Abstract
This study investigated the potential benefit of co-digesting sewage sludge (SS) and settled/mixed fat, oil, and grease (FOG) with BioAmp pretreatment, a bio-additive product, for a utility. BioAmp was found to increase the soluble chemical oxygen demand (SCOD)/COD ratio of FOG (from 4.19 to 4.49), consequently enhancing volatile solid (VS) reduction (from 75.9 to 84.8%). The ratio of SS and FOG for co-digestion was based on their actual daily production volume, resulting in FOG content ranging between 15 and 20% VS for co-digestion assays. Co-digestion of SS and mixed FOG waste after BioAmp pretreatment produced the highest cumulative methane yield (CMY) of 400 mL/g VSadded, which is 98% higher than that of mono-digestion of SS (202 mL/g VSadded). Kinetic analysis demonstrated a synergistic effect in co-digestion of FOG and SS with both increased CMY and degradation rate constant. For the utility, co-digestion can increase methane yield by 141%; however, 7.77% more biosolids are produced and 6% more total nitrogen will return to mainstream, a potential burden to nutrient management. Overall, the results of batch test demonstrated the benefits of co-digestion of SS and FOG with BioAmp pretreatment in terms of increased CMY, degradation rate constant, and VS reduction. The analysis of the utility showed that co-digestion can produce more biogas but also increase more biosolids and total nitrogen return.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
In recent years, co-digestion of sewage sludge (SS) with other organic wastes has gained popularities [2, 11, 45]. Compared with mono-digestion of SS, co-digestion can generate more biogas through balanced C/N ratio, improved organic loading rate (OLR), and supplementation of micronutrients [29]. Furthermore, several works also reported a synergistic effect of co-digestion in improving overall degradation efficiency, shortened lag period, and enhanced biogas production [2, 5, 6]. For the utilities, co-digestion can also gain additional revenue from gate fees or service charges of accepted organic wastes [30]. With all these added benefits, a growing number of wastewater treatment plants are adapting co-digestion to achieve potential energy neutrality [37].
Fat, oil, and grease (FOG) has been attempted as a co-digestion substrate due to its high carbon content and high C/N ratio [16, 44, 46]. FOG has been demonstrated to have higher convertibility (94.8%) to biogas than carbohydrate-rich (50.4%) and protein-rich (71%) substrates [20]. Therefore, theoretically FOG has higher cumulative methane yield (CMY) (1 m3 CH4/kg VSadded) than carbohydrate (0.42 m3 CH4/kg VSadded) and protein (0.63 m3 CH4/kg VSadded) [20]. Xu et al. [46] reported an increase of 45% additional biogas at a FOG loading of 42% VS compared to mono-digestion of SS. Similarly, Wan et al. [41] reported that with a FOG loading of 64% of VS, methane yield was 137% higher than SS mono-digestion. In addition to laboratory experiments, a full-scale study on 6 municipal wastewater treatment plants demonstrated additional 60–300% of biogas production with the addition of FOG at 3–35% based on total solids (TS) [13]. A 10-month study at the Hyperion Wastewater Treatment Plant in Los Angeles showed that co-digestion of FOG at 20% VS generated 31% more biogas than SS mono-digestion. The total cost saving due to energy production from FOG co-digestion was estimated to be $40,000 over the course of the 10-month program [31]. Overall, co-digestion with FOG has been proved to be a promising method to increase methane yield and reduce its environmental impact as a waste.
Despite the benefit of increased biogas yield, FOG co-digestion was also reported to associate with digestion instability and failure [16]. The main reason for digester failure is due to overloading of FOG, which results in the accumulation of long-chain fatty acids (LCFA). LCFA are hydrolyzed from FOG with the help of hydrolytic and fermentative bacteria [35]. However, the degradation of LCFA to volatile fatty acid (VFA) through β-oxidation pathway has been regarded as the rate-limiting process due to a higher energy requirement for LCFA degradation than other processes [23]. In addition, the accumulation of LCFA can change cellular morphology, decrease cell permeability, and impact substrate diffusion and transport into bacteria, consequently resulting in process inhibition [10, 19, 32]. Xu et al. [46] pointed out that when FOG contents reached 55%, biogas production drastically reduced to 40.3% of the mono-digestion of SS. Similarly, a separate study reported that biogas production potential slowed down from 0.752 to 0.63 m3 CH4/kg VSadded coupled with an observed decrease in pH and alkalinity when FOG waste increased from 20 to 40% of the digester feed [42]. In addition, the buildup of FOG residual on flow meter can cause inaccurate reading, and feed pump failure raised from incompatibility between FOG and plastic stator [31]. He et al. [18] found that it is actually FOG deposit generated from LCFA and calcium causing pump and other machine failures. The maintenance problems are more common in treating the diary, slaughterhouse, and restaurant wastewater due to FOG solidification especially at low temperatures [40]. Therefore, co-digestion with FOG should be cautious on OLR and routine equipment maintenance.
BioAmp, a commercial bio-addictive product by ECO Bionics, is designed to facilitate FOG degradation. It consists of 3 species of Bacillus bacteria (Bacillus subtilis, Bacillus licheniformis, and Bacillus thuringiensis) and 2 species of Pseudomonas bacteria (Pseudomonas fluorescens and Pseudomonas putida) [36]. These bacteria strains have been selected for their ability to produce amylase, protease, and lipase degrading carbohydrates, proteins, and fats to small molecules, so that small molecules can enter the metabolic pathways of the bacterium, facilitating their membrane transport and metabolisms [34]. The rate-limiting LCFA degradation can also be facilitated by most Pseudomonas species and a few Bacillus species, avoiding the accumulation of LCFA [1, 14, 15, 21]. During a 3-month treatment study of direct BioAmp addition to a grease trap, a 40% reduction of FOG deposit and a 50% reduction of LCFA were observed [39]. Therefore, BioAmp possesses a potential in remediating the problems that might arise from FOG co-digestion.
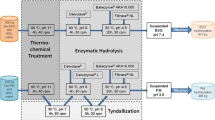
This research focuses on the feasibility study to update the FOG treatment process for Derry Township Municipal Authority (DTMA) Clearwater Road wastewater treatment plant (WWTP), Pennsylvania, USA. The DTMA WWTP currently receives hauled-in FOG wastes with a daily volume of approximately 53,000 gallons. This grease trap waste is delivered to the DTMA WWTP by truck and discharged into an aerobic grease pretreatment digester where it reacts with BioAmp bacteria for 12 h. Currently, the reacted waste is pumped into a primary sedimentation tank for further treatment. To better utilize the FOG waste, it is proposed to send this reacted FOG waste directly to the anaerobic digester for additional biogas production and aeration cost reduction in secondary treatment. The objective of this research was to examine the performance of co-digestion of SS and FOG with/without BioAmp pretreatment of settled and unsettled FOG wastes for the DTMA WWTP in terms of VS reduction, additional methane production, degradation kinetics, digestate quality, and an overall plant assessment.
2 Materials and methods
2.1 Substrates and inoculum
FOG wastes, SS, and inoculum were collected from the DTMA WWTP. An average of 12,000–15,000 gallons of grease per day is introduced to DTMA grease trap. BioAmp is incubated in a biogenerator apparatus at room temperature and neutral pH for 6 h and dispensed to grease trap 4 times a day. Around 123 trillion live cells are introduced to grease trap every day. The detailed BioAmp and grease trap system information can be found in Tang et al. [39] and Schutz [36]. FOG samples were taken from the grease truck upon discharge to the grease reactor and after the waste had been allowed to react with BioAmp for 12 h at around 20 °C. The SS was a combination of sludge from both the primary and secondary sedimentation tanks with 1:1 mixing ratio. The inoculum was taken from the primary anaerobic digester with a sludge retention time of 24 days.
All the samples (inoculum, SS, and FOG samples before and after BioAmp pretreatment) were collected and transported to lab with ice packs within 1 h. FOG samples were then divided into two categories, which are mixed FOG samples and settled FOG samples, since these are two potential options for the plant to co-digest with FOG wastes. The first option is to send all FOG wastes after a 12-h BioAmp pretreatment into the anaerobic digestion tank. The other option is to pump only bottom half of the 1-h settled FOG waste after the 12-h BioAmp pretreatment into the anaerobic digestion tank. Initial sample characteristics were analyzed within 24 h (Table 1). The inoculum was incubated at 35 °C until biogas production ceased. SS and FOG were stored at −20 °C and thawed at 4 °C the day before use.
2.2 Experimental design
Duplicate reactors of nine treatments and one control were set up in each 150-mL serum bottle. Each reactor contained 100 mL of liquid, which includes 0.9 g VS of digestion materials and 0.45 g of inoculum (F/M ratio of 2 as recommended by Braguglia et al. [9]). Digestion materials include SS, mixed FOG waste before BioAmp pretreatment (BM), mixed FOG waste after BioAmp pretreatment (AM), settled FOG waste before BioAmp pretreatment (BS), settled FOG waste after BioAmp pretreatment (AS), inoculum, co-digestion of SS and BM, co-digestion of SS and AM, co-digestion of SS and BS, and co-digestion of SS and AS. Based on the characteristics of each substrate and inoculum and F/M ratio selected in this study, the initial substrate concentration was designed to be 9 g VSadded/L. The co-digestion ratio used was based on the actual volume ratio of daily sludge and FOG with and without settling. This results in a FOG content ranging between 15 and 20% VS for co-digestion assays. The initial pH was adjusted to 7.0 ± 0.1 by using 1 M HCl or 1 M NaOH. Then, the reactors were purged with nitrogen for 2 min, sealed with a rubber sleeve stopper and aluminum crimp cap to create an anaerobic environment devoid of oxygen, and incubated in a shaking water bath (VWR, USA) at 90 rpm and 35 °C for digestion.
2.3 Analytical methods
The characteristics of inoculum, SS, and all FOG samples were tested with TS, VS, pH, COD, soluble chemical oxygen demand (SCOD), total ammonia nitrogen (TAN), and alkalinity. After digestion, digestates were tested for TS, VS, COD, SCOD, pH, TAN, VFA, and alkalinity.
TS and VS were measured based on Standard Method 2540 [4]. The measurement of alkalinity was using Standard Method 2320 [4]. COD, SCOD, and TAN were determined using Hach analysis kits (Cat. Nos. 2415915, 2125925, and 2606945). For SCOD, TAN, VFA, and alkalinity, samples were first centrifuged at 3500×g for 20 min at 4 °C and filtered through a 0.45-μm cellulose filter before analysis. Free ammonia was calculated based on TAN, temperature, and pH as described by Hansen et al. [17], and VFAs were extracted and quantified by gas chromatography as proposed by Bai and Chen [6].
2.4 Biogas and methane quantification
Biogas and methane contents were measured daily until biogas production ceased. The volume of biogas produced was measured based on a liquid displacement method [49]. Methane content was determined using a gas chromatography-flame ionization detector as described by Bai and Chen [6]. The actual methane production of each substrate was calculated by the difference between the methane volume from each digester and the inoculum control. Then, the methane volume was corrected to standard temperature and pressure conditions (0 °C and 1 atm).
2.5 Volatile solid removal calculation
Percent volatile solid removal was calculated based on the actual amount of VS removed in substrates excluding the input from the inoculum (Eq. (1)) as described by Zhang et al. [49].
where F is the total VS of substrate added to reactor (g), I is the total VS of inoculum added to reactor (g), a is the calculated percent VS removal based on initial VS (substrate and inoculum) and after-digestion VS (substrate and inoculum) in the reactor (%), and b is the calculated percent VS removal based on initial VS (inoculum) and after-digestion VS (inoculum) in the blank reactor (%).
2.6 Kinetic model
Both the first-order kinetic model and the modified Gompertz model were used to evaluate degradation kinetics of the digestion. To obtain the potential cumulative methane yield and degradation rate constant in the first-order model, and the potential maximum methane yield, maximum methane production rate, and the length of lag phase in the modified Gompertz model, SigmaPlot 13.0 was used to perform non-linear curve fitting to fit 91 cumulative methane yield data points to corresponding equations. Dynamic fit wizard was used to converge to the best possible solution until the differences between the residual sum of squares no longer decreases significantly.
2.6.1 The first-order kinetic model
The first-order kinetic model (Eq. (2)) assumes that the gas production follows the first-order kinetics [7]. It is used to assess substrate degradability and potential maximum methane yield.
where M(t) is the cumulative methane yield at time t (mL/g VS), Mmax is the potential maximum methane yield (mL/g VS), K is the degradation rate constant (day−1), and t is the duration of the digestion (days).
2.6.2 The modified Gompertz model
The modified Gompertz model (Eq. (3)) is based on the growth of bacteria in batch mode and accounts for an initial lag phase which represents the adaptation and hydrolysis period of microorganisms in anaerobic digestion [24]. The potential maximum methane yield, the maximum methane production rate, and the length of lag phase were determined by fitting the CMY using the modified Gompertz model [25].
where M(t) is the cumulative methane yield at time t (mL/g VS), Mmax is the potential maximum methane yield (mL/g VS), Rmax is the maximum methane production rate (mL/g VS/day), λ is the length of lag phase (days), and t is the duration of the digestion (days).
2.7 Statistical analysis
ANOVA (95% confidence interval) and Tukey’s paired comparison were used for data comparisons. All the statistical analyses were conducted in SigmaPlot 13.0.
3 Results and discussion
3.1 Sample characterization and effect of BioAmp pretreatment
The characteristics of all samples used in this study are shown in Table 1. Compared with FOG, SS has lower VS, TS, VS/TS ratio, SCOD, and COD but higher TAN, pH, and alkalinity, which indicated that FOG has the potential to produce more biogas than SS. The potential drawback of FOG digestion, however, is its low pH and alkalinity because of its acidic nature [22, 35, 47]. Also, anaerobic degradation of FOG can produce glycerol and fatty acids, which can further reduce alkalinity and pH. However, the high COD/N ratio of FOG can balance the low COD/N ratio of SS to optimize digestion. The SCOD/COD ratio of FOG (2.89–4.49%) was higher than that of SS (1.21%), indicating FOG COD is more soluble than SS and thus is expected to have a higher level of biogas production at initial stage.
Comparing between BM and AM or between BS and AS, it is apparent that BioAmp pretreatment can reduce TS, VS, SCOD, COD, TAN, and alkalinity of FOG. With BioAmp pretreatment, both SCOD and COD were reduced, which is consistent with the observations from Tang et al. [39]. In addition, with the increase of SCOD/COD ratio after BioAmp pretreatment, more particulate COD was consumed than SCOD. TAN was also reduced by the pretreatment which is likely due to assimilation by biomass growth despite ammonia release from protein hydrolysis. About 20% of alkalinity was reduced after BioAmp pretreatment which is possibly due to VFA accumulation. Between mixed FOG and settled FOG, settled samples contained higher TS, VS, and COD than mixed samples, while VS/TS, SCOD, TAN, pH, and alkalinity remained similar. Compared with mixed samples, settled samples had more solids, indicating less favorable for anaerobic digestion.
3.2 Volatile solid reduction
In this study, batch anaerobic digesters were operated under mesophilic conditions for a total of 91 days. The percent VS reduction of SS (57.7%) was lower than all 4 FOG assays at 75.9%, 84.8%, 74.0%, and 86.1% for BM, AM, BS, and AS, respectively (Table 2), indicating FOG is more biodegradable than SS. In addition, among FOG assays, BioAmp pretreatment was observed to increase percent VS reduction (BM of 75.9% < AM of 84.8%; BS of 74.0% < AS of 86.1%). One of the key functions of BioAmp is to facilitate the degradation of non-soluble FOG into soluble and easily biodegradable substrates. This function is also observed by the increased ratios of SCOD/COD with BioAmp pretreatment (BM (4.19%) < AM (4.49%); BS (2.89%) < AS (3.25%)). Sedimentation, however, was expected to concentrate more slowly degradable solid materials, thus reducing its overall degradability, but this turned out to be only of minor reduction (BM of 75.9%, BS of 74.0%). Interestingly, when coupled with BioAmp pretreatment, settled sludge exhibited slightly increased %VS reduction, indicating BioAmp can still facilitate degradation of organic solids (BM of 75.9% < AM of 84.8%, BS of 74.0% < AS of 86.1%). Furthermore, the %VS reductions of co-digestion assays (63.0–78.1%) were between those of SS (57.7%) and FOG (74.0–86.1%). Comparing co-digestion assays and mono-digestion of SS, the digestate TS were similar; however, co-digestion resulted in lower VS content. This indicates that co-digestion can result in similar quantities of residual solids but with better stability.
3.3 Methane production
Digestion and gas production were monitored for a total of 91 days. The methane production rate and CMY for all the digesters are shown in Fig. 1. For mono-digestion of FOG with or without BioAmp pretreatment (Fig. 1b–e), a lag phase of 25 days was observed. This indicates a long adaptation phase is needed for FOG mono-digestion, which are likely due to inoculum not previously exposed to FOG. Similar lag phases have also been observed by other researches on the digestion of FOG [33, 41]. If adaptation to FOG is the reason, this lag phase will disappear eventually in subsequent reactor operation with repeated exposure. Another possibility is the lack of alkalinity. This will also result in a lag phase until the reactor recovers and can be avoided with the addition of alkalinity. Without a surprise, the mono-digestion of SS did not exhibit a lag phase since it is the material the inoculum has already been exposed to (Fig. 1a). Interestingly, co-digestion assays reduced the lag phase from over 20 days to less than 0.1 day (Fig. 1f–i). SS is known to contain abundant alkalinity, and therefore, it likely served as a source of alkalinity to FOG digestion, resulting in eliminating the lag phase.
Methane production rate and cumulative methane yield for sewage sludge (SS) only (a), mixed FOG before BioAmp pretreatment (BM) only (b), mixed FOG after BioAmp pretreatment (AM) only (c), settled FOG before BioAmp pretreatment (BS) only (d), settled FOG after BioAmp pretreatment (AS) only (e), co-digestion of SS and BM (f), co-digestion of SS and AM (g), co-digestion of SS and BS (h), and co-digestion of SS and AS (i) incubated at 35 °C. Circle represents methane production rate, and square represents cumulative methane yield. The values are average of two replication bottles
Despite the lag phase, the CMYs of FOG mono-digestion (BM of 792 mL/g VSadded, AM of 1127 mL/g VSadded, BS of 772 mL/g VSadded, and AS of 823 mL/g VSadded) were as high as 3.8–5.6 times the volume observed from SS mono-digestion (202 mL/g VSadded). These are comparable to those reported by other studies on mono-digestion of both SS (173–184 mL/g Added) and FOG (845–1404 mL/g VSadded) [8, 11, 22, 49]. SS is typically characterized as not easily biodegradable material and has poor biogas production potential [48], whereas FOG has been shown to be a methane-rich material due to its high carbon content and degradability [28]. Moreover, there was a 42% CMY increase for the mixed samples with the use of BioAmp pretreatment. For the settled samples, the CMY increase was 6.6% with the use of BioAmp. This increase shows that BioAmp pretreatment can convert FOG into more degradable materials and thus enhance their digestion. Tang et al. [39] confirmed that BioAmp can help increase the readily biodegradable COD fraction of grease trap effluent wastewater. It is also possible that the previously reported LCFA accumulation in FOG digestion is being removed by BioAmp bacteria. In addition, it was also shown that the mixed FOG had a higher CMY than settled FOG. This is likely due to that mixed FOG had a higher SCOD/COD ratio and therefore contained more degradable materials than the settled ones. Grease trap waste was one of the main sources of FOG wastes that DTMA receives, and it was previously reported that FOG mainly retains at the floatable (top) layer, and food particles and other settleable solids stay at the bottom sludge layer [38]. Hence, mixed assays likely contained more degradable FOG while settled assays contained more food wastes, resulting in higher CMY in the mixed assays.
The CMYs of co-digestion assays were 383 mL/g VSadded, 400 mL/g VSadded, 245 mL/g VSadded, and 320 mL/g VSadded for co-digestion of SS and BM, SS and AM, SS and BS, and SS and AS, respectively, which were 89%, 98%, 18%, and 58% more than CMY of SS. These results demonstrated that CMY increased by adding FOG to SS since FOG has higher methane production potential than SS and FOG is more biodegradable with 87% biodegradability compared with 72% biodegradability of SS. The CMYs of co-digestion assays are comparable to other studies with similar FOG load (200–420 mL/g VSadded) [11, 27]. Moreover, for mixed FOG and settled FOG, the CMYs increased by 4.4% and 30.6% with the help of BioAmp pretreatment. This indicates that BioAmp has a stronger effect on converting the particulate FOG waste than the soluble FOG waste, and solubilization of particulate FOG wastes may be the bottleneck of FOG digestion. In addition, sedimentation resulted in 36% and 20% of CMY reduction for assays without BioAmp and assays with BioAmp pretreatment, respectively. This further confirmed that particulate FOG waste is less degradable than the soluble FOG waste, and BioAmp can help facilitate particulate FOG waste digestion. In summary, to achieve maximum methane yield, it is recommended to send all BioAmp-pretreated FOG wastes directly into the anaerobic digester without sedimentation.
To determine whether co-digestion of FOG and SS has a synergistic effect, the expected CMYs of co-digestion were estimated by the combined mono-digestion of SS and FOG based on respective VS addition. The estimated amounts were then compared to the actual observed CMYs. The actual CMYs of co-digestion assays were 40.0%, 20.7%, −5.4%, and 22.1% higher than the expected CMYs which were 273 mL/g VSadded, 306 mL/g VSadded, 259 mL/g VSadded, and 262 mL/g VSadded for co-digestion of SS and BM, SS and AM, SS and BS, and SS and AS, respectively. Statistical analysis showed that actual CMYs were significantly larger than the expected CMYs for co-digestion of SS and BM, SS and AM, and SS and AS (P < 0.05). This increase demonstrated that, other than BS, co-digestion of SS and FOG had a synergistic effect on CMY. The synergistic effects were also observed by other researchers and likely due to an optimized C/N ratio and improved alkalinity [3, 43].
3.4 Kinetic model
3.4.1 The first-order kinetic model
All CMYs of mono-digestions and co-digestions were fitted with the first-order kinetic model. The first-order rate constants (K) of co-digestions ranged between 0.066 and 0.087 day−1, which were all much higher than those of mono-digestion of SS at 0.049 day−1, and 0.001–0.017 day−1 for mono-digestions of FOG (Table 3). This again confirms that co-digestion of SS and FOG can facilitate the digestion process and thus exhibits a synergistic effect. The results also showed that BioAmp pretreatment can facilitate the digestion rate, where K values of BioAmp-pretreated samples were higher than those without BioAmp pretreatment on both mixed samples (without: 0.084 day−1, with: 0.087 day−1) and settled samples (without: 0.066 day−1, with: 0.082 day−1). The mixed samples also showed overall higher degradation rate constants than the settled samples, which is likely due to the relatively higher SCOD/COD ratio of mixed samples. The model also allowed prediction of ultimate CMYs of all treatments. Based on the prediction, co-digestion of AM and SS can produce the highest amount of CMY (387 mL/g VS), followed by BM and SS (372 mL/g VS), AS and SS (307 mL/g VS), and BS and SS (235 mL/g VS).
3.4.2 The modified Gompertz model
The modified Gompertz model was also commonly used to study the kinetics of biogas production and was found to be especially useful when a lag phase is apparent [6, 47]. Figure 2 shows that the modified Gompertz model can categorize digestion into three stages, which includes a lag phase followed by a rapid production phase, and a stabilization phase. Co-digestion of SS and AM was again observed to have the highest maximum methane production rate (Rm) of 22.6 mL/g VS/day, followed by SS and BM (20.8 mL/g VS/day), SS and AS (17.0 mL/g VS/day), and SS and BS (10.4 mL/g VS/day) (Table 3). The Rm values of co-digestion assays were 62.5 to 253.1% more than those of SS mono-digestion. This improvement indicates that the addition of FOG to SS can increase the methane production peak values with more biodegradable COD present. Of all the digesters, only mono-digestion of FOG exhibited a lag phase (λ) between 19.3 and 25.3 days. Co-digestion with SS can eliminate this lag phase and thus reduce the overall digestion period. As discussed previously, the lag phase for FOG is likely due to inoculum adaptation or alkalinity shortage, and SS known to contain high alkalinity can help reduce acid impacts and thus protect methanogens. Not surprisingly, the effective methane production period (Tef) showed the same order of efficiency where co-digestion of SS and AM is the most efficient of all co-digestion assays, followed by SS and BM, SS and AS, and SS and BS. Tef values of all co-digestion assays were all shorter than those of SS mono-digestion, but higher than those of FOG mono-digestions. This is likely due to SS which contained more slowly degradable materials in co-digestion assays.
Comparisons of actual cumulative methane yield and predicted cumulative methane yield from the first-order kinetic model and the modified Gompertz model for sewage sludge (SS) only (a), mixed FOG before BioAmp pretreatment (BM) only (b), mixed FOG after BioAmp pretreatment (AM) only (c), settled FOG before BioAmp pretreatment (BS) only (d), settled FOG after BioAmp pretreatment (AS) only (e), co-digestion of SS and BM (f), co-digestion of SS and AM (g), co-digestion of SS and BS (h), and co-digestion of SS and AS (i). Circle represents actual cumulative methane yield, dash line represents predicted cumulative methane yield from the first-order kinetic model, and solid line represents predicted cumulative methane yield from the modified Gompertz model
Comparing between the two models, it appeared that the first-order kinetic model has a better data fit for co-digestion treatments based on both R-square and the differences of ultimate CMYs between predicted and actual (Table 3). On the other hand, digestions with an apparent lag phase, mainly FOG mono-digestions, were fitted better with the modified Gompertz model.
3.5 Digestate quality
Despite a possible acid accumulation in mono-digestion of FOG at early period, both pH and VFA/alkalinity ratios were all within proper range at the conclusion of experiment. In addition, free ammonia concentrations of all digestates were also all below the critical toxic limit of 200 mg N/L [26]. This indicates that all reactors were stable at the end of digestion. Hence, the cessation of biogas production was because of the completion of degradation rather than inhibition of anaerobic digestion. The stability of digestion was also confirmed by a consistent methane content (70%) at the end of the monitoring period in all the assays.
3.6 Plant impact assessment
The experimental data collected from this bench study was used to assess the impact of co-digestion of SS and FOG for the DTMA WWTP that currently uses BioAmp for FOG treatment but does not co-digest with treated FOG wastes. Based on recent plant data, the average OLR of digester is 0.70 kg VS/m3/day. With the addition of FOG to the digester, the OLR will increase to 0.87 kg VS/m3/day, which is still below the recommended design range of 1.6–1.8 kg VS/m3/day for a municipal anaerobic digester [12]. Therefore, the addition of FOG should not upset current digestion. Compared to mono-digestion of SS, the addition of FOG can increase methane yield by 1137 m3/day, 1173 m3/day, 388 m3/day, and 721 m3/day, corresponding to 137%, 141%, 47%, and 87% increases for co-digestion of SS and BM, SS and AM, SS and BS, and SS and AS, respectively. Co-digestion of SS and FOG also increases total nitrogen (TN) return to mainstream by 14.7 kg/day, 25.9 kg/day, 6.87 kg/day, and 6.09 kg/day, which is 3%, 6%, 2%, and 1% of influent TN for co-digestion of SS and BM, SS and AM, SS and BS, and SS and AS, respectively. In addition, the biosolids are expected to increase to 705.35 kg/day (13.38%), 409.84 kg/day (7.77%), and 1546.58 kg/day (29.33%) and reduce to 208 kg/day (−3.95%) with the TN in biosolid increase of 9%, −4%, −1%, and 17%, for co-digestion of SS and BM, SS and AM, SS and BS, and SS and AS, respectively. Since the DTMA WWTP currently already pretreats FOG with BioAmp, based on our results, it is suggested to send all BioAmp-treated, mixed samples to the digester for co-digestion. With this practice, it is estimated to increase methane yield by 141%, increase biosolids by 7.77%, reduce TN in biosolids by 4%, and increase TN return to mainstream by 6%.
4 Conclusions
It was found that BioAmp can increase the SCOD/COD ratio of FOG, indicating a more favorable substrate for anaerobic digestion. Sending BioAmp-pretreated, mixed FOG to digestion is the optimal choice, providing the highest CMY and the fastest degradation rate constant. The first-order kinetic model demonstrated that FOG digestion is faster with BioAmp pretreatment. Co-digestion showed a synergistic effect with both increases in CMY and degradation rate constant. For the DTMA WWTP, co-digestion can help increase methane yield by 1173 m3/day, corresponding to 141%. However, 7.77% more biosolids with 4% less TN in biosolids will be generated, and 6% more TN will be returned to mainstream.
Data availability
Not applicable
Code availability
Not applicable
References
Ahn JH, Pan JG, Rhee JS (1999) Identification of the tliDEF ABC transporter specific for lipase in Pseudomonas fluorescens SIK W1. J Bacteriol 181(6):1847–1852
Aichinger P, Wadhawan T, Kuprian M, Higgins M, Ebner C, Fimml C, Murthy S, Wett B (2015) Synergistic co-digestion of solid-organic-waste and municipal-sewage-sludge: 1 plus 1 equals more than 2 in terms of biogas production and solids reduction. Water Res 87:416–423
Álvarez J, Otero L, Lema J (2010) A methodology for optimising feed composition for anaerobic co-digestion of agro-industrial wastes. Bioresour Technol 101(4):1153–1158
APHA, AWWA, WEF (2012) Standard methods for the examination of water and wastewater, 22nd edn. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, DC, USA.
Astals S, Batstone D, Mata-Alvarez J, Jensen P (2014) Identification of synergistic impacts during anaerobic co-digestion of organic wastes. Bioresour Technol 169:421–427
Bai X, Chen Y-C (2020) Synergistic effect and supernatant nitrogen reduction from anaerobic co-digestion of sewage sludge and pig manure. Bioresour Technol Rep 100424
Bilgili MS, Demir A, Varank G (2009) Evaluation and modeling of biochemical methane potential (BMP) of landfilled solid waste: a pilot scale study. Bioresour Technol 100(21):4976–4980
Borowski S, Domański J, Weatherley L (2014) Anaerobic co-digestion of swine and poultry manure with municipal sewage sludge. Waste Manag 34(2):513–521
Braguglia C, Mininni G, Tomei M, Rolle E (2006) Effect of feed/inoculum ratio on anaerobic digestion of sonicated sludge. Water Sci Technol 54(5):77–84
Cirne D, Paloumet X, Björnsson L, Alves M, Mattiasson B (2007) Anaerobic digestion of lipid-rich waste—effects of lipid concentration. Renew Energy 32(6):965–975
Davidsson Å, Lövstedt C, la Cour JJ, Gruvberger C, Aspegren H (2008) Co-digestion of grease trap sludge and sewage sludge. Waste Manag 28(6):986–992
Metcalf & Eddy, Burton FL, Stensel HD, Tchobanoglous G (2003) Wastewater engineering: treatment and reuse, 4th edn. Bosten: McGraw-Hill.
Ely C, Rock S (2015) Food waste to energy: how six water resource recovery facilities are boosting biogas production and the bottom line. Report EPA/600/R-14/240, U.S. Environmental Protection Agency, Washington, DC, USA.
Fernández-Valverde M, Reglero A, Martinez-Blanco H, Luengo JM (1993) Purification of Pseudomonas putida acyl coenzyme A ligase active with a range of aliphatic and aromatic substrates. Appl Environ Microbiol 59(4):1149–1154
Frenken L, Egmond MR, Batenburg A, Bos JW, Visser C, Verrips CT (1992) Cloning of the Pseudomonas glumae lipase gene and determination of the active site residues. Appl Environ Microbiol 58(12):3787–3791
Gough HL, Nelsen D, Muller C, Ferguson J (2013) Enhanced methane generation during theromophilic co-digestion of confectionary waste and grease-trap fats and oils with municipal wastewater sludge. Water Environ. Res. 85(2):175–183
Hansen KH, Angelidaki I, Ahring BK (1998) Anaerobic digestion of swine manure: inhibition by ammonia. Water Res 32(1):5–12
He X, Iasmin M, Dean LO, Lappi SE, Ducoste JJ, de los Reyes III FL (2011) Evidence for fat, oil, and grease (FOG) deposit formation mechanisms in sewer lines. Environ Sci Technol 45(10): 4385–4391.
Hwu C-S, van Lier JB, Lettinga G (1998) Physicochemical and biological performance of expanded granular sludge bed reactors treating long-chain fatty acids. Process Biochem 33(1):75–81
Jeganathan J, Nakhla G, Bassi A (2006) Long-term performance of high-rate anaerobic reactors for the treatment of oily wastewater. Environ Sci Technol 40(20):6466–6472
Johnson LA, Beacham I, MacRae I, Free M (1992) Degradation of triglycerides by a pseudomonad isolated from milk: molecular analysis of a lipase-encoding gene and its expression in Escherichia coli. Appl Environ Microbiol 58(5):1776–1779
Kabouris JC, Tezel U, Pavlostathis SG, Engelmann M, Dulaney JA, Todd AC, Gillette RA (2009) Mesophilic and thermophilic anaerobic digestion of municipal sludge and fat, oil, and grease. Water Environ. Res. 81(5):476–485
Lalman J, Bagley DM (2002) Effects of C18 long chain fatty acids on glucose, butyrate and hydrogen degradation. Water Res 36(13):3307–3313
Lay J-J, Li Y-Y, Noike T (1997) Influences of pH and moisture content on the methane production in high-solids sludge digestion. Water Res 31(6):1518–1524
Lay J-J, Li Y-Y, Noike T (1998) The influence of pH and ammonia concentration on the methane production in high-solids digestion processes. Water Environ Res 70(5):1075–1082
Liu T, Sung S (2002) Ammonia inhibition on thermophilic aceticlastic methanogens. Water Sci Technol 45(10):113–120
Long JH, Aziz TN, Francis L III, Ducoste JJ (2012) Anaerobic co-digestion of fat, oil, and grease (FOG): a review of gas production and process limitations. Process Saf Environ Prot 90(3):231–245
Luostarinen S, Luste S, Sillanpää M (2009) Increased biogas production at wastewater treatment plants through co-digestion of sewage sludge with grease trap sludge from a meat processing plant. Bioresour Technol 100(1):79–85
Mata-Alvarez J, Dosta J, Romero-Güiza M, Fonoll X, Peces M, Astals S (2014) A critical review on anaerobic co-digestion achievements between 2010 and 2013. Renew Sust Energ Rev 36:412–427
Nghiem LD, Koch K, Bolzonella D, Drewes JE (2017) Full scale co-digestion of wastewater sludge and food waste: bottlenecks and possibilities. Renew Sust Energ Rev 72:354–362
Palacios RN, Lee H, Fan SS, Soto MA (2012) From grease to gas: anaerobic digestion of fats, oils, grease (FOG) at the Hyperion treatment plant. Proc Water Environ Fed 2012(14):2614–2629
Palatsi J, Laureni M, Andrés M, Flotats X, Nielsen HB, Angelidaki I (2009) Strategies for recovering inhibition caused by long chain fatty acids on anaerobic thermophilic biogas reactors. Bioresour Technol 100(20):4588–4596
Pereira M, Sousa D, Mota M, Alves M (2004) Mineralization of LCFA associated with anaerobic sludge: kinetics, enhancement of methanogenic activity, and effect of VFA. Biotechnol Bioeng 88(4):502–511
Roheim JR (2003) Low cost automated on site system for growing and dispensing vegetative bacteria. Proc Water Environ Fed 2003:634–647
Salama E-S, Saha S, Kurade MB, Dev S, Chang SW, Jeon B-H (2019) Recent trends in anaerobic co-digestion: fat, oil, and grease (FOG) for enhanced biomethanation. Prog Energy Combust Sci 70:22–42
Schutz WA (2008) Aerobic pretreatment of grease trap wastes. Proc Water Environ Fed 2008(3):277–302
Shen Y, Linville JL, Urgun-Demirtas M, Mintz MM, Snyder SW (2015) An overview of biogas production and utilization at full-scale wastewater treatment plants (WWTPs) in the United States: challenges and opportunities towards energy-neutral WWTPs. Renew Sust Energ Rev 50:346–362
Suto P, Gray D, Larsen E, Hake J (2006) Innovative anaerobic digestion investigation of fats, oils, and grease. Proc Water Environ Fed 2006(2):858–879
Tang HL, Xie YF, Chen Y-C (2012) Use of Bio-Amp, a commercial bio-additive for the treatment of grease trap wastewater containing fat, oil, and grease. Bioresour Technol 124:52–58
Vidal G, Carvalho A, Mendez R, Lema J (2000) Influence of the content in fats and proteins on the anaerobic biodegradability of dairy wastewaters. Bioresour Technol 74(3):231–239
Wan C, Zhou Q, Fu G, Li Y (2011) Semi-continuous anaerobic co-digestion of thickened waste activated sludge and fat, oil and grease. Waste Manag 31(8):1752–1758
Wang L, Aziz TN, Francis L (2013) Determining the limits of anaerobic co-digestion of thickened waste activated sludge with grease interceptor waste. Water Res 47(11):3835–3844
Wang X, Yang G, Feng Y, Ren G, Han X (2012) Optimizing feeding composition and carbon–nitrogen ratios for improved methane yield during anaerobic co-digestion of dairy, chicken manure and wheat straw. Bioresour Technol 120:78–83
Wu L-J, Kobayashi T, Kuramochi H, Li Y-Y, Xu K-Q (2016) Improved biogas production from food waste by co-digestion with de-oiled grease trap waste. Bioresour Technol 201:237–244
Xie S, Wickham R, Nghiem LD (2017) Synergistic effect from anaerobic co-digestion of sewage sludge and organic wastes. Int Biodeterior Biodegrad 116:191–197
Xu R, Yang Z, Chen T, Zhao L, Huang J, Xu H, Song P, Li M (2015) Anaerobic co-digestion of municipal wastewater sludge with food waste with different fat, oil, and grease contents: study of reactor performance and extracellular polymeric substances. RSC Adv 5(125):103547–103556
Yalcinkaya S, Malina JF Jr (2015) Model development and evaluation of methane potential from anaerobic co-digestion of municipal wastewater sludge and un-dewatered grease trap waste. Waste Manag 40:53–62
Yang X, Wang X, Wang L (2010) Transferring of components and energy output in industrial sewage sludge disposal by thermal pretreatment and two-phase anaerobic process. Bioresour Technol 101(8):2580–2584
Zhang W, Wei Q, Wu S, Qi D, Li W, Zuo Z, Dong R (2014) Batch anaerobic co-digestion of pig manure with dewatered sewage sludge under mesophilic conditions. Appl Energy 128:175–183
Acknowledgements
The authors are grateful to Penn State University for providing research laboratory facilities. The authors also acknowledge the assistance from the DTMA Wastewater Treatment Plant.
Author information
Authors and Affiliations
Contributions
Xuanye Bai: Methodology, formal analysis and investigation, and writing—original draft preparation. Sarah Folk: Formal analysis and investigation, and writing—original draft preparation. Yen-Chih Chen: Methodology, writing—review and editing, supervision, funding acquisition, and resources.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Bai, X., Folk, S. & Chen, YC. Co-digestion of sewage sludge and fat, oil, and grease with BioAmp pretreatment under mesophilic conditions. Biomass Conv. Bioref. 13, 5023–5034 (2023). https://doi.org/10.1007/s13399-021-01418-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-021-01418-9