Abstract
Green synthesis of ZnO NPs with biological systems is becoming a growing field, especially in plant extracts nanotechnology. Biological reducing agents have been interpreted worldwide to lessen the impact of toxic chemicals applied in development of nanoparticles. In present research, work deals with green synthesis & characterization of ZnO NPs via Psidium guajava leaf extract, also to evaluate their antibacterial action against some selected bacteria. The preparation of ZnO NPs was attained via sol–gel assisted microwave irradiation process. The XRD pattern confirms the hexagonal phase of ZnO and crystalline size to be ~ 15.8 nm. FTIR analysis depicts the bio functional groups present in the surface of the ZnO nanoparticles, SEM predicts the size and morphology of the sample, and it shows rod-shaped surface. Then, the EDAX results showed the purity & elemental stoichiometry of the ZnO nanoparticles. Also, the UV was performed to investigate the optical nature of the prepared ZnO nanoparticles. Also, the antibacterial activity results revealed significantly inhibited both types of bacteria in higher concentrations. This study also suggests that green synthesized ZnO nanoparticles can an excellent antibacterial agent.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
In recent, bio-nanomaterials becomes of more interest due to their projecting biological nature and uses towards biomedical field. The nanoparticles of metal oxides (MONPs) have been introduced into the field of biomedicine, particularly for antibacterial, anticancer, gene conveyance, bio sensing, and cell imaging [1]. Zinc oxide (ZnO) nanoparticle is a versatile material and is an ideal candidate for biological usages owing to their exceptional biocompatibility, environment friendly, and truncated toxicity [2]. In green preparation & bio fabrication of MONPs have stimulate enormous research for use of ZnO NPs in nanomedicine [3]. Owing to its high catalytic nature, strong ability of adsorption, and high chemical stability, the ZnO nanostructures are used more frequently in manufacturing of sunscreens/ceramics/rubber processing, waste-water handling. Moreover ZnO nanoparticles (NPs) has revealed its application in biomedical fields such as anti-cancer, antimicrobial, and antioxidant activities due to its unique physicochemical, pharmacological properties [3, 4]. In addition the plant facilitated biological preparation of NPs is important due to its ease, eco-friendly, biocompatible, safe, and extensive antimicrobial activity [5]. Besides the synthesis of NPs from biomimetic route show higher catalytic nature & bound the uses of high-cost-toxic substances. The strains & plant extract owns certain phytochemicals which acts as a reducing/capping agent [6,7,8,9].
The biosynthesis approach has proven to be useful in preparation of metal oxide (MO) NPs. Green route via natural extract has newly been extended to numerous nanoscale efficient oxides. It can overwhelm the complications associate with physico-chemical routes. Besides, it no detrimental chemicals such as reducing & oxidizing agents are required in this process, as only plant extracts is needed. The NPs preparation via plant extracts act as a bio-reducers, a clean, non-toxic, biocompatible & environmental-friendly method for NPs production at big scale [9]. Green synthesis of nanoparticles is getting significant devotion for their probable usages in opto/nano-electronics, sensors, cosmetics, health care products, food processing, and production of antimicrobials [10]. The branch is mainly used bio molecules to reduce zinc precursors, and it provides non-toxic, pure ZnO nanoparticles were quickly processed [11].
Zinc oxide is a class that has access to inorganic metal oxides and exhibits a broad range of nanoparticles. The advantages of ZnO nanoparticles are low cost, ultraviolet properties, high catalytic activity, and significant applications in the field of medicine and agriculture [12]. It has high excitation energy of 60 meV [13], which permits it to endure high temperatures, large electric fields below high power operation. Due to such characteristics, ZnO nanoparticles are broadly usable in solar cells, chemical/gas sensors [14,15,16], and photocatalysis [17,18,19]. The ZnO powder is commonly used as an additive in a variety of commonly useable products [20] & may also be used in cosmetics like sunscreen, ointments, and lotions. Due to their different morphologies, shapes, size, and their bio-safe nature, ZnO nanoparticles can be represented as an ideal candidate for biomedical applications [21].
Psidium guajava, popularly known as guava, is a small tree of the myrtle family shown in Fig. 1 [22]. It is employed in medicinal ingredients traditionally to advance the exterior wound healing process. Also, guava is proven to be a medicinal plant to have abundant biological functions, like: anti-cough, anti-diabetic, anti-bacterial, anti-spasmodic, and anti-oxidant properties [23]. The leaves have several compounds that perform as fungistatic & bacteriostatic agents, and it also possess significant quantity of antioxidants & owns radio protective capability [24]. The guava leaves extracts are supplemented with photochemical biological composite such as vitamins, flavonoids, and phenolic compounds, catechia, gallic acid [25], which act as reducing & stabilizing agents during the preparation of nanoparticles [26]. Guava leaves minimize breast, prostate, & oral cancers risk, because of high amounts of antioxidant, Lycopene [27, 28]. Numerous studies shown that lycopene acts a substantial role in reducing cancer risk. It contains huge number of organics& inorganics such as tributary metabolites, e.g., polyphenols, antioxidants, antivirals &anti-inflammatory composites. The several parts of plant are employed in general medicine to control malaria, vomiting, diarrhea, dysentery, ulcers, etc. [29, 30]. Biological/living resources/landscape, like: plants & microorganisms, can be employed in a facile, safe, quick & cost-effective to fabricate the required MO NPs. The green method related to medicinal plants is fetching a good line to the synthesis of nanoparticles. The plant extract is used in the green path as capping and reducing agents. They can also act as a biological template to restrict the size, shape & morphology of the NPs [31].
The biosynthesis of ZnO nanoparticle is predominated by physical and chemical processes. The physical method contains thermal evaporation (PVD), pulsed laser deposition (PLD), ball milling, CVD, sputtering technique and molecular beam epitaxy. Generally, such processes need high pressure, consume significant energy, and has low yield [32]. Chemical methods such as sol–gel, solvothermal, spray pyrolysis, hydrothermal, sonochemical, and electro deposition processes are inexpensive and use of toxic chemicals, making both ways are environmentally unfriendly. The biosynthesis of nanoparticles is a hopeful method based on the use of plant extracts. This biological approach has proven to be useful in the synthesis of metal & MONPs [33]. Recently, green synthesis of ZnO nanoparticles has been carried out by different plant sources such as Cassia fistula, Trifolium pratenese, Ocimum basilicum, Catharanthus Roseus, Solanum torvum [34], Aloe vera, Calotropis gigantea, Borassus flabellifer, Sargassum muticum, Tectona grandis, Passiflora caerulea and Glycosmis pentaphylla [35].
Herein, an effort is being made to prepare ZnO NPs using Psidium guajava leaf extract & its antibacterial action was inspected.
2 Materials and Methods
2.1 Materials
Zinc acetate dehydrate of 99% purity, NaOH, Whatman filter paper (WHA10348903), Acetone and distilled water are the ingredients utilized in this work. The psidium guajava (Guava) leaves were composed from in & around sivagiri, Erode, Tamil Nadu. The chemicals are in analytic grade so we used without any refinement.
2.2 Preparation of Leaf Extract
Psidium guajava leaf was collected from the surrounding area. The leaves were washed numerous times using distilled water (DW) to avoid dust particle then shade dried to take away the remaining moisture. Taken 30 g of leaves were mixed with 150 ml of deionized water and boiled at 60 °C for 20 min. After being cooled to 300 K, the yellow colour extract was filtered with filter paper and stockpiled at 4 °C freezer.
2.3 Synthesis of Psidium Guajava Leaf Extract Capped ZnO Nanoparticles
In this method, 5 g of Zinc acetate (ZnC4H6O4) was liquefied with 50 ml of DW and it was stirred about 30 min. After that, 10 ml of yellow colour leaf extract was supplied drop wise in above solution. The pale-yellow colour solution was attained. The mixture was stirred for 30 min. During this process, the sodium hydroxide [NaOH] was drop wise added to maintain the PH as 12. The gelatinous white colour precipitate was continuously stirred for 2 h. It was aged 12 h at room temperature. The white colour precipitation washing was done many times through DW to eradicate impurities. To attain the minimum time consumption microwave oven were used to dry the precipitate at 75 W for 20 min. Finally, the dried sample was grinded by using mortar to get white colour fine powder of psidium guajava leaf extract capped ZnO nanoparticle and the quantity was around 2 g [35].
2.4 Mechanism of ZnO Nanoparticles Formation
ZnO NPs synthesis process has essential oil in leaves and it contains α & β-pinene, limonene, isopropyl, menthol, & β-bisabolene. Oleanolic acid has also been established in leaves of guava [36]. The leaves possess 42% limonene & 21.3% caryophyllene [37]. The guava leaves contain numerous volatile compounds in leaf extract [38, 39], which form complex agents with ZnC4H6O4 precursors that primarily start the nucleation progression, followed by additional reduction and shaping of the nanoparticles [40, 41]. When ZnC4H6O4 is dissolved in water, transparent solution achieved owing to [Zn(CH3COO)2]. 2H2O ions presence. On adding the sodium hydroxide, we attained white colour ZnO nanoparticles precipitate. The capping representative functions as a stabilizer by sticking to NPs surface to form a defensive layer & restricting the size of the particle [42].
3 Characterization Techniques of ZnO Nanoparticles
Synthesized ZnO NPs capped with Psidium guajava leaf extract were inspected by XRD, FTIR, SEM, EDAX, UV, and Antibacterial activity. X-ray diffraction (X’PERT PRO PANalytical, PHILIPS) analysis is used to estimate the size of nanoparticles & phase identification through CuKα radiation, λ = 1.54056 Å in angular range from 10 to 80° [43]. FTIR Spectroscopy is an analytic method to recognize the polymeric, organic, and inorganic materials [44]. The possible functional groups are examined using Fourier Transform Infrared Spectroscopy (Shimadzu, Japan) infrared (IR) double beam spectrophotometer [45]. The SEM (JEOL JSM 6360LA, Japan) was performed to visualize the size & shape of nanoparticles [34]. Elemental analysis and composition of sample was established by EDX (BRUKER, India), these X-rays are used to identify the composition and evaluate the abundance of elements in the sample [46]. Prior to SEM/EDX measurement we have sputter coated the samples through sputtering unit using Au target to avoid the charging the samples. Ultraviolet–visible (UV-1700 Spectrometer of Shimadzu) spectroscopy is used primarily to assess transmission spectra or absorption spectra in liquids and transparent or opaque solids [47].
3.1 Antibacterial Activity
According to the National Committee for Clinical Laboratory Standards (NCCLS), antibacterial action was detected by agar procedure [48]. Sterilization of Liquid Mueller Hinton agar media & Petri plates was done by autoclaving at 121o C for approximately 30 m below pressure of 15 lbs. About 20 ml of agar media was distributed to individual Petri dish at a even depth of 4 mm below aseptic surroundings in the cavity that has airflow as laminar. On solidifying the media, an 18 h culture of Gram + ve like Bacillus cereus (MTCC 430), & Staphylococcus aureus (MTCC 3160), Gram −ve like E.coli (MTCC 1698) & Klebsiella pneumoniae (MTCC10309) attained from IMTECH, Chandigarh was swabbed on agar plates surface. The preparation of well was done via cork borer with 50 & 100 µl of individual sample loading into separate wells using sterile DW as –ve & vancomycin (30mcg/disc) as + ve control. These sample containing plates were then incubated for 24 h at 37 °C. After incubation, the growth inhibition zones diameter was noted in mm [49].
4 Results and Discussion
4.1 XRD Analysis
XRD pattern of green synthesized ZnO NPs is recorded and the most prominent peaks of the sample were indexed and shown in Fig. 2. The diffraction peaks of the prepared sample at 2θ values are 31.82, 34.45, 36.30, 47.51, 56.61, 62.84 and 67.93 likened to hkl planes such as (100), (002), (101), (102), (110), (103) and (112) [50, 51]. The presence of planes helps to ensure that the ZnO nanoparticles have a hexagonal system and well-matched to JCPDS 36–1451. The sharp/intense peaks suggest incredibly crystallinity of sample. The crystalline size of ZnO nanoparticles is 15.8 nm was shown in Table 1. The capping of leaf extract enhances the property by decreasing the crystalline size in the sample. As a result of XRD corroborate with the XRD pattern of ZnO nanoparticles synthesized from various plant extracts [11]. The average crystalline size (D), lattice constraints (a, c) & cell volume (V) were obtained through below rules (1–3) [52,53,54,55],
4.2 FTIR Analysis
FTIR analyses of synthesized ZnO capped with guava (Psidium guajava) leaf extract and biomolecules accountable for the lessening and efficient balance of the bio-reduced ZnO NPs have been carried out [56]. The prepared sample FTIR spectra are demonstrated in Fig. 3. From the result the peak is observed at the range of 648.08 to 3444.87 cm‐1. The vibrations of a variety of groups are present at different wave numbers of IR radiation. The stretching modes of Zn–O bond were approved from peak values of 648.08–1020.34 cm−1. The band at 1413.82 cm‐1 reveals C–C stretching is linked with polyphenols & natural pigment of plant leaf extracts. C-H stretching was authorized from 1560.41 cm‐1 peak. The 1641.42 cm−1 band is allotted to carbonyl and carboxylic (C = O) stretching. Then the broad peak absorbed at 3444.87 cm−1 is for O–H stretching mode due to water absorption on the surface of the metal. Introducing capping agent has created a minor change in the functional group analysis of the samples. The results were reported in Fig. 3 and Table 2. These outcomes are well agreed to ZnO NPs FTIR spectrum previously published in various plant extract [57, 58].
Therefore, from the result of FTIR analysis, it has been concluded that the biomolecules of leaf extract such as phenolic group, primary amines, proteins and steroids are accountable for the biotransformation of Zinc acetate ions into Zinc oxide nanoparticles and its stabilization in aqueous medium [59].
4.3 SEM and EDAX Analysis
SEM study was done to know the size & shape of ZnO nanoparticles. Figure 4a and b, shows the morphological descriptions at different magnifications and scales and elemental composition of prepared samples, respectively. Which shows rod shaped morphology for the prepared sample. The mean size of grains was found noticed approximately, between 70 to 84 nm. The results of SEM in the current study is in line with the SEM image of previously reported for ZnO nanoparticles [58, 60]. Elemental composition and chemical analysis of prepared samples were examined using EDAX. The analysis observed Zn (Zinc), O (Oxygen), C (Carbon) are present in the sample. From this study, the presence of carbon reveals the incorporation of capping agent in the sample. Observations in this regard have been already predicted by Janjal et al. [56, 60]
4.4 UV–vis Spectroscopy
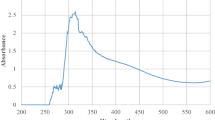
UV–Vis. spectroscopy determines the optical nature & energy gap (Eg) of ZnO nanoparticles. The UV spectrum of ZnO NPs in absorption mode is showed in Fig. 5(a), which contains a band of absorption at ~ 345 nm. Additionally, the Eg acquired through Tauc’s plot estimated from the following relation (4) [61, 62]:
here exponent \(n\) represent the band transitions nature, \(n=\frac{1}{2} and \frac{3}{2}\), which are related to direct allowed & forbidden transition and \(n=2 and 3\) indirect allowed & forbidden transition, correspondingly. The Eg is noticed from extrapolating a straight-line to \((\alpha{\text{hv}})^2\) vs. \({\text{hv}}\) plot to \((\alpha{\text{hv}})^2 = 0\) in Fig. 5b. Hence, the Eg of ZnO NPs is ~ 3.14 eV. The existence of UV shielding solid in extracts of guava leaves can be attributed to UV absorbing polyphenols and flavonoids. These results suggest that the extract of guava leaves was highly effective in blocking ultraviolet light and introduces a protecting character to skin of humans from detrimental UV radiation & also employed in medical usages [23]. Detailed results have been agreed upon by Ansilin et al. [8], who documented that the absorption spectrum of ZnO nanoparticles at 346 nm.
4.5 Antibacterial Activity
The antimicrobial activity of the nanoparticles was tested against for two microorganisms as discussed in Sect. 3.1. The present study points out that the ZnO nanoparticles gives good antibacterial actions against gram + ve & −ve bacteria both. The action for B. subtilis & S. aureus was more efficient. It was shown in Fig. 6a–b, then the gram-positive bacteria is a high resistant than gram negative bacteria Fig. 6c–d. Therefore, this result showed that the synthesized sample obviously have antibacterial activities. The zone of inhibition (ZOI) values are tabulated in Table 3. Vancomycin is applied as a standard for gram + ve & −ve bacteria. Gram + ve bacteria show significant ZOI than negative bacteria due to more reactive oxygen species in gram negative bacteria. Thus, the capped ZnO can inhibit both the bacteria. Thus, ZnO capped with the Psidium guajava can be used as a best antibacterial agent. The similar results have been reported in various plant extracts [58].
A ZnO nanoparticle has other applications on hydrogen gas sensing particularly, compare to commercial nanopowders and film systems. Wide-spread nano-scaled MO solids like nanowires, nanorods, nanotubes, & nanobelts being explored ideally as gas sensor. The NPs of well prominent gas sensitive solids like SnO2, ZnO, In2O3, and WO3 are highly sensitivity and capable of rapid response to the large surface area and fast time recovery, & improved efficiency to identify gases at low contents [63,64,65,66]. The graphene oxide nanosheet provided by the TiO2 and ZnO nanoparticles in ethanol suspension is one of most applications in photocatalytic reduction [67]. Nanostructure has been explored informs of storage of electrochemical energy owed to their large specific surface area, electrical conductivity, exceptional mechano-chemical steadiness. Furthermore, graphene’s high surface area & conductivity allow even packing & binding sites for MO NPs, & fascinatingly improve the conductivity once composite materials are employed in electrodes for storing the energy [17]. Then the Zinc oxide nanoparticles are used in opto-electrical, & piezoelectricity, devices owed to its non-toxicity, full accessibility, cost-effective& steadiness nature [68, 69]. A ZnO usage has been discovered in different as that include catalysis, sensors, solar devices, paints/varnishes/plastics, pharma, etc. It has been commonly used in cosmetics/sunscreen items as defensive representative due to its capability to stop UV radiations. The ZnO nanoparticles are also well-recognized for their antibacterial & antifungal activities [70].
5 Conclusion
The ZnO nanoparticles capped with Psidium guajava leaf extract was synthesized by sol gel assisted microwave irradiation method. The prepared NPs are studied by XRD, FTIR, SEM, EDAX, UV, and antibacterial activity. The XRD reveals the crystalline size of 15.8 nm. The presence of functional groups of the prepared sample was established from FTIR analysis. SEM analysis shows the rod-shaped morphological structure. From the EDAX analysis, the presence of carbon reveals the incorporation of capping agent in the sample. UV analysis confirms the band gap of 3.14 eV. Antibacterial activity shows significant Zone of inhibition ranging from 22 to 14 nm. Thus, this research work reveals that capped Zinc oxide nanoparticles can act as an excellent antibacterial agent.
References
Mishra, P.K.; Mishra, H.; Ekielski, A.; Talegaonkar, S.; Vaidya, B.: Zinc oxide nanoparticles: a promising nanomaterial for biomedical applications. Drug Discov. Today 22(12), 1825–1834 (2017)
Yu, L.; Liu, S.; Yang, B.; Wei, J.; Lei, M.; Fan, X.: Sn–Ga co-doped ZnO nanobelts fabricated by thermal evaporation and application to ethanol gas sensors. Mater. Lett. 141, 79–82 (2015)
Safawo, T.; Sandeep, B.V.; Pola, S.; Tadesse, A.: Synthesis and characterization of zinc oxide nanoparticles using tuber extract of anchote (Coccinia abyssinica (Lam.) Cong.) for antimicrobial and antioxidant activity assessment. OpenNano 3, 56–63 (2018)
Ata, S.; Shaheen, I.; Ul Qurat, A.; Ghafoor, S.; Sultan, M.; Majid, F.; Bibi, I.; Iqbal, M.: Graphene and silver decorated ZnO composite synthesis characterization and photocatalytic activity evaluation. Diamond Related Mater. 90, 26–31 (2018)
Saxena, A.; Tripathi, R.; Singh, R.: Biological synthesis of silver nanoparticles by using onion (Allium cepa) extract and their antibacterial activity. Dig. J. Nanomater. Bios. 5(2), 427–432 (2010)
Yuvakkumar, R.; Suresh, J.; Nathanael, A.J.; Sundrarajan, M.; Hong, S.I.: Novel green synthetic strategy to prepare ZnO nanocrystals using rambutan (Nephelium lappaceum L.) peel extract and its antibacterial applications. Mater. Sci. Eng. C41, 17–27 (2014)
Noorjahan, C.; Shahina, S.J.; Deepika, T.; Rafiq, S.: Green synthesis and characterization of zinc oxide nanoparticles from Neem (Azadirachta indicia). Int. J. Sci. Eng. Technol. Res. 4(30), 5751–5753 (2015)
Ansilin, S.; Nair, J.K.; Aswathy, C.; Rama, V.; Peter, J.; Persis, J.J.: Green synthesis and characterisation of copper oxide nanoparticles using Azadirachta indica (Neem) leaf aqueous extract. J. Nanosci. Technol. 2(5), 221–223 (2016)
Karthik, S.; Siva, P.; Balu, K.S.; Suriyaprabha, R.; Rajendran, V.; Maaza, M.: Acalypha indica–mediated green synthesis of ZnO nanostructures under differential thermal treatment: Effect on textile coating, hydrophobicity UV resistance, and antibacterial activity. Adv. Powder Technol. 28(12), 3184–3194 (2017)
Manokari, M.; Shekhawat, M.S.: Zinc oxide nanoparticles synthesis from Moringa oleifera Lam. Extracts and their characterization. World Sci. News 55, 252–262 (2016)
Saha, R.; Subramani, K.; Petchi Muthu Raju, S.A.K.; Rangaraj, S.; Venkatachalam, R.: Psidium guajava leaf extract-mediated synthesis of ZnO nanoparticles under different processing parameters for hydrophobic and antibacterial finishing over cotton fabrics. Progress Org. Coat. 124, 80–91 (2018)
Parthasarathy, G.; Saroja, M.; Venkatachalam, M.; Shankar, S.; Evanjelene, V.: Green synthesis of zinc oxide nanoparticles-review paper. World J. Pharm. Pharmaceutical Sci. 5(4), 922–931 (2016)
Ngom, B.D.; Mpahane, T.; Manyala, N.; Nemraoui, O.; Buttner, U.; Kana, J.B.; Fasasi, A.Y.; Maaza, M.; Beye, A.C.: Structural and optical properties of nano-structured tungsten-doped ZnO thin films grown by pulsed laser deposition. Appl. Surf. Sci. 255(7), 4153–4158 (2009)
Ngom, B.D.; Mpahane, T.; Manikandan, E.; Maaza, M.: ZnO nano-discs by lyophilization process: Size effects on their intrinsic luminescence. J. Alloy. Compd. 656, 758–763 (2016)
Devi, K.R.; Selvan, G.; Karunakaran, M.; Kasirajan, K.; Shkir, M.; AlFaify, S.: A SILAR fabrication of nanostructured ZnO thin films and their characterizations for gas sensing applications: An effect of Ag concentration. Superlatt. Microstruct. 143, 106547 (2020)
Arun Kumar, K.D.; Valanarasu, S.; Ponraj, J.S.; Fernandes, B.J.; Shkir, M.; AlFaify, S.; Murahari, P.; Ramesh, K.: Effect of Er doping on the ammonia sensing properties of ZnO thin films prepared by a nebulizer spray technique. J. Phys. Chem. Solids 144, 109513 (2020)
Kaviyarasu, K.; Magdalane, C.M.; Manikandan, E.; Jayachandran, M.; Ladchumananandasivam, R.; Neelamani, S.; Maaza, M.: Well-aligned graphene oxide nanosheets decorated with zinc oxide nanocrystals for high performance photocatalytic application. Int. J. Nanosci. 14(03), 1550007 (2015)
Shkir, M.; Hamdy, M.S.; AlFaify, S.: A facile one pot flash combustion synthesis of ZnO nanoparticles and their characterizations for photocatalytic applications. J. Mol. Struct. 1197, 610–616 (2019)
Chandekar, K.V.; Shkir, M.; Al-Shehri, B.M.; AlFaify, S.; Halor, R.G.; Khan, A.; Al-Namshah, K.S.; Hamdy, M.S.: Visible light sensitive Cu doped ZnO: Facile synthesis, characterization and high photocatalytic response. Mater. Character. 165, 110387 (2020)
Suresh, J.; Pradheesh, G.; Alexramani, V.; Sundrarajan, M.; Hong, S.I.: Green synthesis and characterization of zinc oxide nanoparticle using insulin plant (Costus pictus D. Don) and investigation of its antimicrobial as well as anticancer activities. Adv. Nat. Sci.: Nanosci. Nanotechnol. 9(1), 015008 (2018)
Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Khamlich, S.; Maaza, M.: Sageretia thea (Osbeck.) mediated synthesis of zinc oxide nanoparticles and its biological applications. Nanomedicine 12(15), 1767–1789 (2017)
Biswas, B.; Rogers, K.; McLaughlin, F.; Daniels, D.; Yadav, A.: Antimicrobial activities of leaf extracts of guava (Psidium guajava L.) on two gram-negative and gram-positive bacteria. Int. J. Microbiol. 2013, 746165 (2013)
Rehan, M.; Ahmed-Farid, O.A.; Ibrahim, S.R.; Hassan, A.A.; Abdelrazek, A.M.; Khafaga, N.I.; Khattab, T.A.: Green and sustainable encapsulation of Guava leaf extracts (Psidium guajava L.) into alginate/starch microcapsules for multifunctional finish over cotton gauze. ACS Sustain. Chem. Eng. 7(22), 18612–18623 (2019)
Naseer, S.; Hussain, S.; Naeem, N.; Pervaiz, M.; Rahman, M.: The phytochemistry and medicinal value of Psidium guajava (guava). Clin. Phytosci. 4(1), 1–8 (2018)
Barbalho, S.M.; Farinazzi-Machado, F.M.V.; de Alvares Goulart, R.; Brunnati, A.S.; Otoboni, A.M.; Ottoboni, B.: Psidium guajava (Guava): A plant of multipurpose medicinal applications. Med Aromat Plants 1(104), 2167–412 (2012)
Matinise, N.; Kaviyarasu, K.; Mongwaketsi, N.; Khamlich, S.; Kotsedi, L.; Mayedwa, N.; Maaza, M.: Green synthesis of novel zinc iron oxide (ZnFe2O4) nanocomposite via Moringa Oleifera natural extract for electrochemical applications. Appl. Surf. Sci. 446, 66–73 (2018)
Kafle, A.; Mohapatra, S.S.; Reddy, I.; Chapagain, M.: A review on medicinal properties of Psidium guajava. J. Med. Plants 6(4), 44–47 (2018)
Abdelrahim, S.; Almagboul, A.; Omer, M.; Elegami, A.: Antimicrobial activity of Psidium guajava L. Fitoterapia 73(7–8), 713–715 (2002)
Jaiarj, P.; Khoohaswan, P.; Wongkrajang, Y.; Peungvicha, P.; Suriyawong, P.; Sumal Saraya, M.L.; Ruangsomboon, O.: Anticough and antimicrobial activities of Psidium guajava Linn leaf extract. J. Ethnopharmacol. 67(2), 203–212 (1999)
Lutterodt, G.D.: Inhibition of microlax-induced experimental diarrhoea with narcotic-like extracts of Psidium guajava leaf in rats. J. Ethnopharmacol. 37(2), 151–157 (1992)
Khalil, A.T.; Ovais, M.; Ullah, I.; Ali, M.; Shinwari, Z.K.; Hassan, D.; Maaza, M.: Sageretia thea (Osbeck.) modulated biosynthesis of NiO nanoparticles and their in vitro pharmacognostic, antioxidant and cytotoxic potential. Artif. Cells, Nanomed., Biotechnol. 46(4), 838–852 (2018)
Mohamed, H.E.A.; Afridi, S.; Khalil, A.T.; Zia, D.; Iqbal, J.; Ullah, I.; Shinwari, Z.K.; Maaza, M.: Biosynthesis of silver nanoparticles from Hyphaene thebaica fruits and their in vitro pharmacognostic potential. Mater. Res. Express 6(10), 10509 (2019)
Thema, F.T.; Manikandan, E.; Dhlamini, M.S.; Maaza, M.: Green synthesis of ZnO nanoparticles via Agathosma betulina natural extract. Mater. Lett. 161, 124–127 (2015)
Fakhari, S.; Jamzad, M.; Kabiri Fard, H.: Green synthesis of zinc oxide nanoparticles: a comparison. Green Chem. Lett. Rev. 12(1), 19–24 (2019)
Sharmila, G.; Thirumarimurugan, M.; Muthukumaran, C.: Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J. 145, 578–587 (2019)
Begum, S.; Hassan, S.I.; Ali, S.N.; Siddiqui, B.S.: Chemical constituents from the leaves of Psidium guajava. Nat. Prod. Res. 18(2), 135–140 (2004)
Ogunwande, I.A.; Olawore, N.O.; Adeleke, K.A.; Ekundayo, O.; Koenig, W.A.: Chemical composition of the leaf volatile oil of Psidium guajava L. growing in Nigeria. Flavour Fragrance J. 18(2), 136–138 (2003)
Pino, J.A.; Agüero, J.; Marbot, R.; Fuentes, V.: Leaf oil of Psidium guajava L from Cuba. J. Essential Oil Res. 13(1), 61–62 (2001)
Fu, H.-Z.; Luo, Y.-M.; Li, C.-J.; Yang, J.-Z.; Zhang, D.-M.: Psidials A−C, Three Unusual Meroterpenoids from the Leaves of Psidium guajava L. Org. Lett. 12(4), 656–659 (2010)
Sharma, D.; Kanchi, S.; Bisetty, K.: Biogenic synthesis of nanoparticles: A review. Arab. J. Chem. 12(8), 3576–3600 (2019)
Gade, A.; Bonde, P.; Ingle, A.; Marcato, P.; Duran, N.; Rai, M.: Exploitation of Aspergillus niger for synthesis of silver nanoparticles. J. Biobased Mater. Bioenergy 2(3), 243–247 (2008)
Kumar, H.; Rani, R.: Structural and optical characterization of ZnO nanoparticles synthesized by microemulsion route. Int. Lett. Chem., Phys. Astron. 14, 26–36 (2013)
Kumar, G.S.; Rajendran, S.; Karthi, S.; Govindan, R.; Girija, E.K.; Karunakaran, G.; Kuznetsov, D.: Green synthesis and antibacterial activity of hydroxyapatite nanorods for orthopedic applications. MRS Commun. 7(2), 183–188 (2017)
Prasanna, A.; Venkatasubbu, G.D.: Sustained release of amoxicillin from hydroxyapatite nanocomposite for bone infections. Prog. Biomater. 7(4), 289–296 (2018)
Munajad, A.; Subroto, C.: Fourier transform infrared (FTIR) spectroscopy analysis of transformer paper in mineral oil-paper composite insulation under accelerated thermal aging. Energies 11(2), 364 (2018)
Srivastava, R.: Synthesis and characterization techniques of nanomaterials. Int. J. Green Nanotechnol. 4(1), 17–27 (2012)
Thaya, R.; Malaikozhundan, B.; Vijayakumar, S.; Sivakamavalli, J.; Jeyasekar, R.; Shanthi, S.; Vaseeharan, B.; Ramasamy, P.; Sonawane, A.: Chitosan coated Ag/ZnO nanocomposite and their antibiofilm, antifungal and cytotoxic effects on murine macrophages. Microb. Pathog. 100, 124–132 (2016)
Reller, L.B.; Weinstein, M.; Jorgensen, J.H.; Ferraro, M.J.: Antimicrobial susceptibility testing: a review of general principles and contemporary practices. Clin. Infect. Dis. 49(11), 1749–1755 (2009)
Magaldi, S.; Mata-Essayag, S.; Hartung de Capriles, C.; Perez, C.; Colella, M.T.; Olaizola, C.; Ontiveros, Y.: Well diffusion for antifungal susceptibility testing. Int. J. Infect. Dis. 8(1), 39–45 (2004)
Shkir, M.; Al-Shehri, B.M.; Pachamuthu, M.; Khan, A.; Chandekar, K.V.; AlFaify, S.; Hamdy, M.S.: A remarkable improvement in photocatalytic activity of ZnO nanoparticles through Sr doping synthesized by one pot flash combustion technique for water treatments. Colloids Surf. A: Physicochem. Eng. Asp. 587, 124340 (2020)
Shkir, M.; Chandekar, K.V.; Alshehri, B.M.; Khan, A.; AlFaify, S.; Hamdy, M.S.: A remarkable enhancement in photocatalytic activity of facilely synthesized Terbium@Zinc oxide nanoparticles by flash combustion route for optoelectronic applications. Appl. Nanosci. 10(6), 1811–1823 (2020)
Shakir, M.; Kushwaha, S.; Maurya, K.; Bhagavannarayana, G.; Wahab, M.: Characterization of ZnSe nanoparticles synthesized by microwave heating process. Solid State Commun. 149(45), 2047–2049 (2009)
Shkir, M.: Noticeable impact of Er doping on structural, vibrational, optical, dielectric and electrical parameters of flash combustion synthesized NiO NPs for optoelectronic applications. Inorg. Chem. Commun. 121, 108229 (2020)
Shkir, M.; Chandekar, K.V.; Khan, A.; El-Toni, A.M.; AlFaify, S.: A facile synthesis of Bi@PbS nanosheets and their key physical properties analysis for optoelectronic technology. Mater. Sci. Semicond. Process. 107, 104807 (2020)
AlFaify, S.; Shkir, M.: A facile one pot synthesis of novel pure and Cd doped PbI2 nanostructures for electro-optic and radiation detection applications. Opt. Mater. 88, 417–423 (2019)
Janjal, S.; Agale, A.; Rajbhoj, A.; Gaikwad, S.: Synthesis and electrochemical characterization of zinc oxide nanoparticles using green method. Int. J. Appl. Res. 10, 2394–7500 (2017)
El-Arab, N.B.: Synthesis and characterization of zinc oxide nanoparticles using green and chemical synthesis techniques for phenol decontamination. Int. J. Nanoelectron. Mater. 11(2), 179–194 (2018)
Vijayakumar, S.; Vinoj, G.; Malaikozhundan, B.; Shanthi, S.; Vaseeharan, B.: Plectranthus amboinicus leaf extract mediated synthesis of zinc oxide nanoparticles and its control of methicillin resistant Staphylococcus aureus biofilm and blood sucking mosquito larvae. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 137, 886–891 (2015)
Shreema, K.; Kalaiselvi, V.; Mathammal, R.: Green synthesis and characterization of Zinc Oxide nanoparticles using Leaf extract of Evolvulus Alsinoides. Studies Indian Place Names 40(18), 763–778 (2020)
Umar, H.; Kavaz, D.; Rizaner, N.: Biosynthesis of zinc oxide nanoparticles using Albizia lebbeck stem bark, and evaluation of its antimicrobial, antioxidant, and cytotoxic activities on human breast cancer cell lines. Int. J. Nanomed. 14, 87 (2019)
Shkir, M.; AlFaify, S.: Tailoring the structural, morphological, optical and dielectric properties of lead iodide through Nd3+ doping. Sci. Rep. 7(1), 16091 (2017)
Shkir, M.; Abbas, H.; Siddhartha, Z.R.K.: Effect of thickness on the structural, optical and electrical properties of thermally evaporated PbI2 thin films. J. Phys. Chem. Solids 73(11), 1309–1313 (2012)
Tien, L.C.; Wang, H.T.; Kang, B.S.; Ren, F.; Sadik, P.W.; Norton, D.P.; Pearton, S.J.; Lin, J.: Room-temperature hydrogen-selective sensing using single pt-coated ZnO nanowires at microwatt power levels. Electrochem. Solid-State Lett. 8(9), G230 (2005)
Lv, Y.; Guo, L.; Xu, H.; Chu, X.: Gas-sensing properties of well-crystalline ZnO nanorods grown by a simple route. Phys. E. 36(1), 102–105 (2007)
Nwanya, A.C.; Deshmukh, P.R.; Osuji, R.U.; Maaza, M.; Lokhande, C.D.; Ezema, F.I.: Synthesis, characterization and gas-sensing properties of SILAR deposited ZnO-CdO nano-composite thin film. Sens. Actuators, B Chem. 206, 671–678 (2015)
Simo, A.; Mwakikunga, B.; Sone, B.T.; Julies, B.; Madjoe, R.; Maaza, M.: VO2 nanostructures based chemiresistors for low power energy consumption hydrogen sensing. Int. J. Energy 39(15), 8147–8157 (2014)
Khamlich, S.; Abdullaeva, Z.; Kennedy, J.V.; Maaza, M.: High performance symmetric supercapacitor based on zinc hydroxychloride nanosheets and 3D graphene-nickel foam composite. Appl. Surf. Sci. 405, 329–336 (2017)
Darezereshki, E.; Alizadeh, M.; Bakhtiari, F.; Schaffie, M.; Ranjbar, M.: A novel thermal decomposition method for the synthesis of ZnO nanoparticles from low concentration ZnSO4 solutions. Appl. Clay Sci. 54(1), 107–111 (2011)
Tari, O.; Aronne, A.; Addonizio, M.L.; Daliento, S.; Fanelli, E.; Pernice, P.: Sol–gel synthesis of ZnO transparent and conductive films: A critical approach. Sol. Energy Mater. Sol. Cells 105, 179–186 (2012)
Vijayakumar, S.; Vaseeharan, B.; Malaikozhundan, B.; Shobiya, M.: Laurus nobilis leaf extract mediated green synthesis of ZnO nanoparticles: Characterization and biomedical applications. Biomed. Pharmacother. 84, 1213–1222 (2016)
Acknowledgements
The authors from KKU would like to express their gratitude to Deanship of Scientific Research at King Khalid University, Abha, Saudi Arabia for funding this work through Research Groups Program under Grant No. R.G.P.1/102/42.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None to declare.
Rights and permissions
About this article
Cite this article
Ramya, V., Kalaiselvi, V., Kannan, S.K. et al. Facile Synthesis and Characterization of Zinc Oxide Nanoparticles Using Psidium guajava leaf Extract and Their Antibacterial Applications. Arab J Sci Eng 47, 909–918 (2022). https://doi.org/10.1007/s13369-021-05717-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13369-021-05717-1