Abstract
Protists are important microorganisms involved in the transformation of nutrients, but little information is available regarding their roles in composting. This study investigated the changes in the protist community during composting and their relationships with the conversion of nitrogen. The protist community structure varied among the different composting phases, but the communities were similar in the cooling phase and at the end of the thermophilic phase. Phagotrophic protists such as Acantharea-Group-II_XX and Acantharea_XXX dominated in the composting process. The abundance of Acantharea-Group-II_XX increased by 12.8% in the thermophilic phase, whereas that of Acantharea_XXX increased by 74.5% and 275.3% during the cooling and maturation phases, respectively. The protist community was mainly affected by the NO3− and NO2− concentrations and the bacterial community, where NO3− and NO2− inhibited phagotrophic protists. Furthermore, compared with the nitrifying bacterial communities, the denitrifying bacterial communities were more conducive to the growth of Acantharea-Group-II_XX and Acantharea_XXX. Phagotrophic protists were key predators that regulated bacteria with denitrification functions.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Aerobic composting is a highly effective approach for treating livestock and poultry manure to render it harmless, where this biochemical process is mediated by microorganisms [1]. The microbial community during the composting process mainly comprises bacteria and fungi, which can degrade organic matter and synthesize humus, and convert organic nitrogen into a form that can be used by organisms during composting [2, 3]. These microbial processes greatly affect the quality and efficiency of composting [4].
Protists are small, soft-bodied organisms that can survive in all types of natural conditions [5], but they are difficult to preserve or they have few morphological characters that can be observed using conventional microscopy [6]. Protists are crucial for linking the interactions in microbial communities [7], where they may compete with and prey on bacteria and fungi [8]. Fungi and bacteria are consumed primarily by protists, which can control the functions, composition, and changes in the microbial community via predation [9, 10]. For example, Rosenberg et al. (2009) found that amoebae could decrease the abundances of bacteria, and some specific bacterial taxa disappeared 2 days after inoculation [9]. Bacteria and fungi are generally considered to play important roles as catalysts in nutrient cycling during composting, such as in the nitrogen cycle [11]. Previous studies have shown that protists increase ammonia volatilization in soil, mainly because of the higher C/N ratio of protists compared with bacteria [12], and their predation on bacteria may lead to the release of more NH3 [13]. Nitrogen cycling can lead to the release of nitrogen in the form of NH3 during composting, but ammonia can also be transformed into nitrate through nitrification and denitrification to improve the quality of the compost products. Nitrification and denitrification are the most important processes, and nitrifying and denitrifying bacteria that carry the nxrA and nirS genes play key roles during composting [14]. However, protists are often ignored as components of the environmental microbiome [12], and very little is known about the interactions among bacteria, fungi, and protists. Thus, the roles of the protist community members during composting remain unclear.
Protists have diverse functions, such as phagotrophs, bacterivores, parasites, omnivores, and mixotrophs, and these functional groups strengthen the microbial loop [15] because of their important roles in biochemical nutrient cycling [16]. Previous studies have mainly investigated the changes in the protist communities in soil [12, 17,18,19], but little information is available about the variations in the protist communities during composting. The succession of the protist community during composting has not been investigated in previous studies, and the protist community might change during different composting phases in a similar manner to bacterial and fungal communities, but further verification is required.
Microorganisms require suitable environmental conditions to function, and in a similar manner to bacteria and fungi, studies have shown the protist community can be affected by environmental factors (e.g., NO3− and pH [12]) in the soil. In contrast to the soil environment, the environment changes greatly during composting, and the main environmental factors that influence the protist community might be different. In addition, biotic factors such as bacteria and fungi will have mutual effects on the protist community, and they will change during the composting process. However, previous studies have not investigated the relationships between biotic factors and the succession of the protist community. Thus, it is not known how abiotic and biotic factors might affect the protist community, and how they could contribute to these changes. Therefore, it is necessary to explore the abiotic factors and biotic factors that might affect the protist community during composting.
Artificial temperature control was used in this study to simulate the conventional composting process. The protist, bacterial, and fungal communities were identified by high-throughput sequencing. This study aimed (1) to determine the composition of the protist community, (2) to assess the effects of various factors on the changes in the protist community, and (3) to determine the ecological interactions among the protist, bacterial, and fungal communities.
2 Materials and methods
2.1 Experimental design and physical and chemical properties
In this study, a mixture of swine manure (total organic carbon and nitrogen contents of 380.2 g kg−1 and 26 g kg−1, respectively) and wheat straw (total organic carbon and nitrogen contents of 496.3 g kg−1 and 6.5 g kg−1, respectively) was used to simulate the conventional composting process. The temperature was manually controlled over a period as follows: 20–55 °C (0–5 days, mesophilic phase), above 50 °C (6–16 days, thermophilic phase), 50–40 °C (17–21 days, cooling phase), 40–20 °C (22–35 days, maturity phase), and the post-decomposition phase at 20 °C (36–105 days). The volume of each plastic composting reactor was 500 mL (each reactor contained 150 g (dry weight) of the raw material). The C:N ratio and moisture content of the composting material were adjusted to 20:1 and 55%, respectively, as described in a previous study [14]. Samples were collected from three test reactors in the mesophilic phase (day 2), thermophilic phase (day 7 and 14), cooling phase (day 21), and maturity phase (day 35), and the three samples were mixed until they were homogeneous.
Fresh samples were suspended in water at 1:10 (w/w) to measure the pH and water-soluble carbon (WSC) contents. Fresh samples were suspended in 2 mol L−1 KCl at 1:50 (w/w) to measure the NO3−, NO2−, and NH4+ contents. The pH values were determined with a Thermo Orion 3-star pH-meter (San Diego, CA, USA), and WSC was tested using a TOC Analyzer (Elementar, Germany). NO3−, NO2−, and NH4+ were analyzed colorimetrically by flow injection analysis. Kjeldahl analysis was used to determine the total nitrogen (TN) content (for details of the data, please refer to the supplementary material and our previous study [14]).
2.2 DNA extraction and sequencing
The composting samples were pretreated by freezing and crushing, before DNA extraction using a Fast DNA Kit for Soil (MP Biomedicals, USA), as described in a previous study [14].
High-throughput sequencing was conducted using the Ion S5 XL platform with the eukaryotic 18S V9 universal primers 1389F (TTGTACACACCGCCC) and 1510R (CCTTCYGCAGGTTCACCTAC), and ITS5-1737F (GGAAGTAAA AGTCGTAACAAGG) and ITS2-2043R (GCTGCGTTCTTCAT SCGATGC) for fungi. The 16S V4 region bacterial primers 341F (CCTACGGGAGGCAGCAG) and 518R (ATTACCGCGGCTGCTGG) were used for bacteria, and the Illumina HiSeq platform was employed for high-throughput sequencing. All sequencing was performed by Novogene (Beijing, China). The raw sequences were deposited in the NCBI Sequence Read Archive (SRA) database under accession number PRJNA681775.
2.3 Bioinformatics analysis
The low-quality parts of the eukaryote reads and chimera sequences were removed using Cutadapt (V1.9.1). Chimera sequences were then removed according to the barcode data. Comparisons were conducted with the species annotation database to detect chimera sequences (https://github.com/torognes/vsearch/), before finally removing the chimera sequences to obtain the final valid data (clean reads). After clustering all of the effective tags (Uparse v7.0.1001), the sequences were clustered at 97% identity by default clustering to obtain operational taxonomic units (OTUs). QIIME software (Version 1.9.1) and BLAST were used for species annotation, and the pr2_version_4.10 database for species annotation analysis [20, 21]. MUSCLE (Version 3.8.31) was employed for rapid multiple sequence alignment to determine the phylogenetic relationships among all representative OTUs. Finally, the data in each sample were normalized and the sample with the lowest amount of data was used as the standard. Based on previous studies [15, 17, 18, 22], the protist OTUs were divided into different functional groups according to their feeding patterns, i.e., phagotrophs, bacterivores, omnivores, saprotrophs, and parasites. The bioinformatics analyses of bacteria and fungi were based on previous studies [23, 24].
2.4 Statistical analysis
Principal component analysis was conducted based on Euclidean distances to explore differences in the structure of the protist community. Heatmaps of the protist community were generated using TBtools [25]. Random forest analysis (random Forest package in R 3.5.0) was performed to determine the key environmental factors (pH, NO3−, NO2−, WSC, temperature, total nitrogen, and NH4+) and biological factors (including fungal and bacterial communities) associated with differences in the functional groups of protists during composting. The importance of each abiotic and biotic factor was calculated based on the average value obtained using 5000 trees and according to the increase in the mean square error. Redundancy analysis (RDA) was conducted to analyze the positive and negative correlations between the relative abundances of the main protist functional groups in compost and the most important abiotic and biotic factors screened by random forest analysis (CANOCO 5.0). In order to further investigate the bacterial community, the key paths and relative contributions of nxrA-harboring nitrifiers and nirS-harboring denitrifiers to the changes in the protist community were established with AMOS 22.0 to produce structural equation models (SEMs). Network analysis was conducted to obtain insights into the potential interactions among microbes in samples from different composting phases. In these analyses, 60 bacterial genera (divided functions), 60 fungal genera, and 60 protist genera (including six functional groups) were used to construct a network. A similarity matrix was calculated based on the Spearman’s rank correlations. The Gephi platform 0.9.2 was used to visualize the network graphs.
3 Results and discussion
3.1 Protist community structure and composition
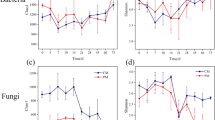
The differences in the protist communities among all samples were determined by principal component analysis during different composting phases (Fig. 1a). The protist communities in the samples collected on days 2, 7, and 35 differed significantly from those in the other phases, but the protist communities were similar on days 14 and 21 (Fig. 1a). Similar changes to those in the protist community structures occur among bacteria during composting [24], and a previous study showed that the degradation of organic matter was determined by the metabolic and enzyme activities of bacteria during the thermophilic phase [24]. The main components of WSC are simple sugars produced by the decomposition of complex organic matter [24], and WSC increased by 55% during the initial thermophilic phase in the present study (Fig. S1). Thus, protists may have been involved in promoting the decomposition of organic matter during the thermophilic phase.
The phagotroph functional group dominated the protist community during composting (Fig. 1b), and previous studies also found that this functional group was dominant in other environments [18, 26]. Acantharea-Group-II_XX was dominant during the mesophilic and thermophilic phases, where its abundance increased by 12.8% during the thermophilic phase but decreased by 84.4% at the end of composting. Murdock and Juniper (2019) showed that Acantharea-Group-II_XX is a member of Rhizaria [27], and it has the ability to resist high temperatures during composting [28], and thus, it may have contributed to the degradation of organic matter by bacteria during the thermophilic phase. Stachyamoeba and Acantharea_XXX dominated during the cooling and maturity phases, where the abundance of Acantharea_XXX increased by 74.5% and 275.3%, respectively, during these phases. A previous study showed that nitrification was stronger than denitrification in the late stage of composting [14], and these conditions may have been closely related to the activity of this protist.
In addition, Haematococcus, Thaumatomonas, Gregarines_XX, Chytriodinium, and Paravahlkampfia were only found in the maturation phase, and these protists may reduce the abundances of pathogenic bacteria in composting products; e.g., Thaumatomonas has a significant predation effect on MS2 phages [29, 30]. However, Platyophrya, Chrysophyceae_Clade-C_X, Ochromonas, and Trinema were only present in the initial stage of composting. Interestingly, Frontonia, Protacanthamoeba, and Pfiesteria disappeared in the thermophilic phase (Fig. 1b), and they may carry pathogenic factors; in particular, Protacanthamoeba is highly pathogenic [31]. Thus, phagotroph protists might have been involved in the transformation of the composting material, and the high temperature could have killed pathogenic protists.
3.2 Abiotic and biotic factors that affected changes in the protist community
According to the distribution and composition of the protist community, the environmental conditions and bacterial and fungal communities may have affected the protist community during composting. Random forest analysis showed that NO2− and NO3−, and the bacterial community were the most important factors (P < 0.05) to affect the protist community (Fig. 2a). A previous study showed that nitrate can inhibit phagotrophic protists when applied to the soil together with organic fertilizers [12]. NO3− and NO2− mainly had positive effects on omnivores and saprotrophs, whereas phagotrophs and parasites were negatively affected by NO2− and NO3− (Fig. 2b). In the maturity phase, the nitrate content was 1.2 times higher than that in the cooling phase (Fig. S1), thereby suggesting that a specific level of nitrate might have inhibited phagotrophic protists. In addition, the bacterial community composition explained 45.7% of the variation in the protist community, and the bacterial composition had positive effects on phagotrophs (Fig. 2b). Phagotrophic protists were dominant in terms of the number of species and their abundances in this study, which is consistent with previous results obtained in soils amended with organic fertilizer [18, 26]. Thus, protists may have interacted with bacteria involved in the transformation of NO3− and NO2−.
3.3 Effects of nitrifying/denitrifying bacterial communities on changes in the protist community
The conversion of NO3− and NO2− mainly involves nitrification and denitrification processes [32, 33], and there may be connections among nitrifiers, denitrifiers, and protists. Based on previous findings [14], nxrA-harboring nitrifiers, nirS-harboring denitrifiers, and the total bacterial community were analyzed to determine the relative contributions of bacteria associated with NO3− and NO2− conversion to the variations in the protist community (Fig. 3). The SEM results indicated that the overall bacterial community mainly affected protists through denitrifying bacteria. The protist community had significant positive effects on nirS-harboring denitrifiers (P < 0.01), but no significant negative effects on nxrA-harboring nitrifiers (P > 0.05). Positive correlations in SEMs indicate that two factors have mutually promoting effects and negative correlations denote the possibility of mutual use between two factors [34]. Thus, the denitrifying bacteria and protists may have mutually promoted growth, mainly because nirS-harboring denitrifiers could relieve the inhibition of nitrate. Indeed, the contribution of nirS to the conversion of NO2− mainly occurred in the early phase and that of nxrA in the maturity phase [14]. The NO3− content decreased in the early phases, but increased in the maturity phase. Therefore, the denitrifying bacterial communities were more conducive to the growth of protist communities compared with nitrifying bacteria.
3.4 Interactions among protists, bacteria, and fungi
The microbial network was constructed based on the bacteria, fungi, and protists to understand the ecological interactions among protists and other microorganisms. The results showed that the network formed three main modules, and some protists connected the bacterial and fungal groups (Fig. 4). Module 1 contained most of the bacteria (24 nodes), fungi (43 nodes), and protists (35 nodes), where the protists mainly belonged to phagotroph and bacterivore functional groups. Thermomonas and Prevotella were the most important bacteria, and Neocosmospora, Fusarium, and Neonectria were the most important fungi. Bacteria (such as Luteimonas, Thalassospira, and Ethanoligenens) and mixotroph protists (such as Dinophyceae_XXX) dominated module 2. Module 3 was dominated by bacteria such as Bacillus and bacterivore protists (such as Echinamoeba). Previous studies also found that protists had key roles in connecting bacterial and fungal communities in soil [12], groundwater [29], and extreme environments [27].
Each module was generally associated with a specific range of functions, and module 1 was mainly important for maintaining phagotroph and bacterivore protist functional groups. Acantharea-Group-II_XX had positive interactions with most other protists, but negative interactions with the main bacteria (such as Steroidobacter) and fungi (such as Neocosmospora, Fusarium, and Neonectria) in this module. Faust and Raes proposed that positive correlations may indicate commensalism or mutualism, whereas negative correlations might be attributed to predation, competition, or unfair competition in an ecological network [35]. The results indicated that Acantharea-Group-II_XX was mainly reliant on predatory fungi for maintaining its activity during the mesophilic and thermophilic phases, and fungi exhibit greater tolerance of high temperature than bacteria [28, 36]. In addition, Fahrbach et al. found that Steroidobacter is an important host of nirS denitrification genes [37], thereby suggesting that Acantharea-Group-II_XX may prey on some denitrifying bacteria. However, Acantharea_XXX had negative interactions with various protists (including Acantharea-Group-II_XX, Prasino-Clade-9-B_X, and Colpodella), fungi, and bacteria (Thermomonas and Prevotella). Acantharea_XXX may have competitive or predatory relationships with many protists, fungi, and bacteria, which may explain the dominance of Acantharea_XXX in the cooling and maturation phases (Fig. 1b). Moreover, similar to Acantharea-Group-II_XX, Acantharea_XXX may prefer to prey on high temperature-tolerant denitrifying bacteria (such as Thermomonas).
Compared with module 1, the microbial relationships were simpler in modules 2 and 3. Thalassospira is a heterotrophic bacterial genus that can degrade organic matter [38], and Dinophyceae_XXX had positive correlations with protists and a negative correlation with Thalassospira in module 2. In module 3, Echinamoeba had positive correlations with other microorganisms, thereby suggesting that module 3 was associated with a bacterivore function. Modules 1, 2, and 3 were mainly connected by protists and bacteria, and protists were most closely related to bacteria with a denitrification function. For example, Prasino-Clade-9-B_X and Nolandellidae_X had negative interactions with Luteimonas (module 2), and Echinamoeba had negative interactions with Thermomonas (module 1) and Oscillospira (module 1). Previous studies also found that protists, especially phagotrophs, were key predators that promoted the cycling of nutrient elements and regulated the structure of the microbial community [5, 39]. In addition, some plant pathogens among fungi (such as Fusarium [40]) had negative correlations with the protists in each module, thereby demonstrating that protists had important roles in rendering the composting harmless.
4 Conclusion
In this study, the structure of the protist community in the cooling phase was similar to that at the end of the thermophilic phase, and the phagotrophic functional group dominated in different phases. Acantharea-Group-II_XX was dominant in the mesophilic and thermophilic phases, whereas Acantharea_XXX dominated the cooling and maturation phases. Protists, bacteria, and fungi formed different hubs, and protists were most closely related to bacteria with denitrification functions. Denitrifying bacterial communities were more conducive to the growth of Acantharea-Group-II_XX and Acantharea_XXX, and phagotrophic protists were key predators that regulated the structure of bacteria with denitrification functions.
References
Robledo-Mahón T, Martín MA, Gutiérrez MC et al (2019) Sewage sludge composting under semi-permeable film at full-scale: evaluation of odour emissions and relationships between microbiological activities and physico-chemical variables. Environ Res 177:108624. https://doi.org/10.1016/j.envres.2019.108624
Shao B, Liu Z, Zhong H et al (2017) Effects of rhamnolipids on microorganism characteristics and applications in composting: a review. Microbiol Res 200:33–44. https://doi.org/10.1016/j.micres.2017.04.005
Pan S, Wang G, Chen H et al (2021) Building a framework of aerobic deer manure/corn stover composting with black liquor/microbial inoculation. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-021-01792-4
Yin Y, Yang C, Tang J et al (2021) Bamboo charcoal enhances cellulase and urease activities during chicken manure composting: roles of the bacterial community and metabolic functions. J Environ Sci 108:84–95. https://doi.org/10.1016/j.jes.2021.02.007
Geisen S, Mitchell EAD, Adl S et al (2018) Soil protists: a fertile frontier in soil biology research. FEMS Microbiol Rev 42:293–323. https://doi.org/10.1093/femsre/fuy006
Minerovic AD, Potapova MG, Sales CM et al (2020) 18S-V9 DNA metabarcoding detects the effect of water-quality impairment on stream biofilm eukaryotic assemblages. Ecol Indic 113:106225. https://doi.org/10.1016/j.ecolind.2020.106225
Hu B, Qi R, An W, Yang M (2012) Responses of protists with different feeding habits to the changes of activated sludge conditions: a study based on biomass data. J Environ Sci 24:2127–2132. https://doi.org/10.1016/S1001-0742(11)61049-8
Kurm V, van der Putten WH, Weidner S et al (2019) Competition and predation as possible causes of bacterial rarity. Environ Microbiol 21:1356–1368. https://doi.org/10.1111/1462-2920.14569
Rosenberg K, Bertaux J, Krome K et al (2009) Soil amoebae rapidly change bacterial community composition in the rhizosphere of Arabidopsis thaliana. ISME J 3:675–684. https://doi.org/10.1038/ismej.2009.11
Cairns J, Jalasvuori M, Ojala V et al (2016) Conjugation is necessary for a bacterial plasmid to survive under protozoan predation. Biol Lett 12:20150953. https://doi.org/10.1098/rsbl.2015.0953
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: the quest for niche specialisation and differentiation. Trends Microbiol 20:523–531. https://doi.org/10.1016/j.tim.2012.08.001
Wang Z, Gong X, Zheng Y et al (2020) Soil protist communities in burrowing and casting hotspots of different earthworm species. Soil Biol Biochem 144:107774. https://doi.org/10.1016/j.soilbio.2020.107774
Sherr BF, Sherr EB, Berman T (1983) Grazing, growth, and ammonium excretion rates of a heterotrophic microflagellate fed with four species of bacteria. Appl Environ Microbiol 45:1196–1201
Yin Y, Yang C, Gu J et al (2019) Roles of nxrA-like oxidizers and nirS-like reducers in nitrite conversion during swine manure composting. Bioresour Technol 297:122426. https://doi.org/10.1016/j.biortech.2019.122426
Xiong W, Jousset A, Guo S et al (2018) Soil protist communities form a dynamic hub in the soil microbiome. ISME J 12:634–638. https://doi.org/10.1038/ismej.2017.171
Nguyen BAT, Chen QL, He JZ, Hu HW (2019) Microbial regulation of natural antibiotic resistance: understanding the protist-bacteria interactions for evolution of soil resistome. Sci Total Environ 705:135882. https://doi.org/10.1016/j.scitotenv.2019.135882
Xiong W, Li R, Guo S et al (2019) Microbial amendments alter protist communities within the soil microbiome. Soil Biol Biochem 135:379–382. https://doi.org/10.1016/j.soilbio.2019.05.025
Zhao Z-B, He J-Z, Quan Z et al (2020) Fertilization changes soil microbiome functioning, especially phagotrophic protists. Soil Biol Biochem 148:107863. https://doi.org/10.1016/j.soilbio.2020.107863
Santos SS, Schöler A, Nielsen TK et al (2020) Land use as a driver for protist community structure in soils under agricultural use across Europe. Sci Total Environ 717:137228. https://doi.org/10.1016/j.scitotenv.2020.137228
de Vargas C, Audic S, Henry N et al (2015) Eukaryotic plankton diversity in the sunlit ocean. Science 348:1261605. https://doi.org/10.1126/science.1261605
Guillou L, Bachar D, Audic S et al (2013) The Protist Ribosomal Reference database (PR2): a catalog of unicellular eukaryote Small Sub-Unit rRNA sequences with curated taxonomy. Nucleic Acids Res 41:597–604. https://doi.org/10.1093/nar/gks1160
Tsao HF, Scheikl U, Herbold C et al (2019) The cooling tower water microbiota: seasonal dynamics and co-occurrence of bacterial and protist phylotypes. Water Res 159:464–479. https://doi.org/10.1016/j.watres.2019.04.028
Rossmann M, Pérez-Jaramillo JE, Kavamura VN et al (2020) Multitrophic interactions in the rhizosphere microbiome of wheat: from bacteria and fungi to protists. FEMS Microbiol Ecol 96:1–14. https://doi.org/10.1093/femsec/fiaa032
Yin Y, Gu J, Wang X et al (2019) Effects of rhamnolipid and Tween-80 on cellulase activities and metabolic functions of the bacterial community during chicken manure composting. Bioresour Technol 288:121507. https://doi.org/10.1016/j.biortech.2019.121507
Chen CJ, Chen H, Zhang Y et al (2020) TBtools: an integrative toolkit developed for interactive analyses of big biological data. Mol Plant 13:1194–1202. https://doi.org/10.1016/j.molp.2020.06.009
Oliverio AM, Geisen S, Delgado-Baquerizo M et al (2020) The global-scale distributions of soil protists and their contributions to belowground systems. Sci Adv 6:1–11. https://doi.org/10.1126/sciadv.aax8787
Murdock SA, Juniper SK (2019) Hydrothermal vent protistan distribution along the Mariana arc suggests vent endemics may be rare and novel. Environ Microbiol 21:3796–3815. https://doi.org/10.1111/1462-2920.14729
Xi B, He X, Dang Q et al (2015) Effect of multi-stage inoculation on the bacterial and fungal community structure during organic municipal solid wastes composting. Bioresour Technol 196:399–405
Deng L, Krauss S, Feichtmayer J et al (2014) Grazing of heterotrophic flagellates on viruses is driven by feeding behaviour. Environ Microbiol Rep 6:325–330. https://doi.org/10.1111/1758-2229.12119
Feichtmayer J, Deng L, Griebler C (2017) Antagonistic microbial interactions: contributions and potential applications for controlling pathogens in the aquatic systems. Front Microbiol 8:1–14. https://doi.org/10.3389/fmicb.2017.02192
Paknejad N, Hajialilo E, Saraei M, Javadi A (2020) Isolation and identification of Acanthamoeba genotypes and Naegleria spp. from the water samples of public swimming pools in Qazvin. Iran J Water Health 18:244–251. https://doi.org/10.2166/wh.2019.074
Cáceres R, Malińska K, Marfà O (2018) Nitrification within composting: a review. Waste Manag 72:119–137. https://doi.org/10.1016/j.wasman.2017.10.049
Maeda K, Toyoda S, Philippot L et al (2017) Relative contribution of nirK- and nirS- bacterial denitrifiers as well as fungal denitrifiers to nitrous oxide production from dairy manure compost. Environ Sci Technol 51:14083–14091. https://doi.org/10.1021/acs.est.7b04017
Wu J, Qi H, Huang X et al (2018) How does manganese dioxide affect humus formation during bio-composting of chicken manure and corn straw? Bioresour Technol 269:169–178. https://doi.org/10.1016/j.biortech.2018.08.079
Faust K, Raes J (2012) Microbial interactions: from networks to models. Nat Rev Microbiol 10:538–550. https://doi.org/10.1038/nrmicro2832
Gu W, Lu Y, Tan Z et al (2017) Fungi diversity from different depths and times in chicken manure waste static aerobic composting. Bioresour Technol 239:447–453. https://doi.org/10.1016/j.biortech.2017.04.047
Fahrbach M, Kuever J, Remesch M et al (2008) Steroidobacter denitrificans gen. nov., sp. nov., a steroidal hormone-degrading gammaproteobacterium. Int J Syst Evol Microbiol 58:2215–2223
Fan X, Zhu Y, Gu P et al (2017) Bacterial community compositions of propylene oxide saponification wastewater treatment plants. RSC Adv 7:22347–22352. https://doi.org/10.1039/c6ra27808f
Thakur MP, Geisen S (2019) Trophic regulations of the soil microbiome. Trends Microbiol 27:771–780. https://doi.org/10.1016/j.tim.2019.04.008
Yuan J, Wen T, Zhang H et al (2020) Predicting disease occurrence with high accuracy based on soil macroecological patterns of Fusarium wilt. ISME J 14:2936–2950. https://doi.org/10.1038/s41396-020-0720-5
Funding
This study was supported by the Natural Science Foundation of Shaanxi Province (No. 2021JQ-501), Key Research and Development Project of Shaanxi Province (No. 2020ZDLNY06-08), China Postdoctoral Science Foundation (No. 2020M673357), Youth Innovation Team of Shaanxi Universities in 2020 year (PI: Dr Haihan Zhang), Scientific Research Program of Shaanxi Provincial Education Department (No. 20JT039), and Shaanxi Provincial Program for Innovative Research Team (No. 2019TD-025).
Author information
Authors and Affiliations
Contributions
Conceptualization: Yanan Yin; Methodology: Yanan Yin, Mengtong Li, Chao Yang; Data curation: Yanan Yin, Mengtong Li, Chao Yang, Xunzhang Hu; Writing — original draft preparation: Yanan Yin; Writing—review and editing: Yanan Yin, Wei Zheng, Manli Duan, Xiaochang Wang, Rong Chen; Funding acquisition: Yanan Yin, Rong Chen; Investigation: Mengtong Li, Chao Yang, Xunzhang Hu; Supervision: Xiaochang Wang, Rong Chen.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yin, Y., Li, M., Yang, C. et al. Phagotrophic protists can change microbial nitrogen conversion patterns during swine manure composting. Biomass Conv. Bioref. 14, 517–524 (2024). https://doi.org/10.1007/s13399-022-02318-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-022-02318-2