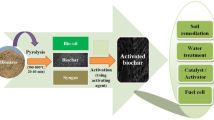
Abstract
Biochar produced from various biomass has been widely used in environmental applications owing to its ability to immobilize or remove the contaminants from soil, water and air. The present work summarizes various pyrolysis variants used for biochar production from rice husk, modification of biochar and its environmental application. The high volatile matter content (70.2–78.5%) and carbon content (35.2–44.7%) favoured production of biochar from rice husk through pyrolysis. Microwave-assisted hydrothermal carbonization showed highest biochar yield from rice husk (57.9%) compared to other process variants, whereas wet pyrolysis produced biochar with the highest carbon content (71.2%). Steam activation of rice husk biochar resulted in a broader pore size distribution with the presence of significant micropores compared to CO2 activation. A substantial improvement in surface area and microporous volume was observed with alkali activation compared to that of acid activation, whereas metal impregnation caused a reduction in surface area. Rice husk biochar with/without modification has been employed for adsorption of pollutants such as cations, dyes, nutrients and tetracycline. The nutrient-loaded rice husk biochar improved the soil fertility and cation exchange capacity. The studies indicated that the choice of suitable pyrolysis variant and biochar modification method is vital to improve the adsorption capacity and nutrient release potential of the rice husk biochar.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Rice husk is the by-product of rice processing and is one of the plentiful resources in Asian countries [1]. India ranks second in global rice production, with 20–22% of the 122 million tons of world’s rice production [2]. Rice husk management involves direct dumping into the soil, composting or burning them out in mass as a fuel [3]. However, composting was found to be ineffective due to low nitrogen content, whereas burning would give rise to the generation of a significant amount of fine particles into the air [4]. Therefore, safe disposing of the rice husk biomass is highly desired.
New practices of biomass management involve several wastes to energy conversion techniques through biochemical or thermochemical routes. The high carbon content in biomass such as rice husk facilitates its conversion into energy-rich biochar upon thermochemical treatment [5]. Among the thermochemical processes adopted, pyrolysis has received much attention in recent years due to its flexibility in both operating condition and the type of feedstock. Pyrolysis involves thermal degradation of biomass at elevated temperature in an oxygen-free atmosphere to obtain value-added by-products such as biochar, bio-oil and non-condensable gas. The quality of by-products, along with the reduction in greenhouse gas emission, makes the method superior to other technologies [6]. Outcomes from previous studies indicated that pyrolysis of rice husk biomass to biochar is an effective method both for biomass disposal and energy recovery [3, 7,8,9,10,11,12]. The present review summarizes the effects of pyrolysis process variants used for conversion of rice husk biomass on the yield and quality of biochar. Further various modifications adopted for enhancing the properties of biochar to be applied as an asset for environmental beneficiation are discussed.
2 Characteristics of rice husk biomass
Biomass is composed of lignin, cellulose and hemicellulose. The cellulose and lignin content in the biomass was one of the essential factors to assess the pyrolysis characteristics. Also, the relative composition of these constituents in biomass influenced the nature of the pyrolysis by-products. Studies revealed that both lignin and cellulose enhanced the biochar yield during pyrolysis. Pyrolysis of biomass with high lignin content resulted in higher biochar content compared to the one with lower lignin content due to the breakdown of relatively weak bonds and consequent formation of the solid structure [13, 14]. Rice husk biomass consists of various proportions of moisture content (MC), volatile matter content (VM), ash content (Ash) and fixed carbon content (FC) depending on its growth condition and geographic location. The reported range of MC, VM, Ash and FC in rice husk is 4.5–10.8%, 70.2–78.5%, 3.4–17% and 3.4–19.8% respectively (Table 1). The high VM, along with low ash and FC content, indicated possible thermal decomposition of rice husk [3].
On the other hand, ultimate analysis showed variation in elemental composition, viz. carbon, hydrogen, oxygen, nitrogen and sulphur (Table 1). High carbon content (35.2–44.7%) favoured the biomass in the production of higher biochar yield. Also, lower nitrogen content (0.2–1.6%) reduced the unfavourable situation for biomass pyrolysis that would lead to the release of toxic GHGs like NOx [15].
3 Pyrolysis process variants for biochar production
Several processes of pyrolysis have been employed for converting biomass into biochar. It is highly crucial to choose the appropriate method to obtain the maximum yield of the desired by-product in pyrolysis. All the process variants are carried out in an oxygen-free atmosphere that results in a reduction of greenhouse gas emission by biomass combustion [15]. Various process variants of pyrolysis based on the heating rate and mode of heating include wet pyrolysis, pyrolysis, torrefaction, slow pyrolysis, fast pyrolysis, flash pyrolysis, and microwave-assisted pyrolysis.
3.1 Wet pyrolysis
Wet pyrolysis or hydrothermal carbonization is a type of thermochemical conversion process for conversion of biomass into a coal-like product hydrochar or hydrothermal biochar. In wet pyrolysis, the feedstock with high moisture content up to 75–90% is subjected to pyrolysis in a pressurized vessel without any pre-drying step. The elevated temperature of ~ 180–250 °C along with high moisture content resulted in dehydration and decarboxylation of biomass constituents and its conversion into carbon-densified product [16]. The process resulted in partial carbonization of biomass to yield biochar with a high concentration of oxygen-containing functional groups and comparatively low H/C, and O/C ratio [17].
3.2 Pyrolysis
Biomass pyrolysis process incorporates thermal degradation of biomass that starts at 350–550 °C and goes up to 700–800 °C. The products obtained from this process include biochar, bio-oil and non-condensable gases such as methane, hydrogen, carbon monoxide and carbon dioxide. Higher moisture waste streams such as sludge and meat processing wastes are pretreated before the pyrolysis to reduce moisture content [6, 16]. In contrast, rice husk biomass, with low moisture content, can be pyrolyzed directly. During the process, more stable by-products are obtained owing to the heating of biomass above its thermal stability limit in an inert atmosphere. Besides, the volatiles produced in the process could be condensed to retrieve bio-oil. Pyrolysis occurs in two stages. Biomass gets cleaved up and devolatilized in the first stage. Biomass gets converted to its main components, viz. lignin, cellulose and hemicellulose by cleavage along with the formation of carboxyl, carbonyl and hydroxyl groups [18]. The process of devolatilization results in decarboxylation, dehydration and dehydrogenation of the biomass. The second stage corresponds to the conversion of larger molecules/heavy compounds in biomass, leading to the formation of biochar, bio-oil or non-condensable gases. The second stage of the process can be accelerated by improving heating rate with the use of a catalyst [19, 20].
3.3 Torrefaction
Torrefaction process is carried out at low pyrolysis temperature of ~ 200–300 °C. In this method, the feed materials are heated up slowly at a heating rate of ≤ 60 °C/min for hours to days [10]. The moisture content and volatiles from biomass are released at a slow rate, thus maintaining the rigid structure carbonaceous biomass. The process results in the partial decomposition of cellulose, hemicellulose and lignin. It tends to yield a higher proportion of solids compared to liquid and non-condensable gases. The solid obtained has high O/C ratio and hence cannot be referred as biochar [21]. Therefore, torrefaction is considered a pretreatment for moisture removal and densification of biomass to increase the heating value [15, 22].
3.4 Slow and fast pyrolysis
Slow pyrolysis is generally carried out at a temperature of ~ 400–500 °C and heating rate (HR) of 0.1–1 °C/s that tends to increase the char yield along with the generation of thick bio-oil like tar [15, 21]. The slow heating rate necessitates the long process time of 5–30 min to complete the process. On the other hand, fast pyrolysis employs heating of the biomass to 400–650 °C at high HR of ~ 10-200 °C/s and for a period of 1–10 s. This process resulted in highest bio-oil yield (~ 60–70%), compared to biochar (15–20%) and syngas (10–20%) [6, 9].
3.5 Flash pyrolysis
Flash pyrolysis or ultrafast pyrolysis is likely to be carried out at a temperature range of 800–1000 °C obtained with HR ≥ 1000 °C/s [23,24,25,26]. The biomass feed is converted to fine particles of < 0.2 mm prior to the pyrolysis process [21]. The flash process yields lesser amount of biochar compared to other by-products (Ibrahim et al., 2012). The biggest challenge of this method is to configure the reactor in which the input biomass can reside for a short amount of time under extremely high temperature and HR, which limits its industrial application [15]. Further, the stability and quality of the bio-oil, the major by-product of the process, is strongly affected by the char content. Char present in the bio-oil can catalyze the polymerization reaction inside the liquid product which in turn causes an increase in the viscosity of oil [12, 27].
3.6 Microwave-assisted pyrolysis
In the conventional heating process, the heat transfer to the material occurs through conduction, convection and radiation, which limits its flexibility in maintaining control over the temperature, whereas microwave-assisted pyrolysis employs microwave radiation for heating that involves selective and volumetric heating of the biomass [3, 28,29,30]. During microwave heating, electromagnetic field enters the material, and it generates thermal energy throughout the penetration depth by dielectric heating due to interaction with dipoles present in the material, which results in volumetric heating from inside. Microwave heating generally requires a material with a high dielectric constant. Microwave absorbers are used along with the biomass to facilitate dielectric heating during the process. Microwave-assisted pyrolysis is conducted at a temperature of 400–800 °C, and it is considered an advancement over the conventional pyrolysis process [3, 16, 31]
4 Characteristics of rice husk biochar obtained from process variants
The properties of rice husk biochar obtained through various pyrolysis variants are listed in Table 1. The results depicted that the yield of biochars produced from wet pyrolysis and pyrolysis process showed a slight reduction from 37.9% to 27.4% and 40.1 to 37% with the rise in temperature from 220 to 280 °C and 350 to 800 °C respectively. This could due to the intensified degradation reaction such as decarboxylation, dehydration and aromatization at higher temperature that resulted in enhanced carbonization [7]. The wet pyrolysis of rice husk indicated a decrease in biochar yield compared to pyrolysis (Table 1). The low biochar yield in wet pyrolysis could be due to reduced degradation of cellulose, hemicellulose and lignin content [32]. The VM and ash content of biochar obtained from wet pyrolysis was high (~ 50%) compared to pyrolysis (~ 14%), indicating partial valorization of biomass in wet pyrolysis (Table 1). Conversely, the lower ash content in the biochar from wet pyrolysis process may be attributed to the higher amount of inorganic compound present in the supercritical water [7].
Slow pyrolysis of rice husk biomass showed that low temperature and longer vapour residence time favoured re-polymerization of rice husk constituents by giving them sufficient time to react and thus improved biochar production [15], whereas high HR during flash pyrolysis of rice husk enhanced the de-polymerization of biomass into volatile primary components, which in turn, retarded biochar yield and improved bio-oil yield [8]. Also, the less retention time in flash pyrolysis reduced thermal cracking, which in turn decreased the formation of non-condensable gases [8]. The presence of high carbon and oxygen content in the biomass leads to more char formation [15]. Microwave-assisted hydrothermal carbonization improved biochar yield from rice husk, with low temperature and lower reaction time, compared to other process variants. Conversely, wet pyrolysis produced biochar with highest carbon content of 71.2% (Table 1).
Further, ultimate analysis of biochar depicted a slight reduction in H content with an increase in pyrolysis temperature due to the release of hydrocarbons such as CH4 and C2H6 at a higher temperature. However, with an increase in temperature, a gradual increase in carbon content along with a considerable depletion in oxygen content was observed, due to the carboxylation of rice husk and subsequent generation of CO2, CO, moisture and carbohydrates (Table 1). Further, the decrease in moisture content (MC) and an increase in C/O ratio improved the high heating value of the terrified rice husk to be used as a source of fuel [10].
5 Modification of rice husk biochar
Although several inherent properties of biochar obtained from the pyrolysis variants suit to various environmental applications, unpyrolyzed biomass and mineral ash block the pores of biochar (Fig. 1). Hence, several modification methods are adopted to enhance the biochar properties based on its end-use, such as soil remediation and pollutant adsorption. Physical modification, chemical modification, and metal impregnation are the common methods adopted to obtain the modified/engineered biochar with improved surface area, morphological properties, and chemical properties compared to the rice husk biochar obtained from pyrolysis.
5.1 Physical modification
The physical or thermal modification method of the rice husk biochar tends to be less costly and more environmental friendly compared to chemical methods. In this method, biochar is subjected to partial gasification using steam and carbon dioxide as activating agents [34].
In steam activation, biochar is exposed to steam, which led to partial gasification of trapped volatiles as per Eq. 1. This step of partial gasification stimulates the formation of crystalline C and results in partial devolatilization of biochar. Hence with steam activation, the pores of the biochar can be increased by the removal of the unpyrolyzed biomass and trapped products formed during pyrolysis. Also, the process facilitates a greater abundance of aromatic and few oxygenated function group being developed on the surface [34, 35].
On the other hand, gas purging is often carried out with gases such as carbon dioxide, which reacts with the available amorphous carbon in biochar in the limited oxygen atmosphere to form carbon monoxide (Eq. 2). Evolution of carbon monoxide enhances the surface area of biochar due to corrosion resulting in the improved microporous structure and increased pore volume (Fig. 2) [34, 35].
Several researchers observed higher gasification rate by steam activation compared to CO2 purging, which could be due to the difference in activation energy in reaction energies using the two activating agents [36,37,38].
5.2 Chemical modification
5.2.1 Alkali modification
Alkali modification of biochar involves a chemical reduction process with reducing agents such as NaOH, KOH and NH4OH. Alkali modification improved porosity and specific surface area of biochar by the removal of unpyrolyzed organic matter and ash content from the pores (Fig. 3). Also, removal of volatile carbon from pores improved the fixed carbon content in biochar. Further, the process caused a reduction in the oxygen-containing functional group, along with an increase in hydroxyl functional group (OH-) [16, 35], thus improving its adsorption capacity of pollutants, mainly, the non-polar pollutants [39].
5.2.2 Acid modification
In acid modification or chemical oxidation process, biochar surface is oxidized using HCl, HNO3, H2O2 and H3PO4. The acid modification improved the hydrophilicity of biochar by increasing the oxygen-containing functional group such as carboxyl group (-COOH) on the surface. Biochar modified by HNO3 comprised of higher oxygen-containing functional groups compared to other oxidants [35, 39]. The process also removed unpyrolyzed organic matter and mineral ash, thus increasing the volume of pores as in the case of physical modification (Fig. 3). Conversely, oxygen-containing functional group caused hydration and subsequent blockage of the pores [39, 40]. Acid modification improved the structure of biochar and provided greater affinity towards adsorbing polar compounds by forming hydrogen bond with highly electronegative surface oxygen as a result of its hydrophilicity [41].
5.3 Metal impregnation
In recent years, several investigators have focused on metal impregnation of biochar, in which metal ions are incorporated on to the surface and pores of the biochar. Magnesium, silver, zinc, copper, iron etc. are the typical metal ions incorporated in biochar. Metal impregnated biochar showed significant improvement in adsorption capacity of various pollutants compared to un-modified biochar owing to the presence of two solid phases, viz. nano-crystals of metal oxides and biochar matrix [42]. Both of these phases contributed to the overall adsorption capacity of composites through various mechanisms such as hydrogen bonding, precipitation, electrostatic precipitation and ligand exchange (Fig. 4). Powdered biochar poses difficulty in separation from the aqueous matrix and unfavourable sorption towards anionic pollutants. The separation of powdered biochar from wastewaters requires a tedious process such as centrifugation/filtration and thereby hindering their usage in real treatment schemes [16]. Metal impregnation by magnetic metals like iron is employed to enhance the separability of powdered biochar after the treatment process through magnetic separation (Fig. 4). Magnetic biochar is synthesized through chemical co-precipitation of Fe2+/Fe3+ onto biomass followed by subsequent pyrolysis [43]. The hybrid nature of magnetic biochar also enables improved sorption of various inorganic and organic pollutants.
5.4 Effect of modification method on biochar properties
Table 2 shows the surface characteristics of modified rice husk biochar obtained from different modification processes. Physical modification by 15-min steam activation of rice husk biochar obtained from fast pyrolysis indicated ~ 3-fold increase in the surface area, with the respective values of 486 m2/g and 1365 m2/g for biochar and modified biochar. The micropore volume (MPV) increased from 0.05 cm3/g to 1.160 cm3/g during steam activation. In contrast, physical modification of the same biochar by CO2 purging for 45 min showed a peak surface area of 1564 m2/g with MPV of 1.045 cm3/g. The faster kinetics of steam activation compared to CO2 activation resulted in a broader pore size distribution with the presence of significant micropores in steam activated biochar [34].
Conversely, acid modification by H3PO4 indicated significant improvement in surface area compared to that of H2SO4 modification (Table 2). However, the chemical modification of rice husk biochar by alkali activation showed considerable improvement in surface area compared to that of acid activation. The surface area and micropore volume of biochar obtained from fast pyrolysis were 34.4 m2/g and 0.028 cm3/g, respectively. Acid and alkali activation showed ~ 1.4 times and ~ 3.4 times improvement in surface area of the biochar with the corresponding value of 46.8 m2/g and 117.8 m2/g respectively. The microporous volumes of acid-activated biochar and alkali-activated biochar were 0.033 cm3/g and 0.073 cm3/g, respectively (Table 2). Even though acid and alkali activation facilitated the removal of unpyrolyzed organic matter and ash content from the surface of biochar, oxygen-containing functional group in the acid activated biochar caused hydration and subsequent blockage of the pores thus hindering physical adsorption capacity [39, 40]. Overall, the chemical modification improved the surface area and porosity of the modified biochar resembling the removal of the unpyrolyzed organic matter and mineral ash and generating favourable surface functional groups such as aromatic (C=C), carboxyl and hydroxyl functional species as shown in Fig. 2.
On the other hand, a reduction in the surface area from 181 to 77.3 m2/g and a reduction in microporous volume from 0.37 to 0.13 cm3/g were observed for the biochar modified with metal impregnation. The decrease in surface area and pore volume was due to the amendment of iron oxide into the pores of the biochar while generating appropriate surface functional groups like metal cations for the adsorption of anionic contaminants from wastewater [43].
6 Environmental application of rice husk biochar
Rice husk biochar is mainly used as functional materials in areas of agriculture and environmental remediation [7, 34, 43]. Use of biochar to soil acts as a carbon sink while reducing the CO2 emission by biomass burning. Also, biochar addition improves the soil property and productivity by enhancing the structure and nutrient content of the soil [16]. Biochar application to soil showed an increase in water retention, soil fertility, cation exchange capacity, soil pH and improved soil microbial activity [46]. The rice husk biochar produced at a higher temperature of about 500–600 °C resulted in high availability of nutrients like phosphorus and potassium. The improvement in the nutrient availability of phosphorus and potassium ranged from 0.22 to 1.8 mg/g and 0.16 to 0.68 mg/g respectively as the temperature varied from 350 to 700 °C [47]. On the other hand, high surface area, microporous structure and surface functional groups led to the use of biochar as an adsorbent for various pollutants from aqueous or gaseous phase [48] [46]. The aromatic functional group in abundance along with hydroxyl group in biochar facilitated adsorption of metal cations by cation-π interaction [15, 21]. Table 3 summarizes various applications of rice husk biochar in environmental remediation. Adsorption of cations like copper and arsenic, anions like iodine and other organic pollutants like dyes and tetracycline indicated its significant removal from aqueous phase by biochar and modified biochar. The adsorption study for the removal of copper, methylene blue and iodine showed better adsorption potential of the biochar obtained from wet pyrolysis compared to that of pyrolysis (Table 3). The improved adsorption potential of wet pyrolysis biochar was due to the retention of higher oxygen-containing functional group compared to that of pyrolysis biochar [7]. Adsorptive removal of trace organic compound by biochar is gaining importance in recent years as a cost-effective treatment method [49]. The alkali- and acid-modified rice husk biochar was used for the removal of tetracycline, a carcinogenic compound, from water. The result indicated higher affinity of alkali-activated rice husk biochar towards adsorbing tetracycline with a maximum capacity of 58 mg/g compared to the acid-modified biochar with a maximum capacity of 23.36 mg/g [40, 50] which was attributed to the large specific surface area and porous structure of the alkali activated biochar (Table 2). In addition, its graphite-like structure facilitated the formation of π-π interaction between the ring structure of tetracycline molecule and graphite sheets (Table 3). Similar results were also observed in adsorption of volatile organic compounds (VOCs) on acid- and alkali-modified coconut shell–based biochar [38]. Further, rice husk biochar obtained from microwave-assisted pyrolysis exhibited excellent nutrient retention capacities of 68 mg/kg of phosphate and 220 mg/kg of nitrate [3]. One kilogram of nutrient-loaded rice husk char released 68 mg of phosphate and 220 mg of nitrate, indicating soil nourishment potential of the used biochar. These extractable nutrients were found to be comparable with the optimum soil nourishment required for the growth of crops [50]. Another study conducted to evaluate the effect of rice husk biochar on soil properties showed the cation exchange capacity (CEC) of the biochar (17.57 c/mol) was found to be higher as compared to that of the compost (10.3 c/mol) used in the study clearly depicting its potential of being a soil amender which resulted in increasing the CEC of the soil. Increased surface area, porosity, negative charges on the biochar surface and increase in pH due to biochar addition resulted in increased CEC of biochar amended soil [51, 52].
7 Conclusion
Rice husk biomass composed of various proportions of moisture content (MC), volatile matter content (VM), ash content (Ash) and fixed carbon content (FC) depending on its growth condition and geographic location. The MC, VM, Ash and FC in rice husk was reported in the range of 4.5–10.8%, 70.2–78.5%, 3.4–17% and 3.4–19.8% respectively. Various pyrolysis variants such as wet pyrolysis, pyrolysis, torrefaction, slow pyrolysis, fast pyrolysis, flash pyrolysis and microwave-assisted pyrolysis have been employed in the past for the conversion of rice husk to biochar. The process variants and operating conditions influenced the yield and property of rice husk biochar. Among physical modification method, steam activation resulted in more microporous biochar compared to CO2 purging. The alkali and acid modification was performed respectively to improve hydrophobicity and hydrophilicity of rice husk biochar. Conversely, metal impregnation was done on rice husk biochar to improve adsorption potential or the post treatment separability. Rice husk biochar is successfully applied for adsorption of pollutants such as heavy copper, methylene blue, iodine, arsenate and tetracycline. The rice husk biochar increased the soil pH, microporosity and cation exchange capacity. Biochar amendment in soil also reduced the leaching of nutrients like nitrate and phosphate from soil.
References
Zhang S, Dong Q, Zhang L, Xiong Y (2016) Effects of water washing and torrefaction on the pyrolysis behavior and kinetics of rice husk through TGA and Py-GC/MS. Bioresour Technol 199:352–361. https://doi.org/10.1016/j.biortech.2015.08.110
Swami Nathen AN, Robert Ravi S (2017) Indian rice husk ash – improving the mechanical properties of concrete: a review. Int J Eng Res Appl 07:76–79. https://doi.org/10.9790/9622-0701017679
Shukla N, Sahoo D, Remya N (2019) Biochar from microwave pyrolysis of rice husk for tertiary wastewater treatment and soil nourishment. J Clean Prod 235:1073–1079. https://doi.org/10.1016/j.jclepro.2019.07.042
Cheng X, Wang B (2017) Yield, composition, and property of biochar obtained from the two-step pyrolysis of rice husk impregnated with boric acid. Energies 10. https://doi.org/10.3390/en10111814
Manoj Tripathi JN, Sahub PG (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: a review. Renew Sust Energ Rev 55:467–481
Nwajiaku IM, Olanrewaju JS, Sato K, Tokunari T, Kitano S, Masunaga T (2018) Change in nutrient composition of biochar from rice husk and sugarcane bagasse at varying pyrolytic temperatures. Int J Recycl Org Waste Agric 7:269–276. https://doi.org/10.1007/s40093-018-0213-y
Jian X, Zhuang X, Li B, Xu X, Wei Z, Song Y, Jiang E (2018) Comparison of characterization and adsorption of biochars produced from hydrothermal carbonization and pyrolysis. Environ Technol Innov 10:27–35. https://doi.org/10.1016/j.eti.2018.01.004
Alvarez J, Lopez G, Amutio M, Bilbao J, Olazar M (2014) Upgrading the rice husk char obtained by flash pyrolysis for the production of amorphous silica and high quality activated carbon. Bioresour Technol 170:132–137. https://doi.org/10.1016/j.biortech.2014.07.073
Wang Y, Yin R, Liu R (2014) Characterization of biochar from fast pyrolysis and its effect on chemical properties of the tea garden soil. J Anal Appl Pyrolysis 110:375–381. https://doi.org/10.1016/j.jaap.2014.10.006
Chen D, Zhou J, Zhang Q, Zhu X, Lu Q (2014) Upgrading of rice husk by torrefaction and its influence on the fuel properties. BioResources 9:5893–5905
Nizamuddin S, Jadhav A, Qureshi SS, Baloch HA, Siddiqui MTH, Mubarak NM, Griffin G, Madapusi S, Tanksale A, Ahamed MI (2019) Synthesis and characterization of polylactide/rice husk hydrochar composite. Sci Rep 9:5445. https://doi.org/10.1038/s41598-019-41960-1
Biswas B, Pandey N, Bisht Y, Singh R, Kumar J, Bhaskar T (2017) Pyrolysis of agricultural biomass residues: Comparative study of corn cob, wheat straw, rice straw and rice husk. Bioresour Technol 237:57–63. https://doi.org/10.1016/j.biortech.2017.02.046
Lathouwers D, Bellan J (2001) Yield optimization and scaling of fluidized beds for tar production from biomass. Energy Fuel 15:1247–1262. https://doi.org/10.1021/ef010053h
Lv D, Xu M, Liu X et al (2010) Effect of cellulose, lignin, alkali and alkaline earth metallic species on biomass pyrolysis and gasification. In: Fuel Processing Technology 91:903–909. https://doi.org/10.1016/j.fuproc.2009.09.014
Tripathi M, Sahu JN, Ganesan P (2016) Effect of process parameters on production of biochar from biomass waste through pyrolysis: A review. Renew Sust Energ Rev 55: 467–481. https://doi.org/10.1016/j.rser.2015.10.122
Vijayaraghavan K (2019) Recent advancements in biochar preparation, feedstocks, modification, characterization and future applications. Environ Technol Rev 8:47–64. https://doi.org/10.1080/21622515.2019.1631393
Nizamuddin S, Siddiqui MTH, Baloch HA, Mubarak NM, Griffin G, Madapusi S, Tanksale A (2018) Upgradation of chemical, fuel, thermal, and structural properties of rice husk through microwave-assisted hydrothermal carbonization. Environ Sci Pollut Res 25:17529–17539. https://doi.org/10.1007/s11356-018-1876-7
Patwardhan PR, Dalluge DL, Shanks BH, Brown RC (2011) Distinguishing primary and secondary reactions of cellulose pyrolysis. Bioresour Technol 102:5265–5269. https://doi.org/10.1016/j.biortech.2011.02.018
Liu C, Wang H, Karim AM et al (2014) Catalytic fast pyrolysis of lignocellulosic biomass. Chem Soc Rev 43:7594–7623. https://doi.org/10.1039/C3CS60414D
Stefanidis SD, Kalogiannis KG, Iliopoulou EF, Michailof CM, Pilavachi PA, Lappas AA (2014) A study of lignocellulosic biomass pyrolysis via the pyrolysis of cellulose, hemicellulose and lignin. J Anal Appl Pyrolysis 105:143–150. https://doi.org/10.1016/j.jaap.2013.10.013
Daful AG, Chandraratne MR (2020) Biochar production from biomass waste-derived material. In: Encyclopedia of renewable and sustainable materials 4:370–378. https://doi.org/10.1016/B978-0-12-803581-8.11249-4
Yang GX, Jiang H (2014) Amino modification of biochar for enhanced adsorption of copper ions from synthetic wastewater. Water Res 48:396–405. https://doi.org/10.1016/j.watres.2013.09.050
Dupont C, Chen L, Cances J, Commandre JM, Cuoci A, Pierucci S, Ranzi E (2009) Biomass pyrolysis: kinetic modelling and experimental validation under high temperature and flash heating rate conditions. J Anal Appl Pyrolysis 85:260–267. https://doi.org/10.1016/j.jaap.2008.11.034
Zhong ZW, Song B, Zaki MBM (2010) Life-cycle assessment of flash pyrolysis of wood waste. J Clean Prod 18:1177–1183. https://doi.org/10.1016/j.jclepro.2010.03.017
Pang S (2019) Advances in thermochemical conversion of woody biomass to energy, fuels and chemicals. Biotechnol Adv 37:589–597
Pandey A, Bhaskar T, Stöcker M, Sukumaran RK (2015) Recent advances in thermochemical conversion of biomass. https://doi.org/10.1016/B978-0-444-63289-0.10000-6
Canabarro N, Soares JF, Anchieta CG, Kelling CS, Mazutti MA (2013) Thermochemical processes for biofuels production from biomass. Sustain Chem Process 1:22. https://doi.org/10.1186/2043-7129-1-22
Motasemi F, Afzal MT (2013) A review on the microwave-assisted pyrolysis technique. Renew Sust Energ Rev 28:317–330. https://doi.org/10.1016/j.rser.2013.08.008
Huang YF, Te Chiueh P, Lo SL (2016) A review on microwave pyrolysis of lignocellulosic biomass. Sustain Environ Res 93:103–109. https://doi.org/10.1016/j.serj.2016.04.012
Lo SL, Huang YF, Te Chiueh P, Kuan WH (2017) Microwave pyrolysis of lignocellulosic biomass. In: Energy Procedia 105: 41–46. https://doi.org/10.1016/j.egypro.2017.03.277
Sahoo D, Remya N (2020) Influence of operating parameters on the microwave pyrolysis of rice husk: biochar yield, energy yield, and property of biochar. Biomass Convers Biorefinery. https://doi.org/10.1007/s13399-020-00914-8
Grønli MG, Várhegyi G, Di Blasi C (2002) Thermogravimetric analysis and devolatilization kinetics of wood. Ind Eng Chem Res 41:4201–4208
Fewer VFRRLCMAGLÁIS (2020) Optimization of slow pyrolysis process parameters using a fixed bed reactor for biochar yield from rice husk. Biomass Bioenergy:132:105412. https://doi.org/10.1016/j.biombioe.2019.105412
Alvarez J, Lopez G, Amutio M, Bilbao J, Olazar M (2015) Physical activation of rice husk pyrolysis char for the production of high surface area activated carbons. Ind Eng Chem Res 54:7241–7250. https://doi.org/10.1021/acs.iecr.5b01589
Sizmur T, Fresno T, Akgül G, Frost H, Moreno-Jiménez E (2017) Biochar modification to enhance sorption of inorganics from water. Bioresour Technol 246:34–47. https://doi.org/10.1016/j.biortech.2017.07.082
Fan D, Zhu Z, Na Y, Lu Q (2013) Thermogravimetric analysis of gasification reactivity of coal chars with steam and CO2 at moderate temperatures. In: Journal of Thermal Analysis and Calorimetry 2013: 599–607, https://doi.org/ 10.1007/s10973-012-2838-9
Hernndez-Montoya V, Garca-Servin J, Ivn J (2012) Thermal treatments and activation procedures used in the preparation of activated carbons. In: Lignocellulosic precursors used in the synthesis of activated carbon - characterization techniques and applications in the wastewater treatment 2: 19–26
Nabais JMV, Nunes P, Carrott PJM, Ribeiro Carrott MML, García AM, Díaz-Díez MA (2008) Production of activated carbons from coffee endocarp by CO2 and steam activation. Fuel Process Technol 89:262–268. https://doi.org/10.1016/j.fuproc.2007.11.030
Yang X, Zhang S, Ju M, Liu L (2019) Preparation and modification of biochar materials and their application in soil remediation. Appl Sci 9(7):1365. https://doi.org/10.3390/app9071365
Liu P, Liu WJ, Jiang H, Chen JJ, Li WW, Yu HQ (2012) Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour Technol 121:121–240. https://doi.org/10.1016/j.biortech.2012.06.085
Zhao L, Zheng W, Mašek O, Chen X, Gu B, Sharma BK, Cao X (2017) Roles of phosphoric acid in biochar formation: synchronously improving carbon retention and sorption capacity. J Environ Qual 46:393–401. https://doi.org/10.2134/jeq2016.09.0344
Zhang M, Gao B, Yao Y, Xue Y, Inyang M (2012) Synthesis of porous MgO-biochar nanocomposites for removal of phosphate and nitrate from aqueous solutions. Chem Eng J 210:26–32. https://doi.org/10.1016/j.cej.2012.08.052
Cope CO, Webster DS, Sabatini DA (2014) Arsenate adsorption onto iron oxide amended rice husk char. Sci Total Environ 488-489:554–561. https://doi.org/10.1016/j.scitotenv.2013.12.120
Mohammad YS (2015) Effect of phosphoric acid modification on characteristics of rice husk activated carbon. Iran J Energy Environ 6. https://doi.org/10.5829/idosi.ijee.2015.06.01.05
Fu Y, Shen Y, Zhang Z, Ge X, Chen M (2019) Activated bio-chars derived from rice husk via one- and two-step KOH-catalyzed pyrolysis for phenol adsorption. Sci Total Environ 646:1577. https://doi.org/10.1016/j.scitotenv.2018.07.423
Yu H, Zou W, Chen J, Chen H, Yu Z, Huang J, Tang H, Wei X, Gao B (2019) Biochar amendment improves crop production in problem soils: a review. J Environ Manag 232:8–21. https://doi.org/10.1016/j.jenvman.2018.10.117
Li L, Zou D, Xiao Z et al (2019) Biochar as a sorbent for emerging contaminants enables improvements in waste management and sustainable resource use. J Clean Prod 210: 1324–1342. https://doi.org/10.1016/j.jclepro.2018.11.087
Nartey OD, Zhao B (2014) Biochar preparation, characterization, and adsorptive capacity and its effect on bioavailability of contaminants: an overview. Adv Mater Sci Eng
Peiris C, Gunatilake SR, Mlsna TE, Mohan D, Vithanage M (2017) Biochar based removal of antibiotic sulfonamides and tetracyclines in aquatic environments: a critical review. Bioresour Technol 246:246–159. https://doi.org/10.1016/j.biortech.2017.07.150
Chen T, Luo L, Deng S et al (2018) Sorption of tetracycline on H3PO4 modified biochar derived from rice straw and swine manure. Bioresour Technol 267. https://doi.org/10.1016/j.biortech.2018.07.074
Jien SH, Wang CS (2013) Effects of biochar on soil properties and erosion potential in a highly weathered soil. Catena. 110:225–233. https://doi.org/10.1016/j.catena.2013.06.021
Ghorbani M, Asadi H, Abrishamkesh S (2019) Effects of rice husk biochar on selected soil properties and nitrate leaching in loamy sand and clay soil. Int Soil Water Conserv Res 7:265. https://doi.org/10.1016/j.iswcr.2019.05.005
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights for review
• Application of different pyrolysis variants for biochar production from rice husk
• Yield and characteristics of rice husk biochar from different process variants
• Modifications of rice husk biochar to improve surface properties
• Environmental applications of as-received and modified rice husk biochar
Rights and permissions
About this article
Cite this article
Bushra, B., Remya, N. Biochar from pyrolysis of rice husk biomass—characteristics, modification and environmental application. Biomass Conv. Bioref. 14, 5759–5770 (2024). https://doi.org/10.1007/s13399-020-01092-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-01092-3