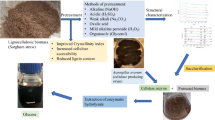
Abstract
In this study, we proposed a simple and effective sodium perborate (SPB) pretreatment method to enhance the enzymatic hydrolysis efficiency of rice straw (RS). The lignin removal rate reached 45.76% under the optimum pretreatment conditions of 8% SPB and 80 °C for 4 h, and the enzymatic hydrolysis efficiency of pretreated RS was 84% at a cellulase loading of 20 FPU/g RS. Through simultaneous saccharification and co-fermentation (SScF) of the pretreated RS solid with Saccharomyces cerevisiae SHY07-1, the maximum ethanol concentration was 15.29 g/L at 72 h, with a fermentation efficiency as high as 91.96%. Scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR) analyses revealed that the RS structure was destroyed by SPB pretreatment, and lignin was effectively removed. The overall data of this study indicate that SPB pretreatment is a promising method to improve enzymatic hydrolysis and bioethanol production from RS by inoculating Saccharomyces cerevisiae SHY07-1.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Increasing global energy consumption like oil not only leads to the rapid depletion of non-renewable energy sources but also global warming, melting polar glaciers, rising sea levels, and other environmental problems, which are considered the main challenges to the sustainable development of human society [1,2,3]. To address these challenges, we need to develop new green renewable energies, such as bioethanol, biodiesel, and other biomass fuels to replace fossil fuels [4]. The first generation of bioethanol is produced from corn, wheat, and other food crops, leading to the consumption of large amount of agricultural products and the competition with people for grain, which has a negative effect on the large-scale production of the first-generation bioethanol [5]. Comparatively, lignocellulose biomass is a cheap and abundant renewable green resource on earth, which, according to the source, can be divided into four categories: forest, agricultural, horticultural, and food industry residues [6]. Therefore, the utilization of waste biomass rich in lignocellulose to produce fuel ethanol, known as the second generation of bioethanol, has become a research hotspot in the field [7,8,9].
Rice straw (RS) is one of the most abundant agricultural lignocellulosic wastes in the world, with a production of about 731–900 million tons every year on the earth [10]. However, only a small amount of RS is used for animal feed or farm manure, while most is discarded or burned directly, causing serious environmental pollution [11,12,13]. RS is mainly composed of cellulose, hemicellulose, and lignin. Cellulose and hemicellulose are polysaccharides available in plant cell walls and can be converted into biofuel through bioconversion [14]. Lignin and hemicellulose form a strong binding layer, which tightly surrounds the cellulose and hinders the contact between cellulose and cellulase [15]. Therefore, direct bioconversion of RS to biofuels is inefficient due to the presence of lignin, which needs to be removed first through pretreatment to disrupt its lignocellulosic structure, increase the surface area, and enhance the enzymatic and microbial attack of cellulose and/or hemicellulose in the hydrolysis process [16, 17].
Sodium perborate (SPB) is widely used in the dental industry, pulp bleaching, and detergent due to its low cost and low toxicity as a bleaching agent [18,19,20]. As shown in Eq. (1), dissolution of SPB in water releases sodium metaborate (NaBO3) and hydrogen peroxide (H2O2). The hydrogen peroxide and hydroxyl radicals resulted from H2O2 can remove lignin and attack almost all types of organic structures, including those containing hydroxyl and ether linkages [21,22,23]. According to previous studies on the effects of adding SPB and hydrogen peroxide on delignification in the oxidation stage of pulp bleaching, at the same active oxygen content of about 0.5%, the degree of delignification increases from 45.56 to 49.42% for hydrogen peroxide and to 52.96% for SPB, which could be further increased to 57.59% at 1% active oxygen content [24]. Therefore, SPB is more effective than hydrogen peroxide in lignin delignification in the oxidation stage. SPB is widely used as a bleaching agent and also reported to be used for the pretreatment of lignocellulose to remove lignin [25, 26]. Thus, the feasibility of using SPB for RS pretreatment was assessed in this study.
The production of fuel ethanol from lignocellulosic biomass mainly consists of five steps: (i) pretreatment of biomass, (ii) enzymatic hydrolysis of cellulose and hemicellulose, (iii) fermentation of monomeric sugars to produce ethanol, (iv) final recovery, and (v) purification of ethanol [27]. Simultaneous saccharification and co-fermentation (SScF) is a promising method for bioethanol production, which, through inoculation of the genetically engineered Saccharomyces cerevisiae, can simultaneously use hexose and pentose (mainly glucose and xylose) from the enzymatically hydrolyzed biomass to produce fuel ethanol, thus reducing costs and saving energy. Compared with separate hydrolysis and fermentation (SHF) and separate hydrolysis and co-fermentation (SHcF), SScF has many advantages, including reducing the inhibition of cellulase production by the accumulation of glucose in the end product of hydrolysis and the risk of contamination, higher ethanol concentration, and fermentation efficiency [28,29,30]. Additionally, SScF is also superior to simultaneous saccharification and fermentation (SSF) in that SScF can not only improve substrate utilization but also can achieve higher ethanol productivity and yield [31, 32].
The purpose of the present study was to evaluate the feasibility of RS pretreatment with SPB and optimize the corresponding parameters. Furthermore, the pretreatment mechanism of SPB was explored by scanning electron microscopy (SEM), X-ray diffraction (XRD), and Fourier transform infrared spectroscopy (FT-IR). Finally, the pretreatment effect was further evaluated by comparing the results of simultaneous saccharification and co-fermentation of RS before and after pretreatment. This research provides useful information for the production of fuel ethanol with SPB pretreatment.
2 Materials and methods
2.1 Materials
The rice straw (RS) was obtained from Lianyungang, Jiangsu, China, crushed and passed through a 20-mesh screen. Sodium perborate (SPB) was purchased from Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). Commercial cellulase Cellic® Ctec2 was purchased from Sigma-Aldrich (Steinheim, Germany) and the activity of the cellulase was 123.33 FPU/mL.
2.2 Pretreatment
The SPB pretreatment was carried out in a 250-mL triangular flask with a solid-to-liquid ratio of 5%. Briefly, 1 g RS was poured into an Erlenmeyer flask under stirring, followed by the addition of a certain amount of SPB which was well mixed with 20-mL deionized water, and sealed with sealing film. Next, the Erlenmeyer flask was quickly put into a digital display constant temperature bath pot for a bath at a constant rotation number. After the pretreatment for a certain period of time, the pretreated solid was washed to neutral pH with tap water and filtered through Buchner funnel with a vacuum pump, followed by drying in an oven at 55 °C overnight and then sealed storage for further use.
2.3 Enzymatic hydrolysis
The enzymatic hydrolysis of RS was carried out in 10-mL penicillin bottles with a hydrolysis volume of 5 mL, 0.1 g RS, a certain amount of 50 mM citric acid buffer (pH 4.8), and cellulase (20 FPU/g RS). Next, the penicillin bottles were sealed with a rubber plug and aluminum lids and incubated in a rotary shaker at 55 °C and 150 rpm for 72 h. The concentration of reducing sugar was measured by the 3, 5-dinitrosalicylic acid (DNS) method [10, 33].
The enzymolysis efficiency was calculated according to the following formula [34, 35]:
where 1.11 is the conversion coefficient between the glucan and the glucose; 1.14 is the conversion coefficient between the xylan and the xylose.
2.4 Cell cultivation and fermentation
2.4.1 Microorganism, culture media, and inoculum
The yeast Saccharomyces cerevisiae SHY07-1 was used in this study, which can simultaneously use glucose and xylose to ferment ethanol. It is preserved in the Fermentation Engineering Research Office of the School of Bioscience and Engineering of South China University of Technology [36]. The seed medium is mainly composed of xylose (20 g/L), yeast extract (10 g/L), and peptone (20 g/L). The fermentation medium mainly consists of (NH4)2SO4 (2 g/L), yeast extract (5 g/L), KH2PO4 (5 g/L), and MgSO4·7H2O (0.5 g/L). The carbon source is derived from the pretreated RS, which contains glucose and xylose, and the initial fermentation broth pH is adjusted to 5.45 with 1 mol/L NaOH.
According to our previous report, the yeast seed solution was prepared and inoculated into the fermentation medium at 10% (v/v) [35].
2.4.2 Simultaneous saccharification co-fermentation
The experiment of simultaneous saccharification and co-fermentation (SScF) of pretreated RS was carried out in 25-mL penicillin bottles. Briefly, 0.6-g pretreated RS was weighed into each bottle, followed by adding a 13-mL fermentation medium to make the RS concentration 4% (w/v). Then, each bottle was sealed with a rubber plug and aluminum lids and sterilized at 115 °C for 20 min. Next, 1.5 mL yeast seed solution was inoculated at an inoculum size of 10% (v/v) into the fermentation medium with 0.5 mL cellulase diluent (20 FPU/g pretreated RS, diluted with fermentation medium). Then, the bottles were transferred to a shaker (30 °C, 180 rpm) for fermentation, with 0.1 mL samples being collected at regular intervals during this process.
The ethanol fermentation efficiency of the SScF process was calculated by the following formula:
where 1.11 is the conversion factor from cellulose to glucose; 1.14, the conversion factor from xylan to xylose; and 0.51 and 0.46, the conversion rates of ethanol produced from glucose and xylose, respectively.
2.5 Structural characterization
The changes in the surface characteristics of RS before and after pretreatment were observed by Merlin field emission scanning electron microscopy (FE-SEM, Carl Zeiss). Briefly, a small amount of completely dry RS powder samples was placed on the loading platform and gold-sputtered. The magnification of the experiment was × 500 and × 2000 at the accelerated voltage of 10.0 kV.
The X-ray diffraction (XRD) analysis was carried out by using the Empyrean sharp X-ray diffraction system (Panalytical BV, Netherlands). The scanning range was set as 5°−50°, the scanning step length was 0.03°, and the scanning speed was 0.2 s/step. The crystallinity index (CrI) was calculated by the following formula:
where Iam is the intensity of the crystallization peak at 2θ = 19°; I002, the intensity of the crystallization peak at 2θ = 22.5° [37].
The FT-IR analysis was performed through the scanning wavelength from 4000 to 400 cm−1 with a resolution of 2 cm−1 and 32 scanning times for each sample using a Nicolet CCR-1 spectrometer (Thermo Nicolet Corporation, USA).
2.6 Analytical methods and data analysis
According to the method of the national renewable energy laboratory, the component content of RS was determined by the two-step acid hydrolysis method [38]. Briefly, 0.1 g completely dry RS and 1 mL of sulfuric acid solution (72% w/w) were mixed in a 50-mL pressure tube, followed by a 30 °C water bath for the first step of acid hydrolysis for 1 h. Next, 28 mL of deionized water was added to dilute the acidolysis solution to 4%, followed by vortex mixing and treating the pressure tube in a 121 °C high-pressure steam sterilizer for 1 h for the second step of acid hydrolysis. After the two-step acid hydrolysis, the content of soluble sugar (glucose and xylose) in the liquid was determined by HPLC and the contents of glucan and xylan were calculated. Lignin content is the sum of acid-soluble lignin content and acid-insoluble lignin content. The content of acid-soluble lignin is measured by UV-Visible spectrophotometer to measure the absorbance value of OD240, while the acid-insoluble residue was dried in a 105 °C oven to measure the lignin content.
As previously described [35], metabolites (soluble sugar and ethanol) in fermentation broth samples were analyzed using Waters 2414 HPLC (Milford, LA, USA) equipped with a refractive index detector and Aminex HPX-87H column, and the mobile phase was 5 mM H2SO4 at a flow rate of 0.6 mL/min.
The SPSS version 17.0 statistical software for Windows (SPSS Inc. Chicago) was used for all statistical analyses and a value of P < 0.05 was considered statistically significant.
The recovery rate of solid, glucan loss, and xylan loss after pretreatment was calculated by the following formula:
3 Results and discussion
3.1 Effects of SPB concentration on the composition and enzymatic hydrolysis of RS
Table 1 shows the effects of different SPB concentrations on the composition and enzyme hydrolysis efficiency of pretreated RS. The unpretreated RS contained 34.4% cellulose, 14.5% hemicellulose, 25.9% lignin, and 48.9% holocellulose. After SPB pretreatment, significant changes can be observed in the content of the related components of RS. Specifically, with the SPB concentration increased from 2 to 10%, the holocellulose content, lignin removal rate, and enzymatic hydrolysis efficiency increased from 61.7 to 70.1%, 26.2 to 40.2%, and 45.6 to 83.6%, respectively. Compared with the unpretreated RS, the enzyme hydrolysis efficiency of SPB-pretreated RS was about 12 to 50% higher. However, the 10% SPB pretreatment had a solid recovery of less than 59.5%, while the 8% SPB pretreatment had a solid recovery of 63.0% and an enzyme hydrolysis efficiency close to 80%. Therefore, 8% SPB is defined as the optimal concentration for further experiments.
3.2 Effects of pretreatment temperature on the composition and enzymatic hydrolysis of RS
Table 2 shows the influence of SPB pretreatment temperatures on the composition and enzymatic hydrolysis efficiency of pretreated RS. When the temperature was increased from 40 to 80 °C (8% SPB and 3 h pretreatment), the holocellulose content, the lignin removal rate, and the enzymolysis efficiency increased from 61.6 to 71.9%, 17.1 to 42.2%, and 34.5 to 83.1%, respectively. Considering that high temperature means high energy consumption and may cause equipment damage, there is no further increase in pretreatment temperature [39]. In addition, too high temperature will lead to the production of inhibitors and degradation of carbohydrates, which may reduce the yield of reducing sugar [40, 41]. Therefore, 80 °C is determined as the optimum temperature for SPB pretreatment.
3.3 Effects of pretreatment time on the composition and enzymatic hydrolysis of RS
Table 3 shows the effects of SPB pretreatment time on the composition and enzymatic hydrolysis efficiency of RS. With the pretreatment time increased from 1 to 4 h, significant changes can be observed in the total cellulose content, lignin removal rate, and enzymatic hydrolysis efficiency, with an increase from 66.5 to 75.4%, 30.5 to 45.8%, and 71.7 to 84.0%, respectively. With the pretreatment time further increased to 5 h, no obvious increase is observed in the values of the above three indices. Therefore, the optimal pretreatment time is determined as 4 h.
The reducing sugar yield was significantly higher in the pretreated RS than that in the hydrogen peroxide pretreatment reported in the previous literature. Reducing sugar yield of 702.4 g/kg was achieved in the present study under the optimum conditions (8% SPB, 80 °C, and 4 h). Li et al. reported the highest yield of reducing sugar was 0.42 g/g Ulva prolifera under the optimum pretreatment conditions (hydrogen peroxide 0.2%, 50 °C, pH 4.0, and 12 h) [42]. In addition, Sarita et al. reported 416.7 kg glucose/ton bagasse was obtained under optimum pretreatment conditions (7.36%, 25 °C, and 1 h) [43]. Therefore, SPB pretreatment is a simple and effective method to enhance the reducing sugar yield of RS.
3.4 Mechanisms for SPB pretreatment
3.4.1 Scanning electronic microscopy analysis
The mechanism for SPB pretreatment of RS was explored by scanning electron microscopy (SEM) analysis of the RS before and after the pretreatment as well as the related changes on the surface (Fig. 1). Previous studies have shown that the unpretreated RS has a dense surface structure and a plurality of silica particles [42]. However, the SPB pretreatment significantly changed the RS surface from a dense structure to a rough and porous structure, leading to damage in the density of RS lignin barrier and exposure of the cellulose [43, 44]. As a result, the rough and porous structure increased the accessibility of the cellulose to the cellulase, contributing to the SSCF of the cellulase and the Saccharomyces cerevisiae in the cellulose and hemicellulose attack [10, 43].
3.4.2 X-ray diffraction analysis
The crystallinity index (CrI) of cellulose is another important factor affecting the enzymatic hydrolysis of RS, and crystallinity can show the recalcitrance of the cellulose content in RS [45, 46]. Figure 2 shows the X-ray diffraction (XRD) patterns of RS before and after pretreatment, with the calculated crystallinity index shown in Table 4. It has been reported that hemicellulose and lignin are amorphous, and the CrI value is inversely proportional to the content of hemicellulose and lignin [35]. The crystallinity index of unpretreated RS was 34.2%, in contrast to 50.9% for RS after pretreatment. The increase in the crystallinity index of pretreated RS can be attributed to the removal of a large amount of lignin and part of hemicellulose by SPB pretreatment, which caused a decrease in the non-crystalline component in RS and an increase in the content of the crystalline component, hence an increase in the crystallinity index, which is consistent with several previous reports [47,48,49,50]. In addition, the increase of crystallinity in this study resulted in a higher sugar yield of the pretreated RS. The reason is that the SPB pretreatment removes lignin and part of hemicellulose and increases the availability of cellulose to cellulase, thus improving the enzymatic saccharification [45, 51].
3.4.3 Fourier transform infrared analysis
Figure 3 shows the FT-IR results of the RS samples before and after pretreatment. A significant reduction was observed in the spectral bands at 1248 cm−1 and 1730 cm−1 of pretreated RS compared with that of the untreated RS, indicating that some acetyl groups may be broken by SPB pretreatment [52, 53]. Meanwhile, the decrease in the spectral bands at 1510 cm−1 and 1322 cm−1 of pretreated RS was also observed, which is attributed to the general lignin and the guaiacyl lignin, indicating that lignin was significantly removed by SPB pretreatment [10, 54]. Furthermore, the spectral bands at 900 cm−1 which corresponded to β-glycosidic linkages in cellulose and hemicellulose were observed to increase after SPB pretreatment, indicating the removal of amorphous components in the pretreatment process, which was in accordance with the previous studies [55, 56].
3.5 Simultaneous saccharification and co-fermentation
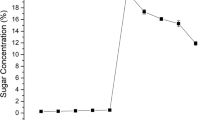
Figure 4 shows the effects of SPB pretreatment on the fermentation performance of RS by SScF inoculated with Saccharomyces cerevisiae SHY07-1. In a certain period of time, the ethanol concentration of the RS before and after pretreatment was increased with the extension of fermentation time. The ethanol concentration of unpretreated RS sharply increased in the first 9 h, slowly increased to the maximum from 9 h to the 15 h, and then maintained until the end of fermentation. The maximum ethanol concentration of the unpretreated RS was 4.8 g/L, the fermentation efficiency was 40.3%, and the ethanol yield was 0.06 g/g RS (Table 5). The ethanol concentration of SPB pretreatment was shown to increase rapidly with the prolongation of fermentation time in the first 18 h, followed by a gradual increase to the maximum value of 15.3 g/L, with a fermentation efficiency of 92.0% and an ethanol yield of 0.38 g/g pretreated RS. Obviously, the ethanol concentration obtained by SScF of pretreated RS was three times that of unpretreated RS, which indicated that the pretreatment with SPB enhanced the accessibility of cellulase hydrolysis and Saccharomyces cerevisiae SHY07-1 attack in the process of SScF and improved the fermentation performance of RS [16, 17].
In the first 3 h, the glucose concentration of the RS before and after pretreatment rapidly increased and reached a maximum value of 2.7 g/L and 7.1 g/L, respectively, followed by a rapid consumption, with a negligible glucose content in the fermentation broth after 6 h, indicating that the sugar produced by the enzymatic hydrolysis was quickly fermented by the Saccharomyces cerevisiae to produce ethanol. In the first 3 h, xylose of the unpretreated RS also reached the maximum value of 1.4 g/L and then rapidly consumed to the lowest point. However, although xylose of the pretreated RS was also rapidly accumulated and reached a maximum value of 2.3 g/L, it was rapidly consumed after 6 h and drop to the lowest value at the 9th hour until the end of the fermentation. The reason for this phenomenon may be that the concentration of glucose and xylose released by the enzyme hydrolysis of unpretreated RS was low due to the low hydrolysis efficiency in the process of SScF. In addition, Saccharomyces cerevisiae SHY07-1 quickly consumed glucose and turned to the utilization of xylose, resulting in the phenomenon that the concentration of glucose and xylose of RS without pretreatment reached the maximum at 3 h and consumed rapidly in the same period time. However, enough glucose was released from pretreated rice straw by enzyme hydrolysis in the process of SScF. Saccharomyces cerevisiae SHY07-1 fermented ethanol with glucose and began to use xylose until glucose was exhausted, resulting in rapid consumption of glucose and xylose at different time points.
The fermentation efficiency was significantly higher in the pretreated RS than that reported in the previous literature. The fermentation efficiency was 66.9% for the bagasse pretreated with steam explosion and 69.9% for the bagasse pretreated with [Cho][OAc]/DMSO [54, 57]. The fermentation efficiency of 91.96 in the present study was similar to that (91.0%) of RS pretreated with the combined alkaline peroxide (AHP) and [Bmim]Cl–water mixtures reported in a previous study [10]. The SScF experimental results indicate that SPB pretreatment is an effective method to improve the fermentation efficiency of ethanol production.
4 Conclusion
In this study, a simple RS pretreatment process using SPB was successfully developed. The SPB pretreatment can destroy the surface structure of the RS, effectively remove the lignin, and improve the enzymolysis efficiency. The pretreated RS and achieve the ethanol production with high fermentation efficiency and yield through SScF. This study provides a new strategy for efficient pretreatment of RS for a high level of bioethanol production through SScF.
References
Liu W, Wu R, Wang B, Hu Y, Hou Q, Zhang P, Wu R (2020) Comparative study on different pretreatment on enzymatic hydrolysis of corncob residues. Bioresour Technol 295:122244. https://doi.org/10.1016/j.biortech.2019.122244
Kapanji KK, Haigh KF, Gorgens JF (2019) Techno-economic analysis of chemically catalysed lignocellulose biorefineries at a typical sugar mill: sorbitol or glucaric acid and electricity co-production. Bioresour Technol 289:121635. https://doi.org/10.1016/j.biortech.2019.121635
Kainthola J, Kalamdhad AS, Goud VV, Goel R (2019) Fungal pretreatment and associated kinetics of rice straw hydrolysis to accelerate methane yield from anaerobic digestion. Bioresour Technol 286:121368. https://doi.org/10.1016/j.biortech.2019.121368
Maggio G, Cacciola G (2012) When will oil, natural gas, and coal peak? Fuel 98:111–123. https://doi.org/10.1016/j.fuel.2012.03.021
Mohapatra S, Ray RC, Ramachandran S (2019) Bioethanol from biorenewable feedstocks: technology, economics, and challenges:3–27. https://doi.org/10.1016/b978-0-12-813766-6.00001-1
Kumar B, Bhardwaj N, Agrawal K, Chaturvedi V, Verma P (2020) Current perspective on pretreatment technologies using lignocellulosic biomass: an emerging biorefinery concept. Fuel Process Technol 199:106244. https://doi.org/10.1016/j.fuproc.2019.106244
Bensah EC, Kemausuor F, Miezah K, Kadar Z, Mensah M (2015) African perspective on cellulosic ethanol production. Renew Sust Energ Rev 49:1–11
Diep NQ, Sakanishi K, Nakagoshi N, Fujimoto S, Minowa T (2015) Potential for rice straw ethanol production in the Mekong Delta, Vietnam. Renew Energ 74:456–463
Pereira SC, Maehara L, Machado CMM (2015) 2G ethanol from the whole sugarcane lignocellulosic biomass. Biotechnol Biofuels 8:44. https://doi.org/10.1186/s13068-015-0224-0
Hong YY, Wang YT, Zhu SM, Luo XC, Li S, Zhuo M, Zhou T, Zhu MJ (2019) Improved enzymatic hydrolysis and ethanol production by combined alkaline peroxide and ionic liquid-water mixtures pretreatment of rice straw. J Chem Technol Biotechnol 94(5):1451–1459. https://doi.org/10.1002/jctb.5895
Sarkar N, Ghosh SK, Bannerjee S, Aikat K (2012) Bioethanol production from agricultural wastes: an overview. Renew Energ 37(1):19–27. https://doi.org/10.1016/j.renene.2011.06.045
Binod P, Sindhu R, Singhania RR, Vikram S, Devi L, Nagalakshmi S, Kurien N, Sukumaran RK, Pandey A (2010) Bioethanol production from rice straw: an overview. Bioresour Technol 101(13):4767–4774. https://doi.org/10.1016/j.biortech.2009.10.079
Tye YY, Lee KT, Wan Abdullah WN, Leh CP (2016) The world availability of non-wood lignocellulosic biomass for the production of cellulosic ethanol and potential pretreatments for the enhancement of enzymatic saccharification. Renew Sust Energ Rev 60:155–172. https://doi.org/10.1016/j.rser.2016.01.072
Chuetor S, Champreda V, Laosiripojana N (2019) Evaluation of combined semi-humid chemo-mechanical pretreatment of lignocellulosic biomass in energy efficiency and waste generation. Bioresour Technol 292:121966. https://doi.org/10.1016/j.biortech.2019.121966
Ho MC, Ong VZ, Wu TY (2019) Potential use of alkaline hydrogen peroxide in lignocellulosic biomass pretreatment and valorization – a review. Renew Sust Energ Rev 112:75–86. https://doi.org/10.1016/j.rser.2019.04.082
Momayez F, Karimi K, Horvath IS (2018) Enhancing ethanol and methane production from rice straw by pretreatment with liquid waste from biogas plant. Energ Convers Manage 178:290–298
Batista G, Souza RBA, Pratto B, dos Santos-Rocha MSR, Cruz AJG (2019) Effect of severity factor on the hydrothermal pretreatment of sugarcane straw. Bioresour Technol 275:321–327
Savic-Stankovic T, Karadzic B, Latkovic M, Miletic V (2019) Clinical efficiency of a sodium perborate - hydrogen peroxide mixture for intracoronal non-vital teeth bleaching. Srp Arh Celok Lek 00:94–94. https://doi.org/10.2298/sarh190504094s
Ramos E, de la Torre MJ, Gutierrez JC (2016) Use of perborate in the bleaching of ethanolamine pulp from olive wood. Afinidad 73(575):176–181
López F, Eugenio ME et al (2002) Hydrogen peroxide and sodium Perborate bleaching of pulp from olive tree residues. Eng Life Sci 2(7):201–208
Attin TPF, Ajam F et al (2003) Review of the current status of tooth whitening with the walking bleach technique. Int Endod J 36(5):313–329
Tran L, Orth R, Parashos P, Tao Y, Tee CW, Thomas VT, Towers G, Truong DT, Vinen C, Reynolds EC (2017) Depletion rate of hydrogen peroxide from sodium perborate bleaching agent. J Endod 43(3):472–476. https://doi.org/10.1016/j.joen.2016.10.043
DI Okan OT, Yildirim I (2013) Bleaching of bamboo (Phyllostachys bambusoides) Kraft-AQ pulp with sodium perborate tetrahydrate (SPBTH) after oxygen delignification. BioResources 8(1):1332–1344
Pesman EKEE, Kirci H (2010) Sodium Perborate usage instead of hydrogen peroxide for the reinforcement of oxygen delignification. Fibers Text East Eur 18(6):83
Davaritouchaee M, Hiscox WC, Martinez-Fernandez J, Fu X, Mancini RJ, Chen SL (2019) Effect of reactive oxygen species on biomass structure in different oxidative processes. Ind Crop Prod 137:484–494
Bulut Y, Aksit A (2013) A comparative study on chemical treatment of jute fiber: potassium dichromate, potassium permanganate and sodium perborate trihydrate. Cellulose 20(6):3155–3164. https://doi.org/10.1007/s10570-013-0049-6
You Y, Li P, Lei F, Xing Y, Jiang J (2017) Enhancement of ethanol production from green liquor-ethanol-pretreated sugarcane bagasse by glucose-xylose cofermentation at high solid loadings with mixed Saccharomyces cerevisiae strains. Biotechnol Biofuels 10:92. https://doi.org/10.1186/s13068-017-0771-7
Ojeda K, Sanchez E, El-Halwagi M, Kafarov V (2011) Exergy analysis and process integration of bioethanol production from acid pre-treated biomass: comparison of SHF, SSF and SSCF pathways. Chem Eng J 176:195–201
Koppram R, Nielsen F, Albers E, Lambert A, Wannstrom S, Welin L (2013) Simultaneous saccharification and co-fermentation for biothanol production using corncobs at lab, PDU and demo scales. Biotechnol Biofuels 6:2. https://doi.org/10.1186/1754-6834-6-2
Liu ZH, Chen HZ (2016) Simultaneous saccharification and co-fermentation for improving the xylose utilization of steam exploded corn stover at high solid loading. Bioresour Technol 201:15–26
Olofsson K, Bertilsson M, Liden G (2008) A short review on SSF- an interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol Biofuels 1(1):7. https://doi.org/10.1186/1754-6834-1-7
Jin MJ, Gunawan C, Balan V, Lau MW, Dale BE (2012) Simultaneous saccharification and co-fermentation (SSCF) of AFEX (TM) pretreated corn stover for ethanol production using commercial enzymes and Saccharomyces cerevisiae 424A(LNH-ST). Bioresour Technol 110:587–594
Zhang CW, Xia SQ, Ma PS (2016) Facile pretreatment of lignocellulosic biomass using deep eutectic solvents. Bioresour Technol 219:1–5
Bu J, Yan X, Wang Y-T, Zhu S-M, Zhu M-J (2019) Co-production of high-gravity bioethanol and succinic acid from potassium peroxymonosulfate and deacetylation sequentially pretreated sugarcane bagasse by simultaneous saccharification and co-fermentation. Energ Convers Manage 186:131–139. https://doi.org/10.1016/j.enconman.2019.02.038
Zhang T, Zhu MJ (2016) Enhancing enzymolysis and fermentation efficiency of sugarcane bagasse by synergistic pretreatment of Fenton reaction and sodium hydroxide extraction. Bioresour Technol 214:769–777. https://doi.org/10.1016/j.biortech.2016.05.032
Zhu Z, Zhu M, Wu Z (2012) Pretreatment of sugarcane bagasse with NH4OH–H2O2 and ionic liquid for efficient hydrolysis and bioethanol production. Bioresour Technol 119:199–207. https://doi.org/10.1016/j.biortech.2012.05.111
Qiu Z, Aita GM, Walker MS (2012) Effect of ionic liquid pretreatment on the chemical composition, structure and enzymatic hydrolysis of energy cane bagasse. Bioresour Technol 117:251–256. https://doi.org/10.1016/j.biortech.2012.04.070
Sluiter AHB, Ruiz R et al (2008) Determination of structural carbohydrates and lignin in biomass. Lab Anal Proced 1617:1–16
Wang SQ, Li F, Zhang PY, Jin SG, Tao X, Tang X, Ye JP, Nabi M, Wang HJ (2017) Ultrasound assisted alkaline pretreatment to enhance enzymatic saccharification of grass clipping. Energ Convers Manage 149:409–415
Ramadoss G, Muthukumar K (2016) Mechanistic study on ultrasound assisted pretreatment of sugarcane bagasse using metal salt with hydrogen peroxide for bioethanol production. Ultrason Sonochem 28:207–217
Jonsson LJ, Martin C (2016) Pretreatment of lignocellulose: formation of inhibitory by-products and strategies for minimizing their effects. Bioresour Technol 199:103–112. https://doi.org/10.1016/j.biortech.2015.10.009
Li Y, Cui J, Zhang G, Liu Z, Guan H, Hwang H, Aker WG, Wang P (2016) Optimization study on the hydrogen peroxide pretreatment and production of bioethanol from seaweed Ulva prolifera biomass. Bioresour Technol 214:144–149. https://doi.org/10.1016/j.biortech.2016.04.090
Rabelo SC, Andrade RR, Maciel Filho R, Costa AC (2014) Alkaline hydrogen peroxide pretreatment, enzymatic hydrolysis and fermentation of sugarcane bagasse to ethanol. Fuel 136:349–357. https://doi.org/10.1016/j.fuel.2014.07.033
Mou HY, Heikkila E, Fardim P (2013) Topochemistry of alkaline, alkaline-peroxide and hydrotropic pretreatments of common reed to enhance enzymatic hydrolysis efficiency. Bioresour Technol 150:36–41. https://doi.org/10.1016/j.biortech.2013.09.093
Zhang HB, Zhang PY, Ye J, Wu Y, Liu JB, Fang W, Xu D, Wang B, Yan L, Zeng GM (2018) Comparison of various pretreatments for ethanol production enhancement from solid residue after rumen fluid digestion of rice straw. Bioresour Technol 247:147–156
Chai L, Liu M, Yan X, Cheng X, Zhang T, Si M, Min X, Shi Y (2018) Elucidating the interactive impacts of substrate-related properties on lignocellulosic biomass digestibility: a sequential analysis. ACS Sustain Chem Eng 6(5):6783–6791. https://doi.org/10.1021/acssuschemeng.8b00592
He YPY, Liu Y et al (2008) Physicochemical characterization of rice straw pretreated with sodium hydroxide in the solid state for enhancing biogas production. Energy Fuel 22(4):2775–2781
Brito JQA, Dias FS, Cunha S, Ramos LP, Teixeira LSG (2019) Multiple response optimization of alkaline pretreatment of sisal fiber (Agave sisalana) assisted by ultrasound. Biotechnol Prog 35(3):e2802. https://doi.org/10.1002/btpr.2802
Akhtar N, Goyal D, Goyal A (2017) Characterization of microwave-alkali-acid pre-treated rice straw for optimization of ethanol production via simultaneous saccharification and fermentation (SSF). Energ Convers Manage 141:133–144
Subhedar PB, Ray P, Gogate PR (2018) Intensification of delignification and subsequent hydrolysis for the fermentable sugar production from lignocellulosic biomass using ultrasonic irradiation. Ultrason Sonochem 40(Pt B):140–150. https://doi.org/10.1016/j.ultsonch.2017.01.030
Yuan ZQ, Long JX, Wang TJ, Shu RY, Zhang Q, Ma LL (2015) Process intensification effect of ball milling on the hydrothermal pretreatment for corn straw enzymolysis. Energ Convers Manage 101:481–488
Mohammadi M, Shafiei M, Abdolmaleki A, Karimi K, Mikkola J-P, Larsson C (2019) A morpholinium ionic liquid for rice straw pretreatment to enhance ethanol production. Ind Crop Prod 139:111494. https://doi.org/10.1016/j.indcrop.2019.111494
Zhu L, O'Dwyer JP, Chang VS, Granda CB, Holtzapple MT (2008) Structural features affecting biomass enzymatic digestibility. Bioresour Technol 99(9):3817–3828
Asakawa A, Oka T, Sasaki C, Asada C, Nakamura Y (2016) Cholinium ionic liquid/cosolvent pretreatment for enhancing enzymatic saccharification of sugarcane bagasse. Ind Crop Prod 86:113–119. https://doi.org/10.1016/j.indcrop.2016.03.046
Reis CLB, Silva LMAE, Rodrigues THS, Felix AKN, de Santiago-Aguiar RS, Canuto KM, Rocha MVP (2017) Pretreatment of cashew apple bagasse using protic ionic liquids: enhanced enzymatic hydrolysis. Bioresour Technol 224:694–701
Zhang ZY, O'Hara IM, Doherty WUS (2012) Pretreatment of sugarcane bagasse by acid-catalysed process in aqueous ionic liquid solutions. Bioresour Technol 120:149–156
Li JWK, Xiao W et al (2014) Effect of antioxidant extraction on the enzymatic hydrolysis and bioethanol production of the extracted steam-exploded sugarcane bagasse. Biochem Eng J 82:91–96. https://doi.org/10.1016/j.bej.2013.11.005
Funding
This work was financially supported by the National Natural Science Foundation of China (grant nos. 51878291 and 51908140).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
• A simple sodium perborate (SPB) process is established for rice straw pretreatment.
• SPB pretreatment can significantly remove lignin with low holocellulose loss.
• SPB pretreatment can effectively improve the enzymatic hydrolysis efficiency.
• SPB pretreatment can improve SScF performance in bioethanol production.
Rights and permissions
About this article
Cite this article
Guo, JM., Wang, YT., Cheng, JR. et al. Enhancing enzymatic hydrolysis and fermentation efficiency of rice straw by pretreatment of sodium perborate. Biomass Conv. Bioref. 12, 361–370 (2022). https://doi.org/10.1007/s13399-020-00668-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-020-00668-3