehmood MA (2021) Characterization of a newly isolated cyanobacterium Trichocoleus desertorum BERC08 as a potential feedstock for the algal biorefinery. Biomass Convers Biorefinery 1–12. https://doi.org/10.1007/s13399-021-01537-3
Shahid A, Usman M, Atta Z, Musharraf SG, Malik S, Elkamel A, Shahid M, Alkhattabi NA, Gull M, Mehmood MA (2021) Impact of wastewater cultivation on pollutant removal, biomass production, metabolite biosynthesis, and carbon dioxide fixation of newly isolated cyanobacteria in a multiproduct biorefinery paradigm. Bioresour Technol 333:125194. https://doi.org/10.1016/j.biortech.2021.125194
Kim BH, Kang Z, Ramanan R, Choi JE, Cho DH, Oh HM, Kim HS (2014) Nutrient removal and biofuel production in high-rate algal pond using real municipal wastewater. J Microbiol Biotechnol 24(8):1123–1132. https://doi.org/10.4014/jmb.1312.12057
Chu HQ, Tan XB, Zhang YL, Yang LB, Zhao FC, Guo J (2015) Continuous cultivation of Chlorella pyrenoidosa using anaerobic digested starch processing wastewater in the outdoors. Bioresour Technol 185:40–48. https://doi.org/10.1016/j.biortech.2015.02.030
Tan XB, Zhao XC, Zhang YL, Zhou YY, Yang LB, Zhang WW (2018) Enhanced lipid and biomass production using alcohol wastewater as carbon source for Chlorella pyrenoidosa cultivation in anaerobically digested starch wastewater in outdoors. Bioresour Technol 247:784–793. https://doi.org/10.1016/j.biortech.2017.09.152
Ling Y, Sun LP, Wang SY, Lin CSK, Sun Z, Zhou ZG (2019) Cultivation of oleaginous microalga Scenedesmus obliquus coupled with wastewater treatment for enhanced biomass and lipid production. Biochem Eng J 148:162–169. https://doi.org/10.1016/j.bej.2019.05.012
Fazal T, Mushtaq A, Rehman F, Khan AU, Rashid N, Farooq W, Rehman MSU, Xu J (2018) Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew Sustain Energy Rev 82:3107–3126
Refaat AA (2009) Correlation between the chemical structure of biodiesel and its physical properties. Int J Environ Sci Technol 6:677–694. https://doi.org/10.1007/BF03326109
Shohaimi NAM, Marodzi FNS (2018) Transesterification of waste cooking oil in biodiesel production utilizing CaO/Al2O3 heterogeneous catalyst. Malays J Anal Sci 22(1):157–165 https://doi.org/10.17576/mjas-2018-2201-20
Farooq M, Ramli A, Subbarao D (2013) Biodiesel production from waste cooking oil using bifunctional heterogeneous solid catalysts. J Clean Prod 59:131–140. https://doi.org/10.1016/j.jclepro.2013.06.015
Qiu F, Li Y, Yang D, Li X, Sun P (2011) Heterogeneous solid base nanocatalyst: preparation, characterization and application in biodiesel production. Bioresour Technol 102(5):4150–4156. https://doi.org/10.1016/j.biortech.2010.12.071
Goli J, Sahu O (2018) Development of heterogeneous alkali catalyst from waste chicken eggshell for biodiesel production. Renew Energy 128:142–154. https://doi.org/10.1016/j.renene.2018.05.048
Knothe G (2008) “Designer” biodiesel: optimizing fatty ester composition to improve fuel properties. Energy Fuels 22:1358–1364. https://doi.org/10.1021/ef700639e
Gendy TS, El-Temtamy SA (2013) Commercialization potential aspects of microalgae for biofuel production: An overview. Egypt J Pet 22:43–51. https://doi.org/10.1016/j.ejpe.2012.07.001
Karmakar R, Kundu K, Rajor A (2018) Fuel properties and emission characteristics of biodiesel produced from unused algae grown in India. Pet Sci 15:385–395. https://doi.org/10.1007/s12182-017-0209-7
Islam M, Magnusson M, Brown R, Ayoko G, Nabi Md, Heimann K (2013) Microalgal species selection for biodiesel production based on fuel properties derived from fatty acid profiles. Energies 6:5676–5702. https://doi.org/10.3390/en6115676
Nascimento IA, Marques SSI, Cabanelas ITD, Pereira SA, Druzian JI, de Souza CO, Vich DV, de Carvalho GC, Nascimento MA (2013) Screening microalgae strains for biodiesel production: lipid productivity and estimation of fuel quality based on fatty acids profiles as selective criteria. Bioenerg Res 6:1–13. https://doi.org/10.1007/s12155-012-9222-2
Predojević ZJ (2008) The production of biodiesel from waste frying oils: a comparison of different purification steps. Fuel 87:3522–3528. https://doi.org/10.1016/j.fuel.2008.07.003
Chen L, Liu T, Zhang W, Chen X, Wang J (2012) Biodiesel production from algae oil high in free fatty acids by two-step catalytic conversion. Bioresour Technol 111:208–214. https://doi.org/10.1016/j.biortech.2012.02.033
Knothe G (2005) Dependence of biodiesel fuel properties on the structure of fatty acid alkyl esters. Fuel Process Technol 86:1059–1070. https://doi.org/10.1016/j.fuproc.2004.11.002
Knothe G (2012) Fuel properties of highly polyunsaturated fatty acid methyl esters. prediction of fuel properties of algal biodiesel. Energy Fuels 26:5265–5273. https://doi.org/10.1021/ef300700v
Acknowledgements
The first author (AP) would like to express his heartfelt gratitude to the Ministry of Human Resource and Development (MHRD) in New Delhi, India, for the Institute PhD fellowship. Authors also wish to thank Head, Department of Biotechnology and Director MNNIT Allahabad for infrastructural facilities and SAIF-IIT Bombay for extending their GC-MS facility. The authors also wish to acknowledge Dr. Sameer Srivastava for critically reviewing the manuscript, supervision and providing financial support to carry out the whole experiment.
Author information
Authors and Affiliations
Contributions
Ashutosh Pandey: conceptualization, experimental designing, collection, analysis and interpretation of data and manuscript writing, Sameer Srivastava: review, editing and supervision, Sanjay Kumar: supervision.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All the presented data in the manuscript are original and not publish anywhere.
Highlights
• Scenedesmus sp. ASK22 was successfully cultivated in BG11 and simulated dairy effluent (SDE).
• Nutrient supplementation in SDE showed enhanced nutrients and COD removal efficiency in both indoors and outdoors.
• The physicochemical properties of biodiesel meet properties of ASTM D7467 and EN14214.
• Scale up of Scenedesmus sp. ASK22 production using raw dairy effluent is possible.
• The cost-benefit of medium showed a cost reduction of ~3–4.5-fold as compared to the standard BG11 medium.
Rights and permissions
About this article
Cite this article
Pandey, A., Srivastava, S. & Kumar, S. Scenedesmus sp. ASK22 cultivation using simulated dairy wastewater for nutrient sequestration and biofuel production: insight into fuel properties and their blends. Biomass Conv. Bioref. 14, 3305–3317 (2024). https://doi.org/10.1007/s13399-022-02596-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02596-w