Abstract
This study presents a classic case of realizing wealth from waste through effective biomass management in two slaughterhouses having a slaughtering capacity of 1000 buffalos per day. On average, 32 to 35 tonnes of dung solids per day, either partially or fully digested, were recovered from the lairage (where animals are retained before slaughtering) and paunch sections (where intestinal contents are removed after slaughtering), respectively. The recovered dung solids with average moisture content (MC) of 85 % were dewatered using a screw press and dried up to ≤ 10% MC with a rotary sludge dryer. Then, briquettes were manufactured without using any binder. Fourier-transform infrared spectroscopy (FTIR) and UV-auto-fluorescence (UV-AF) studies revealed the binding mechanism of dung solids was due to the presence of lignin. Furthermore, owing to the presence of cellulose, hemicellulose, and lignin, the finished biomass briquette’s calorific value was observed to be 3032 Kcal/kg. The manufactured briquettes’ heavy metals concentration was lower than the briquettes derived from wood waste and vine shoots. The toxicity characteristics leachability procedure (TCLP) and waste extraction test (WET) analysis showed that the ash produced after the briquettes’ combustion is safe for disposal/landfilling. The emission of vent gases such as CO, CO2, SO2, and NOx was lower than wood chips and forest waste. The full-scale demonstration of dung management using dewatering, drying, and briquetting system offers a net profit of Rs. 1,560,000 (21,016 USD) annually. The proposed system has huge potential for replication in livestock/dairy farms for dung management.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The “affordable and clean energy,” “responsible consumption and production,” and “increase industry, innovation, and infrastructure” are three of the 17 sustainable development goals (SDGs), 2015 to achieve a better and more sustainable future for all by 2030. The industries are the key components to achieve the aforesaid SDGs by becoming energy and resource-efficient [1]. To do so, it is essential to find an alternative to conventional fuels. Over the past few years, biomass has gained popularity as an alternative to traditional fuels. Biomass-based fuels can be made from numerous waste materials such as peanut shell and sunflower stalk [2], sugarcane bagasse [3], coconut shell [4], groundnut shell [5], oil palm mesocarp fiber [6], palm oil mill sludge [7], and olive mill solid waste [8]. The most widely used biomass energy sources are bioenergy crops, agricultural residues, and forestry waste [9].
In the past, studies have been carried out by several researchers to exploit energy from slaughterhouses. Ware and Power [10] demonstrated that biogas could be produced using anaerobic digestion of slaughterhouse waste such as paunch, soft offal (intestinal residue, fat and meat trimmings, and some blood), and the sludge from the dissolved air flotation (DAF) unit. The methane potential of the waste streams was observed to be in the range of 49.5 to 650.9 mLCH4 .g−1 VS over a period of 30 to 50 days. Similarly, Wang et al. [11] showed that the average BMP of slaughterhouse waste such as manure, blood, viscera, and DAF sludge was 370 mLCH4 .g−1 VS and suggested hydraulic retention time (HRT) of 30 to 60 days in anaerobic digesters. Ali et al. [12] evaluated the biogas production potential of slaughterhouse waste such as blood, rumen, and manure in selected African countries. They suggested an HRT of 30 days for a dome-shaped biogas digester to handle slaughterhouse waste. Rhee et al. [13] calculated BMP of slaughterhouse waste such as heart, liver, lung, and large intestine in Korean slaughterhouse and found it to be in the range of 357.8 to 737.6 mLCH4 .g−1 VS. Likewise, Siddiki et al. [14] evaluated the biogas production of slaughterhouse waste such as rumen, blood, and manure in Bangladesh, and biogas yield was found to be between 300 to 700 mL .g−1 TS. Salehiyoun et al. [15] conducted anaerobic co-digestion of slaughterhouse waste with sewage sludge to improve the BMP. As far as exploiting energy from the slaughterhouses is concerned, the literature revolves mostly around the anaerobic digestion of slaughterhouse waste. The literature review indicated that the minimum HRT required in anaerobic digesters while handling slaughterhouse waste to exploit maximum biogas is 30 days. Given the quantum of waste generated in the slaughterhouses, longer HRT warrants a very high volume of digesters resulting in increased land area requirement, capital cost, and limited practical applicability. The literature review also indicated that biomass briquette manufacturing from the slaughterhouse has yet not been tried. Hence, it is important to come up with an alternative route to use a sizable fraction of organic waste from a slaughterhouse in a techno-economic and sustainable manner. Therefore, the major objectives of the studies were to identify the areas in a slaughterhouse wherein waste can be exploited to generate energy in the form of biomass-based fuels and demonstrate it on a full scale.
2 Materials and methods
2.1 Study area
This study was carried out in two slaughterhouses situated in the state of Uttar Pradesh (U.P.), India. Both slaughterhouses are export oriented and offer fresh boneless buffalo meat. They follow International Standards and are HACCP, ISO 22000:2005, and ISO 9001:2015 certified. Industries are classified as a “large slaughterhouse” since their annual slaughtering capacity exceeds 40,000 cattle or dry live weight per day exceeding 70 tonnes as per EPA Act (India) 1986. They have consent to slaughter 1100 buffalos per day from APEDA, Ministry of Commerce, GoI, and consent to operate from the Uttar Pradesh Pollution Control Board. It is also licensed by the Food Standards and Safety Authority of India (FSSAI) under the Food Safety and Standards Act, 2006.
2.2 Identification of slaughterhouse waste for biomass-based fuel
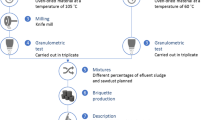
All the slaughterhouse processes and operations were studied in detail, and field investigations studies were conducted in order to assess various categories of effluent generation viz. dung, blood, salt, and fat streams. In addition, a detailed literature review was conducted to identify the areas or sections in a slaughterhouse wherein waste is produced that can be recovered and manufactured into biomass briquettes (Fig. 1).
2.3 Quantification of waste
The screened solids from the solid-liquid separation units, dewatered solids from the filter press, recovered solids from the lairage, and the paunch section are stored in trolleys. The weights of the trolleys were taken using a weighing bridge. It was found that the average net weight of contents in a trolley is 3000 kg. Thereafter, the number of trolleys per day was counted and multiplied by 3000 kg to work out the quantity of separated dung solids.
2.4 Moisture content of waste
The dung solids from the lairage and paunch sections were collected. The moisture content of the dung solids was measured as per ASTM: E871-82, 2014 [16]. The dung solids (50 g) were weighed in a cleaned container using an electronic weighing balance. Then, the samples were dried in an oven at 103 ± 1 °C for 16 h. The dried samples were then allowed to reach room temperature by keeping them in a desiccator. The final weights were taken to estimate the moisture content on a wet weight basis as per Eq. (1).
2.5 Dewatering, drying, and briquetting system
In a slaughterhouse, dewatering such a sizable fraction of organic matter with 85% moisture content is one of the major challenges since it is a well-established fact that the ideal moisture content required for briquetting should be less than 10 %. Thus, while designing biomass dewatering, drying, and briquetting systems, it was necessary to strike a balance between the energy required for dewatering, drying and briquetting, and the energy available from the finished briquettes. Therefore, a multi-intervention approach was necessary to bring down the moisture content from 85 to less than 10 %. Hence, a two-stage dewatering and drying system was developed and adopted to improve biomass handling. Figure 2 presents the overall schematic for the management of dung solids.
The first step is to dewater the recovered dung solids using a screw compactor. The screw compactor squeezes out the moisture of biomass and reduces the moisture. The screw compactor also facilitates feeding dewatered dung to the second stage, i.e., to rotary sludge dryer, where moisture is targeted to bring down up to 10 % or below. The dry solids with moisture content ≤ 10% are then fed to the briquetting plant for manufacturing the briquettes. The technical specifications for the screw compactor, rotary sludge dryer, and briquetting plant are provided in supplementary material in Tables S1, S2, and S3, respectively.
2.6 Characterization of finished briquettes
2.6.1 Proximate and ultimate analysis
The proximate analysis of the dung briquettes was done as per Indian Standard (IS): 1350 Part 1, and the gross calorific value (GCV) was analyzed as per the Indian Standard (IS): 1350 Part 2. The ultimate analysis was done using Elementar Vario MACRO CUBE (CHNS Analyzer). The instrument operates on a principle of high-temperature combustion or pyrolysis of the sample and converting the elements into gaseous products. The gases are then separated by purge and trap chromatography and detected by a thermal conductivity detector (TCD).
2.6.2 Heavy metals analysis
For heavy metals analysis, the briquettes and ash were digested by taking 1 g of the sample and dissolving it in a mixture of 90 mL distilled water and 10 mL nitric acid (Fisher Scientific, 70% v/v, trace metals grade) and heating for 2 h on a hot plate at 150 °C. The digested samples were then analyzed in ICP-OES (Thermo Fisher ICAP 6300 DUO). Merck makes multi-element standard solution (No: IV) containing major concerned elements was taken as reference standards for the analysis.
2.6.3 TCLP and WET analysis
The toxicity characteristics leaching procedure (TCLP) and waste extraction test (WET) were conducted on the ash generated from the combustion of the briquettes in the solid fuel boiler in accordance with the procedure mentioned in USEPA Test Method 1311 (July 1992) and waste extraction test (WET) procedure, Appendix II of section 66261 of Title 22 of California Code regulation (CCR) respectively.
2.6.4 Vent gases emissions
The finished briquette obtained after the biomass dewatering, drying, and briquetting system was crushed using pestle and mortar. The crushed sample was then weighed to 50 g in a crucible and kept in an electrically heated controlled atmosphere chamber (EHCA) furnace (Make Therelek). The temperature in the combustion chamber was maintained at 500 ± 2 °C. This combustion temperature was chosen to more or less mimic the conditions in a solid fuel boiler, which was proposed to be fed with finished briquettes. The flue gases emissions were monitored at every 5-min interval for 165 min using a gas analyzer (Make: ACE 9000XT). The gas analyzer can measure O2, CO2, CO, SO2, and NOx. The measurement of all the gases except CO2 (derived) is sensor based. The O2 sensor consists of an anode, electrolyte, and air cathode. At the air cathode, oxygen is reduced to hydroxyl ions which in turn oxidize the metal anode. The generated current is proportional to the concentration of O2. The measurement of CO, SO2, and NOx was based on the concept of electrochemical sensors of the micro fuel cell type. The sensors use capillary diffusion technology, yielding a low-temperature coefficient and a direct response to concentrations.
2.6.5 Fourier-transform infrared spectroscopy (FTIR)
Fourier-transform infrared spectroscopy (FTIR) measurement was done using PerkinElmer Series FTIR-Spectrum 2 in the spectral range of 400 to 4000 cm−1, and the spectra were collected at a resolution of 4 cm−1 with 16 scans. The results from this study were compared with the FTIR results obtained by Charis et al. [17], who also studied FTIR spectra of sawdust and acacia-based biomass. To confirm the presence of cellulose, hemicellulose, and lignin, the FTIR spectra of the analytical grade cellulose, hemicellulose (Make: Himedia), and lignin (Make: Merck) were taken and compared with that of the biomass from the slaughterhouse.
2.6.6 Ultraviolet (UV)-auto-fluorescence (AF) and brightfield microscopy
The cylindrical biomass briquette was broken using hands along its radius perpendicular to its height. This was done specifically to avoid any cuts on the material while using a knife or blade. The fractured portion of the biomass was then taken for analysis. UV-AF images were taken using Leica DM 2500 (excitation at 320–380 nm, dichroic mirror at 400 nm, emission at 425 nm) to ascertain the spread of lignin within the biomass briquettes. As per Kaliyan and Morey [18], lignin present in a sample shines bluish-white or brilliant blue when subjected to UV. The images are presented along with the scale.
The fractured portion of biomass was also observed using Leica MZ 10F stereomicroscope (brightfield), and an image was captured to study the various binding mechanisms. The image is presented in the grayscale.
2.7 Business model
The cost estimates for operation and maintenance of the briquettes manufacturing unit have been arrived at considering the power consumption, manpower required, maintenance, and repair. The maintenance and repair costs for civil works and mechanical/electrical equipment are taken as actual based on the industries’ secondary data. The power cost is calculated based on the power rating of the equipment, hours of operation, and the existing electricity tariff in the state of Uttar Pradesh.
3 Results and discussion
3.1 Waste for biomass-based fuel
In a slaughterhouse, fully digested dung solids are present in the lairage section (Fig. 1A), wherein buffalos are retained before slaughtering. Usually, dry scrapping of cattle dung is done to recover it as much as possible in the dry form. The rest of the solids on the floor are washed with high-pressure nozzles, which generate wastewater containing suspended solids (SS). In addition to the dung solids from the lairage section, cattle paunch or rumen is another major waste source that contains partially digested food in the stomach of cattle. The wet weight of a paunch material ranges between 22 and 31 kg per cattle [19]. After slaughtering, the paunch is opened, and the contents are emptied in a container (Fig. 1B) before rinsing the paunch in a separate container. The paunch contents that stick onto the wall of rumen are introduced in the wastewater while rinsing. The wastewater characteristics from the lairage and paunch section are described in detail in Shende et al. [20].
In addition to the dry scrapping of dung from the lairage section and recovery of intestinal content from the paunch sections, a primary wastewater treatment system consisting of hydrasieve and externally fed rotary drum filter (EFRFDF) is installed in both the industries to recover dung solids from the lairage and paunch sections, respectively. Furthermore, the primarily settled dung solids from the clarifier are dewatered using a filter press.
3.2 Waste quantification
In a slaughterhouse with a slaughtering capacity of 1000 buffalos/day, it was found that about 30 to 32 tonnes/day of dung solids were recovered, having a moisture content of 86 ± 2 % from the hydrasieve, EFRDF, lairage, and paunch sections. Simultaneously, nearly 2 to 3 tonnes/day of dung solids were recovered from the filter press, and its average moisture content was 65 ± 5 %. Thus, a total of 32 to 35 tonnes/day of the dung solids can be recovered with an average moisture content of 85 %. Based on the quantity and the high moisture content, a biomass dewatering, drying, and briquetting system was designed.
3.3 Performance of biomass dewatering, drying, and briquetting system
The performance of the biomass dewatering, drying, and briquetting system is presented in Table 1. It was found that the screw compactor reduces the moisture content of the separated solids from 85 to 45 ± 5 % with a feed capacity of 5 tonnes per hour (TPH). At the rate of 5 TPH, the dewatered solids’ quantity at the screw press outlet was 1.6 to 2.2 TPH. The dewatered solids from the screw press outlet were fed to a rotary dryer for further drying with the conveyor belt at the rate of 1.6 to 2.2 TPH. The dried solids at the outlet of the rotary dryer had the MC of less than 10 %. It is important to note here that the MC of the dried solids has to be necessarily less than 10 % for making it suitable to feed in the briquetting machine. Thus, 5 to 6 tonnes of briquettes per day are manufactured in a slaughterhouse with a slaughtering capacity of 1000 buffalos per day.
The manufactured briquettes were used in the hot air generator of the rotary dryer, and its consumption was 200 to 300 kg/h. The specifications of the finished briquettes and other important characteristics are shown in Fig. 3. The diameter of the finished briquette was 90 mm, and length varied between 100 and 500 mm with an average density of 1240 kg/m3.
3.4 Proximate and ultimate analysis
As shown in Table 2, the GCV and an ash content of dung briquettes are 3032 Kcal/kg and 28.6 %, respectively, which is comparable with the Grade G15 coal in India (GCV: 2801 to 3100 Kcal/kg and Washery Grade IV coal (ash content: 28 to 35 %) [21]. The calorific value obtained in this study was higher than the previously reported values by Archana et al. [4] for coconut husk (2398 Kcal/kg); Chiou and Wu [22] for pulp (2085 Kcal/kg), and textile (2864 Kcal/kg) sludge and at par with agricultural residues such as corn stalk (3293 Kcal/kg), rice husk (3196 Kcal/kg), and olive refuse (3104 Kcal/kg) [23]. The calorific value of the tannery waste reported by Onukak et al. [24] was between 4450 and 5288 kcal/kg. However, the tannery waste had a very high chromium content of more than 4000 mg/kg; thus, de-chroming was done as a pre-treatment.
It is important to note here that binders in the manufacturing of briquettes add to the finished product’s calorific value. Since no binders were used in this study, the calorific value presented is the actual value of the briquetting material used alone. The dung briquettes’ carbon content was in the range of 35.93 to 36.76 %, which is comparable with briquettes manufactured using textile sludge (32.15 %) and textile industry solid residue (41.4 %), as shown in Table 2. The sulfur content of dung briquettes varied between 0.063 and 0.083%, which is very less than the sulfur content of Indian Coal (0.3 to 0.55%) [27].
3.5 Heavy metals
A comparison of heavy metals concentrations in both briquettes and ash under this study and that reported by Moreno et al. [28] is presented in Table 3. As can be seen, none of the heavy metal concentrations is significant, except Fe and Mn. Nevertheless, the Fe and Mn concentrations in the briquettes and ash were significantly less than the values reported by Moreno et al. [28] for furniture wood waste, solid wood, engineered wood, and vine shoots whose principal source is naturally occurring trees.
3.6 TCLP and WET
As shown in Table 2, the ash content of the biomass briquette was 28.6 %, which may pose difficulties for its ultimate disposal. However, landfilling can be an alternative to manage ash effectively. Hence, TCLP and WET analysis of the ash was carried out. The results are presented in Table 4. The leachable concentration of all the heavy metals from the ash is less than the concentration limits of class A and is safe for disposal/landfilling, etc. Moreover, the possibility of using ash for bricks manufacturing and soil amendments has to be explored to earn incentives from it.
3.7 Vent gases emissions
The average concentration of the vent gases is presented in Table 5 (detailed results are provided in Table S4 of Appendix), along with the comparison with the previously published data. It is evident that the emissions from the finished briquettes in this study were lower than the emissions from olive mill waste, rice husk, wood chips, spent coffee beans, and forest waste. The lower values of SO2 and NOx can be attributed to very low concentrations of S (0.063 to 0.083 %) and N (0.68 to 0.70 %) in the briquettes, as mentioned in Table 2.
3.8 FTIR analysis
The results from the FTIR analysis are shown in Fig. 4. In the biomass briquettes, the peak around 3339.45, 1731, and 1029.50 cm−1 correspond to hydrogen-bonded OH stretch, C=O stretch, and esters/ethers, respectively that may be associated with cellulose, hemicellulose, and lignin. Thus, in order to further ascertain, the FTIR spectra were matched with that of the cellulose, hemicellulose, and lignin, as shown in Fig. 4. The peaks in lignin around 1651 and 2929 cm−1 resemble peaks in biomass from slaughterhouse, i.e., 1650 and 2974 cm−1. Similarly, the peak around 896 cm−1 due to ß-glucosidic linkages between monosaccharides is common in hemicellulose and biomass [33]. The peak around 2885 and 2906 cm−1 corresponding to C-H stretch in glucose unit is common in both cellulose and biomass [34]. It is possible to quantify cellulose, hemicellulose, and lignin as per National Renewable Energy Laboratory’s (NREL’s) Biomass compositional analysis procedures. However, in this case, the same was not applicable owing to the interference of ash content which should be less than 10 %. However, the presence of cellulose, hemicellulose, and lignin contributed to the calorific value of the finished biomass briquettes.
3.9 UV Auto-fluorescence
The results are presented in Fig. 5. The lignin can be seen in bright blue color almost across all parts of the fractured parts of the biomass briquettes. The presence of lignin accompanied by suitable glass transition temperature (Tg) and lower moisture content favors the natural binding process, as stated by Tumuluru [35].
In this study, it is likely that the required Tg must have been generated during high-pressure densification in the briquetting machine because of the force of friction. This was also evident from the temperature measurement of the finished briquettes immediately which ranged between 55 and 68 °C. The temperature was measured using an infrared thermometer (Make: Hanna). The Tg of lignin is reported as 50 to 100 °C [36]. Above Tg, lignin behaves as a viscous material, and once it cools down, it solidifies and binds materials with which it comes in contact. In a study conducted by kaliyan and Morey [18], while making pallets from corn stover and switchgrass, the lignin can be seen in abundance in the fractured portion of their biomass. However, in the present study, lignin is not as abundant as compared to Kaliyan and Morey [18]. The probable reason is that lignin from the grass, leaves, or straw might have been altered during the digestion process.
3.10 Brightfield microscopy
The presence of lignocellulose such as straw, grass, and hay in paunch content has been reported by Tritt and Schuchardt [37]. In this study, the straws are distinctly visible as shown in Fig. 6 in elongated circles. The grass and hay might have been bitten well and digested by buffalos. Nevertheless, the color of paunch materials is green, as shown in Fig. 1b, thus indicating the presence of grass, hay, or any other plant’s leaves-based contents.
As shown in Fig. 5, lignin was spread well across the biomass of briquettes. Moreno et al. [28] reported that the lignin, starch, and carbohydrates in waste materials act as a natural binder since they form inter-particle solid bridges under pressure. In this study, the presence of starch and carbohydrates was not evaluated. However, as stated by Tumuluru [35], lignin is a basic binder in herbaceous and woody biomasses. In Fig. 6, various binding mechanisms such as mechanical inter-locking, adhesion, and solid bridges are clearly visible and in line with the findings of Moreno et al. [28], Kaliyan and Morey [18], and Tumuluru [35]. Moreover, the void spaces (around 0.1 to 0.5 mm size) were observed in between biomass which may be due to the non-uniform size of biomass leading to differential densification during briquetting, thus creating void spaces.
3.11 Business model
Recurring cost estimates and economics of dung dewatering, drying, and briquetting plant is shown in Table 6. In a slaughterhouse with a capacity of 1000 buffalos per day, the net production of briquettes is 3.6 TPD, and the daily expenditure required for its manufacturing is Rs. 10,766 (145 USD). Considering the market prize of the finished briquettes as Rs. 5000 (67.36 USD) per ton, the net profit which can be gained by selling the finished product is Rs. 7324 (98.67 USD) per day. Alternatively, the finished briquettes can also be used in the slaughterhouse operating an in-house solid fuel boiler. Moreover, the capital cost on the briquetting plant can be saved if the dried dung can be fed directly in the solid fuel boiler.
An industry usually operates 26 days a month. Considering the average profit of Rs. 5000 (67.36 USD) per day (industry does not operate on full slaughtering capacity daily); the monthly and annual profit gained by selling finished materials is Rs. 130,000 (1751.35 USD) and Rs. 1,560,000 (21,016 USD) respectively. The total expenditure incurred for setting up the dung dewatering, drying, and briquetting plant was Rs. 5,500,000 (74,095 USD). Thus, the return on investments (RoI) would be achieved after 3.5 years.
The cost of managing the 32 to 35 TPD dung solids with a moisture content of 85 % will also be saved by implementing the dung dewatering, drying, and briquetting plant. The cost saved to handle 32 to 35 TPD dung solids in the absence of the dung dewatering, drying, and briquetting plant is not considered while arriving at the RoI.
The total power consumption per day to run the briquette manufacturing plant is 796 kW/day (68,4437 Kcal/day), as shown in Table 6. The biomass net production is 3.6 TPD having a calorific value of 3032 Kcal/kg, which implies that the total calories available from the finished briquettes are 10.915 million Kcal/day. Thus, this study demonstrates a classic case of effective dung management in a slaughterhouse with the added advantage of net gains and meeting the SDGs.
4 Conclusions
The slaughterhouse having a slaughtering capacity of 1000 buffalos per day has the potential to recover nearly 32 to 35 tonnes of dung solids per day (either partially or fully digested). The recovered solids were converted to useful resource “biomass” following dewatering, drying, and briquetting without the use of binders. For briquetting, the MC of dung was reduced from 85 to 10 %. The calorific value of the finished biomass briquette from the slaughterhouse was 3032 Kcal/kg, and the volatile percentage by mass was 49.1 %. Heavy metal concentration in the briquettes and the ash was lower than the previously reported values for biomass-based fuels. The TCLP and WET analysis showed that the ash produced after the briquettes’ combustion is safe for disposal/landfilling. The vent gases emissions from the burning of biomass briquettes were lower than wood chips, forest waste, etc. The binding mechanism was due to the presence of lignin which enabled solid bridges, adhesion of biomass. By implementing the developed dewatering, drying, and briquetting system, it is possible to gain a net profit of Rs. 1,560,000.00 (21,016 USD) annually. The system can even be replicated in livestock/dairy farms.
Abbreviations
- APEDA:
-
Agriculture and Processed Food Export Development Authority
- AF:
-
Auto-fluorescence
- ASTM:
-
American Society for Testing and Materials
- BMP:
-
Biochemical methane potential
- DAF:
-
Dissolved air flotation
- EFRDF:
-
Externally fed rotary drum filter
- EHCA:
-
Electrically heated controlled atmosphere chamber
- EPA:
-
Environment Protection Act
- FSSAI:
-
Food Standards and Safety Authority of India
- FTIR:
-
Fourier-transform infrared spectroscopy
- GCV:
-
Gross calorific value
- HACCP:
-
Hazard Analysis and Critical Control Points
- HRT:
-
Hydraulic retention time
- ICP-OES:
-
Inductively coupled plasma-optical emission spectrometry
- IS:
-
Indian Standards
- ISO:
-
International Standards Organization
- Kcal:
-
Kilocalories
- Kg:
-
Kilograms
- MC:
-
Moisture content
- NREL:
-
National Renewable Energy Laboratory
- ppm:
-
Parts per million
- SDG:
-
Sustainable Development Goal
- TCLP:
-
Toxicity characteristics leachability procedure
- Tg:
-
Glass transition temperature
- TPD:
-
Tonnes per day
- TPH:
-
Tonnes per hour
- TS:
-
Total solids
- USD:
-
US dollars
- UV:
-
Ultraviolet
- VS:
-
Volatile solids
- WET:
-
Waste extraction test
References
Berawi MA (2019) The role of industry 4.0 in achieving Sustainable Development Goals. Int J Technol. https://doi.org/10.14716/ijtech.v10i4.3341
Rizvi T, Xing P, Pourkashanian M, Darvell LI, Jones JM, Nimmo W (2015) Prediction of biomass ash fusion behaviour by the use of detailed characterisation methods coupled with thermodynamic analysis. Fuel. https://doi.org/10.1016/j.fuel.2014.10.021
Bot BV, Sosso OT, Tamba JG, Lekane E, Bikai J, Ndame NK (2021) Preparation and characterization of biomass briquettes made from banana peels, sugarcane bagasse, coconut shells and rattan waste. Biomass Conv Bioref. https://doi.org/10.1007/s13399-021-01762-w
Archana A, Vijay Pradhap, Singh M, Chozhavendhan S, Gnanavel G, Jeevitha S, Muthu Kumara Pandian A (2019) Coconut shell as a promising resource for future biofuel production. In: Praveen Kumar R, Bharathiraja B, Kataki R, Moholkar VS (eds) Biomass Valorization to Bioenergy. Springer, Singapore, Singapore, pp 31–43
Lubwama M, Yiga VA, Lubwama HN (2020) Effects and interactions of the agricultural waste residues and binder type on physical properties and calorific values of carbonized briquettes. Biomass Conv Bioref. https://doi.org/10.1007/s13399-020-01001-8
Mitchual SJ, Katamani P, Afrifa KA (2019) Fuel characteristics of binder free briquettes made at room temperature from blends of oil palm mesocarp fibre and Ceiba pentandra. Biomass Conv Bioref 9:541–551. https://doi.org/10.1007/s13399-019-00410-8
Obi OF, Okongwu KC (2016) Characterization of fuel briquettes made from a blend of rice husk and palm oil mill sludge. Biomass Conv Bioref. https://doi.org/10.1007/s13399-016-0206-x
Khlifi S, Lajili M, Tabet F, Boushaki T, Sarh B (2020) Investigation of the combustion characteristics of briquettes prepared from olive mill solid waste blended with and without a natural binder in a fixed bed reactor. Biomass Conv Bioref. https://doi.org/10.1007/s13399-019-00449-7
Smeet EMW, Faaij APC, Lewandowski IM, Turkenburg WC (2007) A bottom-up assessment and review of global bio-energy potentials to 2050. Prog Energy Combust Sci. https://doi.org/10.1016/j.pecs.2006.08.001
Ware A, Power N (2016) Biogas from cattle slaughterhouse waste: energy recovery towards an energy self-sufficient industry in Ireland. Renew Energy. https://doi.org/10.1016/j.renene.2016.05.068
Wang S, Jena U, Das KC (2018) Biomethane production potential of slaughterhouse waste in the United States. Energy Convers. https://doi.org/10.1016/j.enconman.2018.07.059
Ali MM, Ndongo M, Bilal B, Yetilmezsoy K, Youm I, Bahramian M (2020) Mapping of biogas production potential from livestock manures and slaughterhouse waste: a case study for African countries. J Clean Prod. https://doi.org/10.1016/j.jclepro.2020.120499
Rhee C, Kim DW, Yu SI, Lee ME, Shin J, Kim HW, Chung JW, Shin SG (2021) Biogas potential assessment and characterization of Korean slaughterhouse waste for anaerobic digestion. Environ Technol Innov. https://doi.org/10.1016/j.eti.2021.101858
Siddiki SYA, Uddin MN, Mofijur M, Fattah IMR, Ong HC, Lam SS, Kumar PS, Ahmed SF (2021) Theoretical calculation of biogas production and greenhouse gas emission reduction potential of livestock, poultry and slaughterhouse waste in Bangladesh. J Environ Chem Eng. https://doi.org/10.1016/j.jece.2021.105204
Salehiyoun AR, Maria FD, Sharifi M, Norouzi O, Zilouei H, Aghbashlo M (2020) Anaerobic co-digestion of sewage sludge and slaughterhouse waste in existing wastewater digesters. Renew Energy. https://doi.org/10.1016/j.renene.2019.08.001
ASTM International: E871–82 (2014) Standard test method for moisture analysis of particulate wood fuels 1. Annu B ASTM Stand 82:2
Charis G, Danha G, Muzenda E (2020) Characterizations of biomasses for subsequent thermochemical conversion: a comparative study of pine sawdust and Acacia tortilis. Processes. https://doi.org/10.3390/pr8050546
Kaliyan N, Morey RV (2010) Natural binders and solid bridge type binding mechanisms in briquettes and pellets made from corn stover and switchgrass. Bioresour Technol. https://doi.org/10.1016/j.biortech.2009.08.064
Carawan RE, Chambers JV, Zall RR (1979) Meat processing water and wastewater management. North Carolina Agricultural Extension Service, Raleigh, North Carolina
Shende AD, Dhenkula S, Waghambare A, Rao NN, Pophali GR (2021) Water consumption, wastewater generation and characterization of a slaughterhouse for resource conservation and recovery. Water Pract Technol. https://doi.org/10.2166/wpt.2021.122
Ministry of Coal, Coal Controller’s Organisation, Kolkata, Government of India (2014) Coal directory of India: coal statistics (2011-2012). Available at http://www.coalcontroller.gov.in/writereaddata/files/download/coaldirectory/Coal%20Directory%20of%20India%202011-12.pdf
Chiou IJ, Wu IT (2014) Evaluating the manufacturability and combustion behaviors of sludge-derived fuel briquettes. Waste Manag. https://doi.org/10.1016/j.wasman.2014.05.013
Dinesha P, Kumar S, Rosen MA (2019) Biomass Briquettes as an alternative fuel: a comprehensive review. Energy Technol 7. https://doi.org/10.1002/ente.201801011
Onukak IE, Mohammed-Dabo IA, Ameh AO, Okoduwa SIR, Fasanya OO (2017) Production and characterization of biomass briquettes from tannery solid waste. Recycling 2. https://doi.org/10.3390/recycling2040017
Avelar NV, Rezende AAP, Carneiro A, de CO, Silva CM (2016) Evaluation of briquettes made from textile industry solid waste. Renew Energyhttps://doi.org/10.1016/j.renene.2016.01.075
Sawadogo M, Tchini Tanoh S, Sidibé S, Kpai N, Tankoano I (2018) Cleaner production in Burkina Faso: case study of fuel briquettes made from cashew industry waste. J Clean Prod. https://doi.org/10.1016/j.jclepro.2018.05.261
Chandra A, Chandra H (2004) Impact of Indian and imported coal on Indian thermal power plants. J Sci Ind Res (India) 63:156–162
Moreno AI, Font R, Conesa JA (2016) Physical and chemical evaluation of furniture waste briquettes. Waste Manag. https://doi.org/10.1016/j.wasman.2016.01.048
Wongwuttanasatian T, Sakkampang C (2016) Combustion characteristics and emission of briquette fuel from biomass mixed with glycerin. Combust Sci Technol. https://doi.org/10.1080/00102202.2015.1136298
Mustafa BG, Aji MM, Yaumi AL, Highina BK, Sulaiman SI (2014) Comparative studies on the combustion performance of briquettes produced from selected biomass residues in Maiduguri. World J Energy Sci Eng 1:1–8
Pilusa TJ, Huberts R, Muzenda E (2013) Emissions analysis from combustion of eco-fuel briquettes for domestic applications. J Energy S Afr 24:30–36
Ullah S, Noor SZ, Sanaullah, Gang T (2021) Analysis of biofuel (briquette) production from forest biomass: a socioeconomic incentive towards deforestation. Biomass Convers Biorefin. https://doi.org/10.1007/s13399-021-01311-5
Liang T, Wang L (2016) Thermal treatment of poplar hemicelluloses at 180 to 220 °C under nitrogen atmosphere. Bioresources. https://doi.org/10.15376/biores.12.1.1128-1135
Abderrahim B, Abderrahman E, Mohamed A, Fatima T, Abdesselam T, Krim O (2015) Kinetic thermal degradation of cellulose, polybutylene succinate and a green composite: comparative study. World J Environ Eng. https://doi.org/10.12691/wjee-3-4-1
Tumuluru JS (2018) Effect of pellet die diameter on density and durability of pellets made from high moisture woody and herbaceous biomass. Carbon Resour Convers. https://doi.org/10.1016/j.crcon.2018.06.002
Kong L, Zhao Z, He Z, Yi S (2018) Effects of steaming treatment on crystallinity and glass transition temperature of Eucalyptuses grandis x E. urophylla. Results Phys. https://doi.org/10.1016/j.rinp.2017.02.017
Tritt WP, Schuchardt F (1992) Materials flow and possibilities of treating liquid and solid wastes from slaughterhouses in Germany. A review. Bioresour Technol. https://doi.org/10.1016/0960-8524(92)90008-L
Acknowledgements
The authors gratefully acknowledge the help rendered by M/s FEPL, Rampur and M/s FEPL, Barabanki (U.P.). The authors also acknowledge Dipin, Rothish, Amal, Vasanthragavan for their assistance during FTIR analysis and interpretations; Jasmine and Suryalekshmi for their assistance in UV-AF and Bright-field microscopy; and M P Patil for his assistance during TCLP and WET analysis.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• Recovered dung from lairage and paunch sections was dewatered and dried, and briquettes were manufactured without using any binder.
• The briquettes have moderate gross calorific value (GCV), very low concentration of heavy metals and leachability, and minimum vent gases emissions.
• A slaughterhouse with the capacity of 1000 buffalos per day offers 5–6 tonnes of briquettes/day with a net annual profit of Rs. 1,560,000 (21,016 USD).
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shende, A.D., Khan, M.S., Dhenkula, S. et al. Waste to wealth in a slaughterhouse through effective biomass management. Biomass Conv. Bioref. 14, 269–281 (2024). https://doi.org/10.1007/s13399-022-02374-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02374-8