Abstract
This work evaluated the influence of process conditions on the chemical characteristics and yield of polymers based on castor oil. Castor oil maleate and styrene copolymers (MACO-Sty) were produced by bulk polymerization using benzoyl peroxide (BPO) and cobalt naphthenate as free radical initiators and reaction accelerators, respectively. The effects of temperature (100 to 140 °C), molar ratio between styrene and castor oil maleate (2:1 to 4:1), BPO concentration (0.10 to 0.20 wt%), and cobalt naphthenate concentration (0.10 to 0.20 wt%) were evaluated on the number average molecular weight (Mn), weight average molecular weight (Mw), dispersity (Đ), molar fraction of styrene in the copolymer, reaction yield, and viscosity of the copolymer. A wide range of molecular weights (Mw from 15,809 to 38,656) could be produced, with dispersity ranging from 2.0 to 4.8, and high yields into copolymers (> 80%). The physical characteristics ranged from resins of low viscosity (1.583 Pa s) to solid polymers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The development of new polymers using biological sources, such as vegetable oils, has been the focus of many investigations. Concerns regarding environmental protection induced the search for polymers that can be biodegradable or that can degrade faster than conventional polymers such as polyethylene and polyvinyl chloride.
Vegetable oils are widely available, biodegradable, relatively cheap, and can be modified to form monomers for polymerization [1,2,3]. Currently, vegetable oils are used in the production of polyurethanes, polyesters, polyethers, and polyolefin copolymers [4]. Castor oil is comprised of more than 90% of ricinoleic acid, having a more homogeneous composition than most oils. It presents a hydroxyl group and a double-carbon bond that are prone to chemical reactions, having a higher versatility to be used in different reactions [5, 6]. Castor oil can be modified onto castor oil maleate (MACO) to present better reactivity towards polymerization reaction [7].
Castor oil maleate is the product of the reaction of castor oil with maleic anhydride [8, 9]. This reaction can be carried out through thermal reaction [8] or free radical reaction [10]. The thermal reaction produces monomers of castor oil maleate while the free radical reaction produces a mixture of castor oil maleate oligomers.
Copolymers produced from castor oil maleate and styrene, divinylbenzene, vinyl toluene, and methyl methacrylate have been synthesized [11, 12]. Typical applications for these copolymers are as varnishes, paints, and resins [13, 14]. From an ecological point of view, the hydrophilic characteristic of the maleate group increases the interaction of the produced copolymer with water accelerating potential biodegradation of these copolymers [15].
Castor oil maleate copolymers can be produced by bulk, suspension, and emulsion polymerization using free radicals as initiators [15, 16]. The choice of reaction system influences the molecular weight and dispersity of the polymer with consequent effect in the physical, chemical, and mechanical properties of this copolymer.
Castor oil and castor oil maleate are biodegradable materials that have limited application in varnishes, coatings, paints, and adhesives due to their physical and mechanical properties. These materials are hydrophobic but are washed away by water or soap solutions having little application as resin substitutes. The copolymerization of castor oil maleate with styrene produces a resin that continues to be hydrophobic, but that does not wash away by water or soap solutions, thus that can be used in paints, coatings, and adhesives.
This work investigates the production of a castor oil maleate and styrene copolymer (MACO-Sty) using free-radical polymerization. This study evaluates the effects of temperature, the molar ratio between castor oil maleate and styrene, the weight ratio of castor oil maleate, and free-radical initiator (benzoyl peroxide) on the molecular weight distribution. The effect of cobalt naphthenate as co-catalyst in the reaction was also carried out.
2 Materials and methods
2.1 Materials
Castor oil was obtained from Olveq Indústria e Comércio de Óleos Vegetais (Quixadá, Brazil). Maleic anhydride was purchased from Vetec Química Fina (Rio de Janeiro, Brazil). Styrene (≥ 99%), BPO (benzoyl peroxide), NafCo (cobalt naphthenate, 6%), and THF (tetrahydrofuran for HPLC, ≥ 99.9%) were obtained from Sigma-Aldrich.
2.2 Synthesis of castor oil maleate (MACO)
Castor oil maleate was produced by reacting castor oil and maleic anhydride at a molar ratio of 1:1. Castor oil (0.16 mol–150 g), maleic anhydride (0.16 mol–15 g), and benzoyl peroxide (0.010% w/w) were mixed and fed to a 400-mL stainless-steel reactor (Metalquim, Brazil). The reaction was carried out for 3 h at 120 °C, with constant mechanical agitation (800 rpm). These operating conditions were based on the optimal conditions determined in previous work. [10]
2.3 Synthesis of the MACO and styrene copolymer (MACO-Sty)
The MACO-Sty copolymer was produced by bulk polymerization in a glass reactor inserted in a dry block heater (Tecnal model TE005/50, Brazil) equipped with a digital temperature controller. Castor oil maleate, styrene, and initiator (benzoyl peroxide) were mixed and fed into the glass reactor. The reaction was carried out for 4 h. Table 1 presents the molar ratio between castor oil maleate and styrene, reaction temperature, and the weight ratio of initiator used in the reactions. The experiments were based on a 23 Box, Hunter and Hunter experimental design. All experiments were carried out in triplicates. The values for the operating conditions were based on previous studies on MACO-Sty production. [15]
2.4 Synthesis of MACO-Sty using cobalt naphthenate as a reaction accelerator
The same experimental design was carried out to produce the MACO-Sty copolymer using benzoyl peroxide and cobalt naphthenate as co-catalyst. Castor oil maleate, styrene and benzoyl peroxide, and cobalt naphthenate were mixed and fed into a 200-mL glass reactor. The reaction was carried out for 4 h. Cobalt naphthenate was added in a weight ratio ranging from 0.10 and 0.20 g/gOIL (Table 3).
2.5 Statistical analysis
The response surface methodology (RSM) was used to analyze the effects of the operating conditions on the molecular weight distribution of the copolymer. The data was handled and analyzed using the software Statistica v13.
2.6 Chemical characterization
The molecular weight distribution of the MACO-Sty copolymer was determined by gel permeation chromatography (GPC). The analysis was carried out in a Varian GPC system equipped with a refraction index detector (IR—Varian model Pro Star 355, USA), a Rheodyne-automated sample injector, and a column heater. A TSK Gel G2500HHR column (30 cm × 7.88 mm, 5 μm) was used. THF was used as a solvent at a 1-mL/min flow rate. The temperature of the column was set at 30 °C. The molecular weight distribution was determined based on a calibration curve built using five polystyrene standards (266 to 45,500 g/mol).
The molecular structures of the copolymers were analyzed by Fourier-transform infrared spectroscopy (FTIR). The FTIR spectra were collected using a Cary 630 FTIR equipment (Agilent, USA) within the wavelengths from 400 to 4000 cm−1 at a 1 cm−1 spectral resolution. All samples were applied directly to the spectrometer without any previous treatment.
2.7 Reaction yield
The yield of the reaction was calculated based on the FTIR peak absorption measurements at wavelength 910 cm−1, which corresponds to the CH=C-H out of plane bending vibrations of styrene.
where Abs(t) is the absorbance at 910 cm−1 of the sample collected at time t, AbsMACO is the absorbance at 910 cm−1 of castor oil maleate (MACO); Abssty is the absorbance at 910 cm−1 of styrene.
2.8 Viscosity
The viscosity measurements of the copolymer resins were carried out by the Quimis digital display viscometer (model Q860M21, Brazil) coupled with a small sample adapter at 30 °C. The rotation speed of the viscometer and spindle were selected according to the viscosity of the sample.
3 Results and discussion
3.1 Castor oil maleate
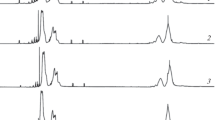
The GPC analysis of the castor oil maleate produced by the method applied herein showed the production of a mixture of castor oil maleate and castor oil maleate oligomers (Fig. 1), presenting an Mn of 2200 g/mol, Mw of 4614 g/mol, and dispersity of 2.1. The reaction of castor oil with maleic anhydride was carried out to provide a carboxylic acid functionality with maleate unsaturation, that may result in a plasticizing effect, improvement of polymer adhesion and that can be copolymerized with vinyl monomers. The production of castor oil maleate dimer, trimer, and small chain oligomers were also reported by Wang and collaborators [9], which obtained a castor oil maleate with about 16 to 20% of dimers, trimers, and oligomers.
3.2 Production of castor oil maleate-styrene copolymer using BPO as free-radical initiators
The polymerization, under the conditions applied herein, produced polymers with Mw ranging from 15,809 to 34,534 g/mol and dispersity ranging from 2.0 to 4.0 (Table 1). GPC analysis indicated that the polymerization product comprised of a mixture of polymer chains (Fig. 2). The lower molecular weight compounds of the mixture are comprised mostly of castor oil maleate bonded to short styrene chains, while the higher molecular weight compounds are comprised by long chains of castor oil maleate bonded by styrene “bridges” (Fig. 3). All copolymers presented molecular weight distributions such as the distribution presented in Fig. 2. The number average molecular weight of the copolymers produced herein was lower than the reported by Mamat and collaborators [15], which obtained copolymers ranging from 27,300 to 44,800 g/mol in emulsion polymerization using higher styrene to castor oil maleate molar ratio and lower concentration of initiator.
Scheme of the chemical reaction between castor oil and maleic anhydride (a), chemical reaction between styrene and castor oil maleate using a free radical initiator (b), propagation of styrene in the styrene - castor oil maleate copolymer (c), and propagation of two styrene—castor oil maleate chains (d)
An increase in the polymerization temperature (from 100 to 140 °C) increased both Mn and Mw of the copolymer. The increase was proportionally higher in Mn than in Mw, resulting in a decrease in the dispersity. An increase in the polymerization temperature tends to increase all reactions rates (initiation, propagation, and termination). On conventional polymerization, the increase in the initiation rate tends to generate more polymer chains. When castor oil maleate is used, the increase in the initiation rate may increase the number of radicals in the castor oil maleate molecule leading to an increase of both Mn and Mw. The temperature increase allowed greater mobility of the monomers, due to the lower viscosity of the reaction media, increasing the propagation reaction rate, and therefore the molecular weight of the copolymers. This trend may have been followed by a higher increase in the termination reaction rate than in the propagation reaction rate, favoring the formation of a larger amount of short polymer chains, leading to a higher increase in Mn and lower increase in Mw.
The molar ratio between styrene and castor oil maleate had little effect at low temperature (100 °C). Small changes in Mn and Mw were observed when the molar ratio increased from 2:1 to 4:1.
The effect of the concentration of benzoyl peroxide (BPO) on the molecular weight distribution was significant. An increase in the concentration of initiator (from 0.1 to 0.2% wt) increased both Mn and Mw but reduced the dispersity. On traditional free-radical polymerization, an increase on the concentration of initiator results in a decrease on both number average and weight average molecular weight because more chains will polymerize at the same time competing for the available monomers. Castor oil maleate may contain one, two, or three maleates in the castor oil chain. Thus, the molecule may have three polymerization points. The increase on the initiator concentration increased the number of radicals in each castor oil maleate molecule, resulting in larger copolymer chains.
The analysis of perturbation of factors corroborated with the observations made previously. Table 2 presents the statistical analysis of the independent variables (temperature, the molar ratio between styrene and castor oil maleate, and weight fraction of BPO) on the dependent variables (Mn, Mw, and Đ).
The weight fraction of initiator was the variable that presented the highest effect on the production of the MACO-Sty copolymer, followed by the effect of temperature. The concentration of the initiator has a direct effect on the production of the copolymer by initiating the polymerization process. The molar ratio between styrene and castor oil maleate did not show any statistical significance. The lack of significance of this variable may be due to the substantial difference of the molecular weight of these two monomers. The statistics show that the control of the molecular weight distribution of the copolymer can be achieved preferably by controlling the concentration of initiator and temperature.
Figure 4 presents the surface plots for the number average, weight average molecular weight, and dispersity of the MACO-Sty copolymer. Based on the surface plots, a statistical correlation for the molecular weights and dispersity was obtained using the surface response methodology (Eqs. 2 to 4).
where I is the initiator mass fraction; M is the molar ratio between styrene and castor oil maleate; and T the temperature.
The statistical F test was applied to evaluate the suitability of the correlations presented by Eqs. 2 to 4. Table 3 presents the ANOVA for Mn, Mw, and Đ. The correlations were significant at a 95% level of confidence for Mn and Đ and a 75% level of confidence for Mw. Figure 5 presents the observed and predicted values obtained by applying the statistical model for Mn, Mw, and Đ after 4 h of polymerization.
The yield into the copolymer was higher than 80%. The increase in the reaction temperature increased the yield into the copolymer. The increase in BPO concentration and styrene concentration in the reaction did not affect the yield significantly.
The molar ratio of styrene to castor oil maleate in the feed was the most significant variable on the content of styrene in the copolymer chain. The molar fraction of styrene in the copolymer was higher than 0.5 indicating that styrene has been effectively incorporated in the polymer chain.
3.3 Production of castor oil maleate-styrene copolymer using BPO as a free-radical initiator and cobalt naphthenate as a reaction accelerator
Table 4 presents the molecular weight distribution for the synthesis of the MACO-Sty copolymer by bulk polymerization using BPO as a free-radical initiator and cobalt naphthenate (NafCo) as a reaction accelerator. The effect of the temperature followed the same trend observed when only BPO was applied. An increase in the polymerization temperature increased both the number average and weight average molecular weight and decreased the dispersity.
The use of cobalt naphthenate induced a lower variability on the molecular weight distribution of the polymer at each processing temperature. At 100 °C, the Mn ranged from 4000 to 5000 g/mol, and the Mw ranged between 19,000 and 22,000. When cobalt naphthenate was not used, the Mn ranged from 3500 to 11,500 g/mol at this same temperature. Considering that the number average molecular weight is highly influenced by the number of chains being formed, the presence of cobalt naphthenate showed a more significant influence in the initiation of polymer chains.
The dispersity of the copolymer increased with the addition of cobalt naphthenate, which may have been influenced by a gel effect. The reaction mixture showed a higher viscosity when cobalt naphthenate was used, resulting in lower mobility of the molecules, especially the large castor oil maleate and its oligomers (Table 5).
The analysis of perturbation of factors showed that temperature, BPO weight fraction and cobalt naphthenate weight fraction were essential variables influencing the molecular weight distribution (Table 6). This statistical analysis corroborates with the observation that the use of cobalt naphthenate significantly influenced the molecular weight distribution and can also be used to control it.
Figure 6 presents the surface plots for the number average, weight average molecular weight, and dispersity of the MACO-Sty copolymer produced using BPO and NafCo. Based on the surface plots, a statistical correlation for the molecular weights and dispersity was obtained using the surface response methodology (Eqs. 5 to 7).
where C is the co-catalyst mass fraction; I is the initiator mass fraction; M is the molar ratio between styrene and castor oil maleate; and T the temperature.
The statistical F test was applied to evaluate the suitability of the correlations presented by Eqs. 5 to 7. Table 7 presents the ANOVA for Mn, Mw, and Đ. The correlations were significant at a 95% level of confidence. Figure 7 presents the observed and predicted values obtained by applying the statistical model for Mn, Mw, and Đ after 4 h of polymerization.
4 Conclusion
This work showed that the production of styrene—castor oil maleate copolymer is possible using benzoyl peroxide as a free-radical initiator. The range of molecular weights and distributions produced by different operating conditions (styrene to castor oil maleate molar ratio, temperature, BPO concentration, and cobalt naphthenate concentration) allowed the obtention of both liquid resins (with different viscosities) and solid polymers. The use of cobalt naphthenate as reaction accelerator allowed a new degree of freedom to control the molecular weight and dispersity of the polymer.
References
Mosiewicki MA, Aranguren MI (2016) Recent developments in plant oil based functional materials. Polym Int 65:28–38. https://doi.org/10.1002/pi.5033
Zhang C, Garrison TF, Madbouly SA, Kessler MR (2017) Recent advances in vegetable oil-based polymers and their composites. Prog Polym Sci 71:91–143. https://doi.org/10.1016/j.progpolymsci.2016.12.009
Alam M, Akram D, Sharmin E, Zafar F, Ahmad S (2014) Vegetable oil based eco-friendly coating materials: a review article. Arab J Chem 7:469–479. https://doi.org/10.1016/j.arabjc.2013.12.023
Miao S, Wang P, Su Z, Zhang S (2014) Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater 10:1692–1704. https://doi.org/10.1016/j.actbio.2013.08.040
Mubofu EB (2016) Castor oil as a potential renewable resource for the production of functional materials. Sustain Chem Process 4:11. https://doi.org/10.1186/s40508-016-0055-8
Kunduru KR, Basu A, Haim Zada M, Domb AJ (2015) Castor oil-based biodegradable polyesters. Biomacromolecules 16:2572–2587
Vibhute BP, Khotpal RR, Karadbhajane VY, Kulkarni AS (2013) Preparation of maleinized castor oil (MCO) by conventional method and its application in the formulation of liquid detergent. Int J Chem Tech Res 5:1886–1896
de Espinosa LM, Meier MAR (2011) Plant oils: the perfect renewable resource for polymer science? Eur Polym J 47:837–852. https://doi.org/10.1016/j.eurpolymj.2010.11.020
Wang HJ, Rong MZ, Zhang MQ, Hu J, Chen HW, Czigány T (2008) Biodegradable foam plastics based on castor oil. Biomacromolecules 9:615–623
Maia DLH, Alves Filho EG, Barros Junior AF, Fabiano FAN (2018) Kinetics of the production of castor oil maleate through the autocatalyzed thermal reaction and the free radical reaction. In J Chem Kinet 50:112–121. https://doi.org/10.1002/kin.21145
Indrajati IN, Dewi IR (2017) Performance of maleated castor oil based plasticizer on rubber: rheology and curing characteristic studies. IOP Conf Ser Mater Sci Eng 223:012001. https://doi.org/10.1088/1757-899X/223/1/012001
Campanella A, Zhan M, Watt P, Grous AT, Shen C, Wool RP (2015) Triglyceride-based thermosetting resins with different reactive diluents and fiber reinforced composite applications. Compos A Appl Sci Manuf 72:192–199. https://doi.org/10.1016/j.compositesa.2015.02.009
Sharma V, Kundu PP (2006) Addition polymers from natural oils-a review. Prog Polym Sci 31:983–1008. https://doi.org/10.1016/j.progpolymsci.2006.09.003
Mao W, Li S, Li M, Huang K, Xia J (2017) Design, preparation and properties of novel flame retardant thermosetting vinyl ester copolymers based on castor oil and industrial dipentene. Polish J Chem Technol 19:1–8
Mamat X, Wang Y, Eli W (2012) New potentially environmentally friendly copolymer of styrene and maleic acid-castor oil monoester. Polym Adv Technol 23:1271–1275
Echeverri DA, Jaramillo F, Rios LA (2015) Curing copolymerization kinetics of styrene with maleated castor oil glycerides obtained from biodiesel-derived crude glycerol. J Appl Polym Sci 132:1–9
Funding
This study is the financially supported by the Brazilian funding institutes CNPq and CAPES.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Maia, D.L.H., Fernandes, F.A.N. Effect of process conditions on the properties of castor oil maleate and styrene copolymer produced by bulk polymerization. Biomass Conv. Bioref. 9, 411–420 (2019). https://doi.org/10.1007/s13399-018-0359-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13399-018-0359-x