Abstract
In this study, biomass was converted into new products with catalyst (H3BO3, Na2CO3, Al2O3) and without catalyst at 300, 325, and 350 °C by the hydrothermal liquefaction method. The products obtained were analyzed by GC-MS, FT-IR, SEM, elemental analysis, and 1H NMR methods. Based on the trials, the highest liquid product yield (total bio-oil) was determined as 29.69% in the trial without catalyst at 350 °C. The higher heating value (HHV) has been calculated by Dulong’s formula, and the HHV values of all the (light bio-oil, heavy bio-oil, and solid residue) were determined to be higher than the HHV value of the feedstock. The highest HHV value was obtained from heavy bio-oil as 31.32 MJ/kg with the catalyst at 350 °C. This HHV value is higher than the HHV value attained by the pyrolysis and supercritical liquefaction method. The products obtained generally consisted of monoaromatics, polyaromatics, oxygen compounds, and aliphatics. Based on the results of the elemental analysis, HHV values varying between 16.22 and 31.78 MJ/kg were found for all products.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Fossil resources are used as the main resources for various energy requirements around the world. The energy, transportation, and manufacturing industries have the largest share in the usage of fossil resources [1]. There may be cases in which the use of renewable energy resources is not possible for the chemical and manufacturing industries, but the usage of fossil resources can be decreased by supporting the energy production and transportation industries that have the highest percentage of usage of renewable energy resources. In this way, the problems arising from the usage of these resources can be eliminated. One of the crucial features of biomass, one of the renewable energy resources, is that it keeps CO2 emissions in the atmosphere constant. When biomass is burned and converted into energy, the CO2 released into the atmosphere was used by the biomass to be transformed into carbohydrates and other structural substances by photosynthesis.
Biomass is a renewable natural substance which stores the energy of the sun as chemical energy by using photosynthesis. Biomass consists of polymers which are constituted from macromolecules containing C–C bonds. While the C–C bond constitutes the main frame for the polymers, there may also be bonds that are constituted from C–O, C–H, C–N, C–S, or other elements. The polymers constituting the biomass are generally macromolecular materials consisting of micro-units. Cellulose is constituted from glucan (C6H10O5)n units, called anhydroglucose, which is formed when the glucose molecule loses one molecule of water. It is a high molecular-weighted, non-polar, long chain polysaccharide with a high degree of polymerization of which the basic cellulose chemical unit is (C6H10O5)n and solubility increases with temperature [2]. The hemicellulose is a biopolymer consisting of branched amorphous structure pentoses and hexoses. The structure of hemicellulose varies depending on xylan, mannan, glucan, and galactan, which are present in woody and herbaceous plants [3]. The hemicellulose disintegrates and decomposes easily due to leaner intramolecular bonds compared to cellulose. Lignin, which is a natural biopolymer of aromatic hydrocarbons constituted from the hydroxyl and ethoxy groups and phenyl-propane interconnected with ether bonds, is one of the basic components of biomass [4]. Lignin, like hemicellulose, has an amorphous structure morphologically, but it resembles cellulose due to its low solubility and its strengthening of the plant cell. Compared to other biomass components, lignin is the biopolymer most resistant to biological disintegration; this can be attributed to the multiple number of bonds between the units in its structure. Lignin has a high energy value, thanks to its valuable phenol compounds [5].
The elemental analysis of biomass revealed that it mostly consists of C, H, N, S, and O. C is the most significant part of the content of biomass; its resource is atmospheric CO2 and added to the structure of the plant at the end of photosynthesis. The large part of thermal energy, which is acquired by the phenomenon of combustion, is obtained from carbon, and then, it is released into the atmosphere in the form of CO2 [6]. In combustion, C does not burn in its entirety, CO and polycyclic aromatic hydrocarbons (e.g., naphthalene, acenaphthylene, acenaphthene, phenanthrene, fluoranthene, pyrene, benzo(a) anthracene, and dibenzo(a,h)anthracene) generated as the output [7]. In general, the carbon amount of the biomass can be estimated from the ratios of cellulose, hemicellulose, and lignin; it also supports the generation of lignin char, and therefore, the carbon amount is low in herbaceous plants of which the lignin content is high. One of the fundamental components of biomass is hydrogen, and as known, hydrogen is involved in the structure of carbohydrate and phenolic polymers. Then, hydrogen, which significantly affects the energy obtained during combustion, is converted into H2O. Hydrogen content is generally lower in herbaceous plants compared to woody plants. Nitrogen is the vital nutrient for biomass. The nitrogen amount in herbaceous plants is higher than in woody plants [8, 9]. Moreover, the existence of nitrogen contributes to the energy value since it is not oxidized in the combustion phenomenon and it contributes to the phenomenon of disintegration. Another significant component is S. Like nitrogen, S is essential for growth, and it is involved in the structure of amino acids, proteins, and enzymes. The higher S content in herbaceous plants is attributed to one of the causes of faster growth of herbaceous plants compared to woody plants. While the ratio of S in some woody plants may be 0%, this ratio varies between 0 and 0.2% in herbaceous plants. The most significant effect of S is the corrosion that it causes in the generations of environmentally hazardous SOx and gasification operations [8]. Oxygen has vital importance for life, and it is largely formed at the end of photosynthesis. The oxygen amount controls the energy value in combustion, and the excess oxygen content is the most significant factor in limiting the use of biomass [10]. The liquefaction process involves very complex reaction mechanisms such as the fragmentation of the oxygen bond present in the structure of phenolic compounds or the increase of the heating value by removing oxygen from the structure of the compound in different ways. The content of oxygen is not directly detectable, but it is detectably generated by removing other components (C, H, N, S) from the composition scale accepted as a total of 100.
The most common method used for obtaining energy from biomass is direct burning. The phenomenon of combustion is comprised a series of exothermic reactions which result in heat generation and chemical conversion between the fuel and the oxidant. The heat is generated by the oxidation reaction of oxygen or carbon, hydrogen, oxygen, and inflammable sulfur and nitrogen compounds present in the air environment during the combustion of biomass. Combustion is a very low-cost method of which the mechanism is known. Converting it into products with high enthalpy density instead of directly burning the biomass is significant in terms of both transportation and obtaining high energy efficiency. Biomass is converted into products with high enthalpy density by the thermochemical methods such as burning, pyrolysis, gasification, and liquefaction. Water is also used in the liquefaction method as a solvent, as well as organic compounds such as ethanol, acetone, methanol, and butanol. When the working conditions are reviewed, studies in which the use of organic solvents and water vary depending on the temperature and pressure.
Hydrothermal liquefaction is a thermochemical procedure used for converting biomass into liquid products that have high energy content. In general, conversion is conducted under subcritical water conditions and at high pressure, and an organic liquid called bio-oil is obtained [10, 11]. This procedure is comparable to the way fossil fuels are generated. However, liquid fuel (bio-oil) is obtained in the hydrothermal liquefaction process within periods expressed in hours and even in minutes while fossil fuels are created by the exposure of biomass to high temperature and pressure under the ground for a period of many years [12]. The following aspects make hydrothermal liquefaction desirable: water is a unique, environmentally friendly solvent, the procedure can be applied to wet biomasses, there is no need to dry the biomass, it can be conducted at lower temperatures compared to procedures such as pyrolysis, and it has a high energy yield [13]. The goal of hydrothermal liquefaction is to obtain a biofuel with a high enthalpy density following the conversion of biomass. The most significant and remarkable feature of the procedure is the cost and risk arising from the high pressure.
The decomposition of biomass mainly consists of the stages of dehydration in which water molecules are eliminated, decarboxylation reactions in which CO2 molecules are eliminated, and deamination in which amino acid molecules are eliminated. The dehydration and decarboxylation reactions facilitate the removal of O2 from the biomass in the form of H2O and CO2. The biomass, which is constituted from macromolecules to a considerable extent (biopolymer), converts into polar oligomers and monomers by hydrolysis. This leads to the generation of glucose monomers by breaking the hydrogen bonds available in the structure of cellulose because of its water-soluble feature at high temperature and high pressure. Fructose is more reactive than glucose, and these molecules are converted into micro products by dehydration, reverse-aldol defragmentation, isomerization, hydrolysis, rearrangement, and recombination reactions [14, 15].
The products obtained at the end of the hydrothermal liquefaction process have lower oxygen content and, therefore, a higher heating value compared to pyrolysis [16]. Miscellaneous factors such as reaction period, temperature, pressure, size of biomass particles, use of the catalyst, and reaction environment affect the yield and composition of the bio-oils obtained. While there is an increase in bio-oil yield up to approximately 300–350 °C in the hydrothermal liquefaction generally conducted at 250–350 °C, the yield of the gaseous product increases at higher temperatures. Moreover, the bio-oil composition obtained varies depending on the composition available in the structure of the biomass [17]. Phenol, acetic acid, fatty acids, furfural, 2-methoxyphenol, docosane and cholestane, cycloalkane, 1 and 2 benzenediol, aldehydes, ketones, and alcohols are examples of some basic products that form as a result of the hydrothermal liquefaction of biomass.
Miscellaneous biomass resources such as woody biomasses, weeds, and algae have been used in hydrothermal studies. In a study in which Nannochloropsis sp., a microalga, was liquified with a hydrothermal procedure in a waiting period of 60 min between 200 and 500 °C, it was reported that the highest bio-oil yield (43%) was obtained at 350 °C. The heating value of the bio-oil obtained was 39 MJ/kg, which is close to the crude oil value [18]. In a study in which Dunaliella tertiolecta, another microalga, was liquified with a hydrothermal procedure that had a retention period of 10–100 min at 300–380 °C, it was reported that the highest bio-oil yield (36.9%) was obtained from the experiment conducted at 360 °C within a retention period of 30 min. The heating value of the bio-oil obtained was 26.62 MJ/kg [19]. In a study in which the Datura plant was converted into new products with and without catalyst at 250–380 °C by the hydrothermal liquefaction method, the highest conversion ratio obtained was 79% at 380 °C in the presence of the colemanite catalyst. It was determined that the highest liquid product yield at which the catalysts were effective on the conversion and liquid product yield was 40.20% at 300 °C in the presence of the colemanite catalyst. Moreover, 64 chemical compounds were isolated by GC-MS analysis [20]. In a study in which the hydrothermal liquefaction of lignin, rice husk, and wood chip was conducted at 280 °C with a retention period of 15 min, the highest bio-oil amount (8.6%) was obtained from the wood chip [21]. Since cellulose, hemicellulose, and lignin compounds are present in the woody biomasses while carbohydrates, proteins, nucleic acids, and oils are present in the structures of algal biomasses, different product compositions were obtained as a result of hydrothermal processing of these two types of biomasses. Since the wood components are more durable, the use of a catalyst is preferred for obtaining high bio-oil yield.
In this study, the Anchusa azurea plant, an annual that grows in nature, was used. This plant was converted into new products by a supercritical liquefaction method using organic solvents and pyrolysis. In this study, considering the energy value of liquid products, the hydrothermal liquefaction method, which has a lesser effect on the environment, was found to be more advantageous to pyrolysis and supercritical liquefaction process.
2 Materials and methods
2.1 Materials
Anchusa azurea samples were harvested in July, collected from the Van region of Turkey. The stems were removed from the leaves and tops which were dried naturally in the open air. Then, they were ground, milled, and screen-sieved to a 0.6-mm pore size. The raw material powder was extracted with petroleum ether (b.p. 40–60 °C) in a Soxhlet extractor for 6 h. The milled feedstock was uniformly mixed before the experiments. Ultimate and approximate analysis of the Anchusa azurea were conducted before the experiments, and the results are given below. The ultimate analysis was as follows: carbon, 40.82%; hydrogen, 5.56%; nitrogen, 0.63%; oxygen, 52.99%; higher heating value (MJ/kg), 12.40. The proximate analysis was as follows: lignin, 18.11%; cellulose, 40.67%; hemicellulose, 24.23%; moisture, 6.03%; ash, 10.61%; and soxhlet extractives, 0.7% as the percentage of dry feedstock [22]. An elemental analyzer (LECO CHNS-932) was used for performing the ultimate analysis of the sample. The key features of Anchusa azurea were determined by Tappi Test methods. Lignin and cellulose were determined by the Tappi T222 and Tappi T202 methods, respectively [23]. The holocellulose content was determined by using the chloride method. The ash and moisture content were determined according to the Tappi T211 and Tappi T264, respectively. The higher heating value (HHV) was calculated with Dulong’s formula by using the results of the ultimate analysis. A Fourier-transform infrared (FT-IR) spectroscopy of the raw material was taken by using potassium bromide as transparent pellets with a Perkin Elmer Spectrum One FT-IR Spectrometer for identification of structural groups within the raw material. The raw material was characterized by FT-IR in the middle region, including the wave numbers ranging between 4000 and 550 cm−1. According to the literature [23], the bands in the analysis of the raw material indicate that it is mainly composed of lignin, cellulose, and hemicelluloses. The band at 3343.85 cm−1 was formed by the hydroxyl group of lignin in Anchusa azurea. Absorption at wave number 1737.62 cm−1 is a feature of xylenes of hemicellulose. The absorptions that peak at about 2922.30 and 1320.09 cm−1 are features of cellulose. Generally, the spectrum of lignin gives similar absorption peaks. The absorptions at 2922.03, 1621.83, and 1029.71 cm−1 represent lignin; in particular, the sharp peak at 1029.71 cm−1 is due to the ether bonds that are present in the raw material.
2.2 Experimental procedure
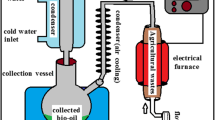
The procedures applied to the biomass within the process of hydrothermal liquefaction and the analyses methods used for examining the products obtained are given in Fig. 1. The hydrothermal liquefaction experiments were carried out in a batch stainless steel reactor (uniterm high-pressure reactor). The reactor is shown in Fig. S1. The reactor was designed to operate at a maximum temperature of 400 °C and a maximum pressure of 26 MPa. The reactor was heated with an external fabric mantle and cooled by an internal air system. The temperature was monitored using an inert thermocouple and controlled by a proportional integral derivative module (PID). The experimental stage and analysis methods used in characterization are shown in Fig. 1. In a typical run, 5 g of Anchusa azurea powder and 60 mL distilled water was charged into the reactor. Then, the reactor was purged three times with nitrogen to remove the air inside. In catalytic experiments, the desired quantity of the catalyst (10 wt%) was dissolved, first in the solvent and then added to the reactor. The experiments were performed at three different temperatures (300, 325, and 350 °C) with the catalysts (H3BO3, Na2CO3, Al2O3) and without a catalyst. The catalyst ratio was kept constant as 10% in all runs. The reactor was plugged with a screw bolt, placed in a furnace, and heated to the reaction temperature at a heating rate of 10 °C/min and held at this temperature for 0 min. During the hydrothermal liquefaction, the temperature of the reaction vessel was measured using a thermocouple and checked at 300 ± 2, 325 ± 2, and 350 ± 2 °C. Upon completion of the reaction, the reactor was cooled to room temperature by an air system. The maximum pressures that occurred in the reactor at these temperatures were 87.2, 123.5, and 173.6 bar, respectively. After reaching the desired temperature, the heating was stopped, and the system was cooled to room temperature. After cooling, the reactor was opened, uncondensed gases were vented, and then the contents (unconverted raw material and liquids) of the reactor were poured into a 200-mL beaker for separation. The residual oils and solids inside the reactor were washed several times with distilled water until all of them recovered. The liquid phase was filtered in a 20-mL glass crucible to separate the solid (unconverted raw material) from the mixture. The solid was washed several times with distilled water to remove impurities. The ether solution obtained by extracting the liquid part with an equal amount of diethyl ether was dried with anhydrous sodium sulfate, and then it was filtered. The solvent ether was removed by a rotary evaporator at room temperature. The amount obtained at the end of the removal of the diethyl ether was determined and called light bio-oil. The solid product remained on the filter paper, and the autoclave was washed with acetone (250 mL). The amount obtained by removing the acetone in the rotary evaporator under low pressure was measured and called heavy bio-oil. The total amount is the sum of bio-oil, light bio-oil, and heavy bio-oil. The solid substance remaining on the filter paper (solid residue) was called biochar. The solid residue was dried in the oven at 105 °C for 2 h. Following the drying procedure, the filter paper and solid residues were brought to room temperature, and then they were weighted and quantified.
3 Product analysis
Molecules such as polar organic molecules, glycolaldehydes, furfurals, phenols, and organic acids have high water solubility under the HTL process conditions. The hydrothermal liquefaction procedure is a thermochemical transformation process in which gaseous, aqueous, and solid phase products are obtained from biomass in an aquatic environment at 250–380 °C and under high pressure (10–25 MPa) to gain a bio-liquid product [8]. Various complex reactions arise during this procedure, and a quasi-oil product is obtained. The depolymerization of biomass is the process of the decomposition of macromolecules depending on their chemical and physical properties. The hemicellulose and cellulose biopolymers make a positive contribution to the thermal stability of bio-oil. The resistance of lignocellulosic biomass to decomposition is overcome with the depolymerization processes and the natural geological processes of producing fossil fuels. The effective temperature and pressure criteria change the bond structure of biopolymers and positively affect the generation of short chain polymers consisting of hydrogen, oxygen, and carbon [24].
The bio-oils obtained at 350 °C were analyzed and characterized by chromatographic and spectroscopic techniques such as GC-MS, elemental, FT-IR, and 1H NMR; they were then compared with each other. Elemental analysis was performed with a LECO CHNS 932 analyzer and infrared analysis with a PerkinElmer Spectrum 100 Spectrophotometer. The GC-MS analysis was conducted with the Agilent GC-MS 7890A/5975C series (Agilent Technologies, Santa Clara, CA, USA). The column (HP–INNOWAX, length, 60 m; I.D., 0.250 mm; film, 0.25 μm; and temperature limits, from 40 to 260 °C) and injector temperatures were the same as those for GC. The carrier gas was helium at a flow rate of 1.7 mL/min. Samples of 1 μL were injected with a split ratio of 1:30. The GC oven temperature program was as follows: started at 40 °C; held for 10 min, raised from 40 to 200 °C with a 5-°C/min heating rate; held for 15 min, raised to 240 °C with a 10-°C/min heating rate; held for 15 min, raised to 260 °C with a 10-°C/min heating rate; held at this final temperature for 10 min. The column was directly introduced into the ion source of an Agilent 5975 series mass selective detector operating with an electron impact (EI) ionization mode. Chemical constituents were identified by a comparison of their retention indices with the literature and their mass spectral data with those from the PMW_Tox3.l, Wiley7n.1, and NIST05a.L mass spectral databases.
4 Results and discussion
4.1 Effect of temperature on product yields
The temperature, pressure, usage of catalyst, and physicochemical properties of water are significant factors in terms of the disintegration of biomass. The disintegration temperature of each component available in the structure of biomass is different. While the disintegration temperature is 220 °C for hemicellulose, it is 200–500 °C for lignin and approximately 280 °C for cellulose. While high temperatures lead to gasification, low temperatures and long holding periods lead to carbonification [25].
In the hydrothermal liquefaction process, most organic compounds do not react with water although water acts both as a solvent and reactant. However, a change occurs to this tendency along with the changes arising in the structure of water when the temperature reaches 250–350 °C, and the water molecules participate in the chemical reactions with the organic compounds. Under normal conditions, water has high dielectric constant (ε~80) because of its hydrogen bonds. But, as it is heated up to the critical point, its dielectric constant value (ε~5) decreases. The water having dielectric constant within this range can solve the hydrophobic materials. This decrease in dielectric constant pushes the water’s structure from polar to apolar structure, and it makes water an effective solvent for organic compounds, gases, lignin, and carbohydrates [10]. Since the ion multiplication of water around the critical temperature ([H+][OH−]~10–11) is higher than it is under normal conditions (~ 10–14), the water can be an efficient medium for acid- and base-catalyzed organic reactions. The viscosity of water increases as the temperature increases, and around the critical point, it becomes closer to the viscosity of water vapor. Since the lower viscosity offers a high diffusion coefficient, the reaction velocities increase. Besides that, the water at high temperature may act as a reactant in the hydrothermal reaction medium. Water molecules may participate in the hydrolysis reactions under hydrothermal conditions, and they might act as hydrogen resources [26].
The macromolecules available in the structure of biomass first decompose into their water-soluble oligomers and monomers with hydrolysis, and these microstructured monomers and oligomers may be exposed to disintegration, and polymerization occurs again or functional loss of group [16].
The results of the liquefaction and distribution of products obtained in this study at different temperatures with and without catalyst are given in Table 1. The results obtained in the trials are given in the graphics in Fig. 2. As seen in the graphic, the temperature is an effective parameter for the trials both with and without catalyst in terms of the hydrothermal liquefaction process. In the trials, light oil and heavy oil, and the solid residue at which the conversion was not achieved were obtained. The product yield of light bio-oil was increased up to 325 °C and decreased after this temperature in all experiments. This may be attributed to the continuance of depolymerization at the end of the ongoing secondary reactions and the increase of gaseous product conversion.
As seen in Fig. 2, the temperature was effective on total bio-oil yield. When the bio-oil yield graphic is reviewed, it is seen that the highest product yield was obtained at 350 °C in all trials with catalyst. In the trial with catalyst, the highest total bio-oil yield was determined to be 27.52%, 21.35%, and 29.25% for the H3BO3, Na2CO3, and Al2O3 catalysts, respectively. Here, the most significant issue is the quality of the product as well as the value of the product yield.
In the hydrothermal liquefaction process, macromolecules in the biomass structure break down into water-soluble oligomers and monomers by hydrolysis at first, and these smaller monomers and oligomers may degrade, repolymerize, or lose functional groups. The glucose monomers present in the cellulose structure are linked to each other by β-(1-4) glyosidic bonds, and these bonds lead to the formation of strong intramolecular and extramolecular hydrogen bonds and crystal structure [16].
4.2 Effect of catalyst on product yields
The aim of using a catalyst in the hydrothermal liquefaction process is to decrease the solid product amount and to increase the liquid product yield and quality. In this regard, homogenous and heterogenous catalysts are used. The alkali salts such as NaOH, KOH, Na2CO3, and K2CO3 are mostly used as homogeneous catalysts. It was determined in the studies that, in general, the alkali salts decreased the solid product amount by accelerating the water-gas shift and increased the liquid product yield. The esters were generated by the decarboxylation reactions between the hydroxyl groups coming from the biopolymers in the biomass because of the effect of the catalyst and the ions generated in the alkali carbonates. The dehydrogenation of micromolecular compounds with a series of dehydration, deoxygenation, and decarboxylation follows the generation of ester. The alkali salts lead to the generation of unstable and unsaturated molecules by increasing the pH and decreasing dehydration reactions [27, 28]. The lower char production with the usage of NaOH may be attributed to neutralization of the molecules that cause the polymerization by OH-ion. Char is generated with the polymerization reaction between the hydroxyl groups on a solid surface and ester bonds which are produced in an aqueous environment. As NaOH neutralizes the carboxylic acids, it prevents the polymerization reactions and therefore suppresses the generation of char [29]. The catalytic effect of alkali salts such as K2CO3 in the disintegration of macromolecules such as cellulose and hemicellulose into micromolecular products arises in the presence of CO, which is generated during conversion [30]. The cellulose type macromolecules decompose into smaller compounds with reactions such as dehydration, dehydrogenation, decarboxylation, and ring-opening. This mechanism explains the catalytic effects of alkali carbonates during the generation and decomposition of intermediate products.
When Fig. 2 was reviewed, it was seen that the catalysts came into play in both the liquid products and solid residue amounts. The highest yield for light bio-oil was determined to be 7.25% in the presence of Al2O3 catalyst at 325 °C and 7.07% in the presence of the H3BO3 catalyst. When the heavy bio-oil yields were reviewed, the highest yield was obtained in the trials without catalyst. In the trials with catalyst, the highest yield was obtained at 325 °C in the presence of H3BO3 and Al2O3.
4.3 Characterization of bio-oils and biochars by FT-IR, GC-MS, SEM, 1H NMR, and elemental analysis
In the hydrothermal liquefaction procedure, the water-soluble part of the biomass starts to be dispersed in water at 100 °C, then the hydrolysis phenomenon above 15 °C begins, and the fragmentation occurs in monomeric chains of cellulosic and hemicellulosic biomass fractions. Then, the slurry generates at 200 °C under 1-MPa pressure, and the mechanism progresses towards the liquefication or gasification. These substances make a substantial contribution to the generation of bio-oil since the hemicellulose decomposes easily due to its amorphous structure, and the cellulose may disintegrate due to its reasonable polymerization feature while the lignin is responsible for the solid generation due to its complex structure and limited depolymerization capability [27].
The polysaccharides constitute the monosaccharides with hydrolysis and then phenols or cyclic ketones with isomerization, cyclization, and dehydration during hydrothermal liquefaction while the carbohydrates generate the aromatic compounds with the ring-opening and then cyclization and condensation reactions [31]. The nitrogen-containing compounds are mainly constituted from amine, pyrroles, and pyrazines. The proteins consisting of amino acids either lead to carbonic acid and amines with the decarboxylation reactions or generate the ammonium and organic acids with the deamination reaction in the hydrothermal liquefaction. The hydrolysis of cellulose is more limited compared to other components in the biomass mainly consisting of cellulose and hemicellulose because the cellulose microfibers are wrapped with the lignin and hemicellulose, and this provides extra protection for the cellulose. Together with the hydrothermal liquefaction process, the organic contents of organic materials decreased from 40% by 10–15%, the oxygen is removed from the environment in the form of CO2 or CO. Moreover, in addition to the gas phase, the organic compounds are formed in the aqueous phase and smaller molecular structures. The resultant products can be easily transported and stored [32]. The elemental analyses and HHV values of the liquid products obtained at 350 °C are given in Table 2. It is seen that the carbon values of the obtained heavy bio-oil were higher than the values of light bio-oil. The highest carbon ratio for heavy bio-oil was obtained from the trial with the Na2CO3 catalyst (71.53), the trial without catalyst (69.98), the trial with the H3BO3 catalyst (67.91), and the trial with the Al2O3 catalyst (67.77), respectively. The highest carbon ratio for light bio-oil was obtained from the trials with the Na2CO3 catalyst (67.49), with the Al2O3 catalyst (65.47), without catalyst (64.75), and with the H3BO3 catalyst (63.28), respectively.
The pre-treatment HHV value of the pure Anchusa azurea sample was calculated as 12.40 MJ/kg. When HHV values of both liquid products and solid residues in Tables 2 and 3 are reviewed, it is seen that values varying between 25.84 and 26.84 MJ/kg for light bio-oil, 28.34–31.32 MJ/kg for heavy bio-oil, and 13.88–19.05 MJ/kg for solid residue were obtained. All these values were higher than the HHV values obtained from the untreated biomass.
In Fig. 3, the percentage of energy value ratios to the total energy value of light bio-oil, heavy bio-oil, and bio-char obtained from the trials are given. When the energy ratios of products obtained by the trials without catalyst to total mass were reviewed, it was seen that the highest share of the energy value belongs to heavy bio-oil, bio-char, and light bio-oil, respectively. In the trials conducted in the presence of the H3BO3 and Na2CO3 catalysts, the ratios to total energy value were comparable to the trials without catalyst, and the highest share belonged to heavy bio-oil, bio-char, and light bio-oil. This gradation differed in the trials with the Al2O3 catalyst. The shares of the products obtained in Al2O3 trials among the total energy value was determined to be heavy bio-oil, bio-char, and light bio-oil, respectively. This may be attributed to the generation of compounds with high carbon and hydrogen content by the reactions caused by an acidic catalyst and the removal of oxygen from the environment in a more effective way.
With NMR analysis, the aromatic and aliphatic compounds in the structure may be revealed. The locations of signal groups in the spectrums give information on the type of compounds available in the structure. The π electrons in the aromatic molecule generate ring flows. These create a force against the magnetic area applied externally, and thereby, backward shielding occurs. Hence, the chemical shifts of the 1H and 13C nuclei are affected [33]. The NMR signals are observed in a lower area (at high frequency). The 1H NMR spectrums of light and heavy bio-oils obtained by the hydrothermal conversion of the Azurea biomass with catalyst (H3BO3, Na2CO3, Al2O3) and without catalyst at 350 °C are given in Fig. S2-S3. The spectrum peaks in the range of 6.2–8.1 ppm indicate the protons belonging to the aromatic groups, the peak in the range of 4 ppm indicates the proton belonging to methoxy (-OCH3), and the peaks in the range of 0.8–3.0 ppm indicate the protons belonging to the alkali (-CH2, -CH3) groups. The peak in the range of 9–10 ppm is the typical aldehyde peak [33]. The shifts were observed at the estimated peak values of some basic compounds in the GC-MS analysis. This is because the mesomeric effect is more dominant than the inductive effect. As the bio-oils obtained are constituted from an oxygenic hydrocarbon mixture, the chemical shift values were affected, and, moreover, there may be delacerations and disintegrations in the structure of the compounds due to the conditions of the reaction environment.
In the GC-MS technique, the mixture components are diverged and quantified in accordance with the difference in the molecule masses. The mixture is carried at a fixed phase with gases such as nitrogen and helium throughout the colon where there are fixed and mobile phases. The quantifications and qualifications can be done through these divergent components depending on the retention periods. The components obtained from gas chromatography can be quantified more precisely with fast scanning and high sensitivity by means of MS which serves as the detector in the system. The electronic ionization spectrums obtained from mass spectroscopy are compared with library (NIST) spectrums, and the structural analysis of the compound is completed. The GC-MS analyses of liquid products obtained from trials made at 350 °C are given in Table S1.
In the analyses, the infrared spectroscopy, another method for illustrating the structures of organic substances, was used. The infrared is located between the visible region and microwaves in the electromagnetic spectrum, and it is the radiation of which the wavelength is 0.8–500 μm. The infrared spectrums give information on whether (i) the functional groups in the structure of organic compounds and (ii) two organic components are identical or not. KBr or NaCl pellets, which fully transmit the light or the film layer, is used for measuring the absorption of the substance. The FT-IR graphics of heavy bio-oil, light-oil, and bio-char obtained from the trials are given in Fig. S4-S5. The vibrations between 3500 and 3200 cm−1 are the characteristics of O–H, and groups that indicated the presence of phenols and alcohols in bio-oils obtained only ethanol. The sharp C–H and = C-H, stretching vibrations between 2900 and 3000 cm−1, indicated the presence of alkanes and alkenes. The stretching vibrations between 2200 and 2400 cm−1 are the characteristics of the C ≡ N and C ≡ C groups, which show the presence of nitriles and alkynes. The typical carbonyl group (C=O,) stretching vibrations between 1710 and 1665 cm−1, indicated the presence of aldehydes, ketones, or carboxylic acids in bio-oils obtained in acetone. The C=C stretching vibrations between 1600 and 1585 cm−1, which are found only in bio-oils of ethanol and methanol, indicate the presence of alkenes and aryl groups. The presence of alcohols and esters can be confirmed by C–H bending vibrations between 900 and 1350 cm−1 and C–O stretching vibrations between 1000 and 1350 cm−1 in bio-oils. Aromatic compounds are confirmed by the aromatic C=C stretching vibrations between 1350 and 1500 cm−1. The C-Br stretching vibrations between 690 and 515 cm−1 indicate the presence of alkyl halides [34,35,36,37,38]. FT-IR results support the GC-MS and 1H NMR results.
The classification of compounds obtained from the GC-MS analysis of heavy bio-oil and light bio-oil obtained at the end of the trials at 350 °C is given in Fig. 4. As seen in the graphic, the ratio of macroaromatics was very high for light bio-oil in the trial without catalyst, and moreover, there were too few polyaromatics and oxygen compounds. The ratio of mono-aromatics declined in the trials without catalyst. While the amount of oxygen compounds and polyaromatics increased with the H3BO3 catalyst, only the polyaromatics decreased substantially for the Na2CO3 and Al2O3 catalysts. When heavy bio-oil content was reviewed, it was seen that there was a higher variety of compounds for all trials. The mono-aromatics and polyaromatics were obtained substantially in the trial without catalyst; these ratios varied in the trials with catalyst, and moreover, the aliphatics were observed at insignificant amounts in the trials with the Al2O3 catalyst.
The analysis method used for characterization of the solid products obtained was SEM (scanning electron microscope). SEM analyses of the pure sample and the samples obtained from the trials were made. The SEM images obtained are given in Fig. 5. Differences are seen between the untreated pure sample and the solid product obtained at the end of trials made at 350 °C. When the images obtained from the trial without catalyst were compared with the pure sample, the differentiations were seen on the surface in the form of cracks. The distinctive changes occurred in the trials with catalyst. In the trials made with the H3BO3 catalyst, there were differentiations on the surface in the form of fragmentation and lamination. The changes in the form of friability were observed on the surface in the presence of the Na2CO3 and Al2O3 catalysts. Since the catalysts affect the biomass surface by various mechanisms due to their structure, they lead to the generation of different surfaces.
4.4 Pyrolysis and the supercritical liquefaction process
In two studies conducted with the aim of obtaining new products from the biomass of Anchusa azurea, the pyrolysis and supercritical liquefaction methods were used. In the pyrolysis study, the trials were conducted with catalyst (ZnCl2, Al2O3, Ca (OH)2, Na2CO3) and without catalyst at 350, 450, and 550 °C. Elemental and GC-MS analyses of the liquid products obtained were made. In the elemental analysis, energy values varying between 17.54–23.75 MJ/kg for 350 °C and 17.43–24.18 MJ/kg for 550 °C were obtained in terms of the HHV values of the liquid products. The energy values varying between 9.06–13.64 MJ/kg for 350 °C and 6.01–10.30 MJ/kg for 550 °C were obtained in terms of the HHV values of bio-char. Based on GC-MS results, 124 different chemical compounds were isolated at 350 °C, and 164 different chemical compounds were isolated at 550 °C. The highest product yield was determined to be 34.05% at 450 °C in the presence of Na2CO3 [23].
In the supercritical liquefaction method, methanol and isopropanol were used as the solvent. The trials were conducted with catalyst (Borax, FeCl3) and without catalyst at 260, 280, and 300 °C. The liquid products obtained were analyzed with the elemental, GC-MS, and FT-IR methods. The highest liquid product yield was obtained as 64.7% from the methanol solvent and 29.20% from the isopropanol solvent in the presence of a borax catalyst at 300 °C. Based on the elemental results, the HHV values varying between 18.71 and 23.00 M/kg−1 were obtained in the methanol trials while the values varying between 25.06 and 26.86 MJ/kg were obtained in the isopropanol trials. Based on the GC-MS results, 73 and 65 different chemical compounds, respectively were isolated in the methanol and isopropanol trials conducted at 300 °C. In general, the compounds can be classified as monoaromatics, oxygenated compounds, polycyclic compounds, and nitrogenated compounds [22].
5 Conclusion
At the end of trials, the Anchusa azurea plant was converted into solid (biochar) and liquid products (heavy bio-oil, light bio-oil) using the hydrothermal liquefaction method with catalyst (H3BO3, Al2O3, Na2CO3) and without catalyst at 300, 325, and 350 °C. The products obtained were evaluated with the GC-MS, FT-IR, 1H NMR, SEM, and elemental analysis methods. The analysis results were consistent. In these trials, the highest liquid yield (total bio-oil) was determined as 29.69% in the trial without catalyst at 350 °C. The HHV values of all products obtained (light bio-oil, heavy bio-oil, and solid residue) were determined to be higher than the HHV value of the pure substance. The highest HHV value was obtained from heavy bio-oil as 31.32 MJ/kg with the Na2CO3 catalyst at 350 °C. This HHV value was higher than the HHV value obtained with pyrolysis and the supercritical liquefaction method. Based on the research results, the hydrothermal liquefaction method may yield more advantageous results compared to the supercritical liquefaction method in which pyrolysis and organic solvents are used.
References
Bhaskar T, Bhavya B, Singh R, Naik D, Kumar A, Goyal H (2011) Thermochemical conversion of biomass to biofuels. Biofuels; Alternative Feedstocks and Conversion Processes, pp 51–77
Kumar S, Gupta RB (2008) Hydrolysis of microcrystalline cellulose in subcritical and supercritical water in a continuous flow reactor. Ind Eng Chem Res 47:9321–9329
Delmer DP, Amor Y (1995) Cellulose biosynthesis. Am Soc Plant Physiol 7:987–1000
Saisu M, Sato T, Watanabe M, Adschiri T, Arai K (2003) Conversion of lignin with supercritical water – phenol mixtures. Energy Fuel 17:922–928
Wahyudiono Kanetake T, Sasaki M, Goto M (2007) Decomposition of a lignin model compound under hydrothermal conditions. Chem Eng Technol 30:1113–1122
Wang W, Kuang Y, Huang N (2011) Study on the decomposition of factors affecting energy-related carbon emissions in Guangdong province, China. Energies 4:2249–2272
Vallero D (2008) Fundamentals of air pollution, 4th edn. Elsevier Inc., San Diego
Gollakota ARK, Kishore N, Gu Sai (2017) A review on hydrothermal liquefaction of biomass. Renew Sust Energ Rev
Kreith FGY (2007) Handbook of energy efficiency and renewable energy. Taylor and Francis, Boca Raton
Elliott D (2011) Thermochemical processing of biomass. Wiley, Chichester
Toor SS, Rosendahl L, Rudolf A (2011) Hydrothermal liquefaction of biomass: a review of subcritical water technologies. Energy 36:2328–2342
Patil V, Tran KQ, Giselrød HR (2008) Towards sustainable production of biofuels from microalgae. Int J Mol Scı 9:1188–1195
Tekin K, Karagöz S, Bektaş S (2012) Hydrothermal liquefaction of beech wood using a natural calcium borate mineral. J Supercrit Fluids 72:134–139
Martin JR, Kornsulle R (2011) Process for the extraction of macromolecules from biomass using the stillage. US Patent
Zhang X, Wilson K, Lee AF (2016) Heterogeneously catalyzed hydrothermal processing of C5-C6 sugars. Chem Rev 116:12328–12368
Savage PE, Levine RB, Huelsman CM (2010) Hydrothermal processing of biomass: thermochemical conversion of biomass to liquid fuels and chemicals. In: Crocker M (ed) RSC Publishing, Cambridge, pp 192–215
Huber GW, Iborra S, Corma A (2006) Synthesis of transportation fuels from biomass: chemistry, catalysts and engineering. Chem Rev 106(9):4044–4098
Brown TM, Duan P, Savage PE (2010) Hydrothermal liquefaction and gasification of Nannochloropsis sp. Energ Fuel 24:3639–3646
Zou S, Wu Y, Yang M, Li C, Tong J (2010) Bio-oil production from sub- and supercritical water liquefaction of microalgae Dunaliella tertiolecta and related properties. Energy Environ Sci 3:1073–1078
Durak H, Aysu T (2016) Structural analysis of bio-oils from subcritical and supercritical hydrothermal liquefaction of Datura stramonium L. J Supercrit Fluids 108:123–135
Karagöz S, Bhaskar T, Muto A, Sakata Y, Oshiki T, Kishimoto T (2005) Low-temperature catalytic hydrothermal treatment of wood biomass: analysis of liquid products. Chem Eng J 108:127–137
Aysu T, Durak H (2016) Catalytic effects of borax and iron (III) chloride on supercritical liquefaction of Anchusa azurea with methanol and isopropanol. Energ Source Part A 38(12):1739–1749
Aysu T, Durak H, Güner S, Bengü AŞ, Esim N (2016) Bio-oil production via catalytic pyrolysis of Anchusa azurea: effects of operating conditions on product yields and chromatographic characterization. Bioresour Technol 205:7–14
Jae J, Tompsett GA, Lin YC, Carlson TR, Shen J, Zhang T, Yang B, Wyman CE, Conner WC, Huber GW (2010) Depolymerization of lignocellulosic biomass to fuel precursors: maximizing carbon efficiency by combining hydrolysis with pyrolysis. Energy Environ Sci 3:358–365
Shen DK, Gu S, Luo KH, Bridgwater AV (2009) Analysis of wood structural changes under thermal radiation. Energ Fuels 23:1081–1088
Guo Y, Wang SZ, Xu DH, Gong YM, Ma HH, Tang XY (2010) Review of catalytic supercritical water gasification for hydrogen production from biomass. Renew Sust Energ Rev 14:334–343
Kumar M, Oyedun AO, Kumar A (2017) A review on the current status of various hydrothermal technologies on biomass feedstock. In: Renew Sust Energ Rev
Arturi KR, Kucheryavskiy S, Søgaard EG (2016) Performance of hydrothermal liquefaction (HTL) of biomass by multivariate data analysis. Fuel Process Technol 150:94–103
Sugano M, Takagi H, Hirano K, Mashimo K (2008) Hydrothermal liquefaction of plantation biomass with two kinds of wastewater from paper industry. J Mater Sci 43:2476–2486
Minowa T, Murakami T, Dote Y, Ogi T, Yokoyama S (1995) Oil production from garbage by thermochemical liquefaction. Biomass Bioenergy 8(2):117–120
Liu HM, Li MF, Yang S, Sun RC (2013) Understanding the mechanism of cypress liquefaction in hot-compressed water through characterization of solid residues. Energies 6:1590–1603
He W, Li G, Kong L, Wang H, Huang J, Xu J (2008) Application of hydrothermal reaction in resource recovery of organic wastes. Resour Conserv Recycl 52:691–699
Gomes JANF, Mallion RB (2001) Aromaticity and ring currents. Chem Rev 101(5):1349–1384
Durak H (2015) Thermochemical conversion of Phellinus pomaceus via supercritical fluid extraction and pyrolysis processes. Energy Convers Manag 99:282–298
Akalın MK, Tekin K, Karagöz S (2012) Hydrothermal liquefaction of cornelian cherry stones for bio-oil production. Bioresour Technol 110:682–687
Peterson AA, Vogel F, Lachance RP, Fröling M, Antal MJ Jr, Teste JW (2008) Thermochemical biofuel production in hydrothermal media: a review of sub- and supercritical water technologies. Energy Environ Sci 1:32–65
Durak H (2018) Trametes versicolor (L.) mushrooms liquefaction in supercritical solvents: Effects of operating conditions on product yields and chromatographic characterization. J Supercrit Fluid 131:140–149
Çolak U, Durak H, Genel S (2018) Hydrothermal liquefaction of Syrian mesquite (Prosopis farcta): Effects of operating parameters on product yields and characterization by different analysis methods. J Supercrit Fluid 140:53–61
Funding
This work was supported by the Van Yuzuncu Yil University Research Fund. The authors gratefully acknowledge the Van Yuzuncu Yil University Research Fund for financial support (No. 2014-SHMYO-B076).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Durak, H. Characterization of products obtained from hydrothermal liquefaction of biomass (Anchusa azurea) compared to other thermochemical conversion methods. Biomass Conv. Bioref. 9, 459–470 (2019). https://doi.org/10.1007/s13399-019-00379-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-019-00379-4