Abstract
A highly sensitive method for real-time air-monitoring of mustard gas (bis(2-chloroethyl) sulfide, HD), which is a lethal blister agent, is proposed. Humidified air containing a HD simulant, 2-chloroethyl ethyl sulfide (2CEES), was mixed with ozone and then analyzed by using an atmospheric pressure chemical ionization ion trap tandem mass spectrometer. Mass-spectral ion peaks attributable to protonated molecules of intact, monooxygenated, and dioxygenated 2CEES (MH+, MOH+, and MO2H+, respectively) were observed. As ozone concentration was increased from zero to 30 ppm, the signal intensity of MH+ sharply decreased, that of MOH+ increased once and then decreased, and that of MO2H+ sharply increased until reaching a plateau. The signal intensity of MO2H+ at the plateau was 40 times higher than that of MH+ and 100 times higher than that of MOH+ in the case without in-line ozonation. Twenty-ppm ozone gas was adequate to give a linear calibration curve for 2CEES obtained by detecting the MO2H+ signal in the concentration range up to 60 μg/m3, which is high enough for hygiene management. In the low concentration range lower than 3 μg/m3, which is equal to the short-term exposure limit for HD, calibration plots unexpectedly fell off the linear calibration curve, but 0.6-μg/m3 vapor was actually detected with the signal-to-noise ratio of nine. Ozone was generated from instrumentation air by using a simple and inexpensive home-made generator. 2CEES was ozonated in 1-m extended sampling tube in only 1 s.

ᅟ
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Chemical warfare agents (CWAs) [1, 2] are lethal toxic compounds that are used as chemical weapons. In 1997, the Chemical Weapons Convention [3] mandated its contracting countries to destroy their remaining chemical weapons. Accordingly, for managing the health and safety of workers and populations in CWAs-related areas, air monitoring of CWAs is required [4].
Conventional methods for air monitoring of CWAs, based on gas chromatography [5, 6], provide high specificity. These methods can achieve practically high sensitivity when the CWAs are preconcentrated using thermal desorption tubes [7]; however, they are time-consuming and require frequent maintenance of preconcentration devices. Consequently, direct air-sampling mass spectrometry (MS) [8–11] has received substantial interest in regard to continuous air-monitoring of CWAs [12–16]. Soft ionization followed by multiple-stage mass spectrometry (MSn) not only gives information on molecular weight but also on molecular structure; therefore, it provides high specificity comparable to that attained by gas-chromatographic methods. Ion-trap mass analyzers, which are capable of MSn, are suitable for field use because of their compactness and robustness. Atmospheric pressure chemical ionization (APCI) ion-trap MSn with a counter-flow ion introduction (CFI) [17] configuration provides sufficient sensitivity for hygiene management of a wide range of CWAs [18, 19].
Mustard gas [bis(2-chloroethyl) sulfide, HD] is one of the common CWAs. For example, the Japanese military used HD for chemical weapons during World War II [20], and many of those weapons that were left in China after the war are being destroyed [21]. HD contains a divalent sulfur atom and can be oxygenated to form quadrivalent and hexavalent sulfur compounds or corresponding sulfoxide and sulfone of HD [22–24]. It was demonstrated that HD provides dominant mass-spectral ion peaks attributed to the [HD+OH]+ ion [14], but the mechanism of the ion formation was not elucidated. Under irradiation by ultraviolet light in air, HD was monooxygenated by ozone to form mustard sulfoxide (bis(2-chloroethyl) sulfoxide), which was protonated and its mass spectrum was observed [25].
In the case of CFI-APCI-MSn [18, 19], HD is efficiently protonated so that it can provide dominant mass-spectral ion peaks under low humidity. However, as air humidity increases, the peak intensities sharply decrease owing to interference of protonation by moisture and fall below those of the protonated monooxygenated HD. Oxygenation of HD is possibly caused by reactive oxygen species (ROS) such as ozone and NOx generated in the corona discharge (CD) used for initiating APCI [26]. In the case of the CFI configuration, reactant ion species (such as H3O+ and O2 –) with a polarity of interest are drawn out of the CD region in the direction opposite to the air flow through the CD region by the strong electric field used for generating the CD. The reactant ion species then react with analyte molecules in the air flow and ionize them. The ionized analyte molecules are introduced into a mass analyzer. This CFI configuration removes ROS effectively from the chemical-ionization region and suppresses interference of negative ionization by NO3 (which has high electron affinity). However, residual ROS can react with HD. The oxygenation ratio from HD to monooxygenated HD is estimated to be low (<1%). Proton affinity of monooxygenated HD has been demonstrated in a numerical simulation as being higher than that of HD [26]. As a result, monooxygenated HD would be detected more intensely than intact HD. In a previous study [19], the limit of detection, defined as three times the standard deviation of the background signals (σBG), was 0.6 μg/m3 (in 2-s measurement) when monooxygenated HD was detected instead of intact HD. The limit of quantification (LOQ), defined as 10 times σBG, is therefore calculated to be 2 μg/m3. This LOQ is lower than the short-term exposure limit (STEL, 3 μg/m3) [27]. Repeating the 1-s measurement 100 times and averaging them, which takes only 2 min, decreases the LOQ to 0.2 μg/m3 (to one-tenth) which is lower than the worker population limit (WPL, 0.4 μg/m3) [27], because σBG is proportional to the square root of the number of averaging times. Signal averaging over 200 min is expected to allow for quantification of the general population limit (GPL, 0.02 μg/m3) [27]. However, evaluation of the analytical performance of CFI-APCI-MSn with such long measurement time will be unrealistically laborious. In addition, the sensitivity, and correspondingly the LOQ, can be affected by interferences in practical air monitoring.
In this study, aimed at improving the sensitivity of APCI-MSn for HD, a new method of in-line ozonation followed by detection of the dioxygenated product was developed for a HD simulant, 2-chloroethyl ethyl sulfide (2CEES). Reversing the air-flow through the CD region, which allows analyte molecules to react with ROS more efficiently, only increased the signal intensity of monooxygenated 2CEES a few times.
2CEES is a good simulant for HD in that it has a divalent sulfur atom and can be oxygenated to its sulfoxide and sulfone forms [28] similarly to HD [25]. HD and 2CEES are ionized by protonation in the CFI-APCI-MS, and proton affinities [29] of these compounds are similar in that their protonation are similarly affected by humidity increase [26]. On the other hand, 2CEES contains a chlorine atom, whereas HD contains two chlorine atoms. By this difference, HD and 2CEES are differently hydrolyzed (i.e., HD is doubly hydrolyzed to form thiodiglycol, whereas 2CEES is only singly hydrolyzed). However, hydrolysis of HD and 2CEES is not evident in the CFI-APCI-MS [26].
With this method, it is impossible to distinguish dioxygenated HD formed by in-line ozonation from that formed during storage and destruction of HD. Since the dioxygenated HD is also vesicant [30] like HD and should be fully converted to less toxic products during the destruction process, discrimination between differently formed dioxygenated HD may be less important. In case the discrimination is needed, switching between ON and OFF ozonation and comparing the signal intensities of the dioxygenated HD is expected to allow the discrimination.
Experimental
Chemicals and Gases
2CEES (>98% purity) was purchased from Wako Pure Chemical Industries (Osaka, Japan). Standard solutions of 2CEES were prepared in n-hexane of analytical grade (Wako Pure Chemical Industries). Purified water was purchased from Nichi-Iko Pharmaceutical Co., Ltd. (Toyama, Japan). Pressurized dry air (dew point: –60°C) was supplied in-house.
Instrument
A CFI-APCI-MSn instrument (CP-2000; Hitachi High-Tech Solutions Corporation, Mito, Japan) was used for this study. A quadrupole ion trap was used for MSn analysis. The variable temperatures of the instrument’s sampling tube, ionization chamber, and ion inlet into the vacuum system were set at 120°C (which is the same as those set in previous studies [18, 19, 26]).
Generation of Sample Air
A humidified-air stream was generated by splitting a dry-air stream (2 L/min) into two streams and mixing them after immersing one stream in a purified-water bath at room temperature (23°C). Humidity of the humidified air was controlled by adjusting the split ratio of the dry-air stream. The humidified air was sucked by the instrument through a sampling tube at the rate of 1.2 L/min. The excess stream (0.8 L/min) was ejected, and its humidity was measured.
Vapor of 2CEES was generated in ambient air by a head-space (HS) method. An open bottle containing 0.2 mL of liquid 2CEES was put inside a larger bottle (40 mL) sealed with a septum, which was pierced with two tetrafluoroethylene-perfluoroalkylvinylether copolymer (PFA) tubes with an inner diameter of 0.025 mm. The HS ambient air (containing vapor of 2CEES) was pushed out through one of the PFA tubes by introducing ambient air into the HS through the other PFA tube with a syringe pump, and infused into the sampling tube. Concentration of 2CEES in the HS ambient air was estimated to be 1.3 g/m3 (250 ppm) by comparing the mass-spectral signal intensity of 2CEES with that obtained when a standard solution was infused. In this estimation, it was assumed that the signal intensity responds linearly to 2CEES vapor concentration, and the matrix effect due to the solvent (n-hexane) was neglected. The concentration of the tested vapor was controlled by adjusting the infusion rate of the HS ambient air.
Set-up for In-Line Ozonation
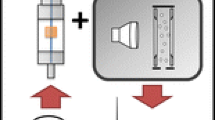
The experimental set-up for in-line ozonation is shown in Figure 1. Ozone gas was generated by a home-made ozone generator based on dielectric-barrier discharge in a dry-air stream. A stainless-steel rod was inserted into a glass tube, which was coaxially inserted into a stainless-steel tube. The pure air was fed through the 1-mm gap between these tubes. High voltage (HV), in the form of a sine wave with frequency of 2–800 Hz and amplitude of ±4–10 kV, was applied to the rod, while the stainless-steel tube was grounded. The dry-air stream containing ozone gas was mixed with the sample-air stream in the sampling tube.
The pressurized dry air was controlled with a mass flow controller to generate a dry-air stream with flow rate of 0.05 L/min. Ozone concentration in the sampling tube was measured by detection tubes (no. 18 L and no. 18 M, Gastec Corp., Ayase, Japan) located 5-cm downstream of the position where the dry-air and sample-air streams were mixed. The ozone concentration was found beforehand to be correlated to the amplitude and frequency of the applied HV. The sampling tube (15-cm long in original form) was extended up to 1 m by connecting an additional tube. Both the original and the additional tubes were made of stainless steel with inner diameters of 4 mm. Since the flow rate in the sampling tube was 1.2 L/min, the time for ozonation was calculated to be 1 s1 s at most.
Results and Discussion
Assignment of Mass-Spectral Peaks
In a previous report [26], vapors of 2CEES, one with and one without in-line ozonation, were analyzed by APCI-MSn. Mass-spectral ion peaks at m/z of 125 (127), 141 (143), and 157 (159) are assigned to protonated 35Cl (37Cl) isotopic species of intact, monooxygenated, and dioxygenated 2CEES, respectively. MS3 transitions of these ion peaks [26] are consistent with these assignments. The monooxygenated and dioxygenated products of 2CEES are considered to be known sulfoxide and sulfone forms of 2CEES [28].
In this study, a major MS3 product ion, which is listed in Table 1, was monitored for the detection of each compound of interest. Comparison against monitoring of MS2 product ion(s) and multiple MS3 product ions may be useful to further improve the analytical performance presented in this study.
Effect of In-Line Ozonation on Peak Intensity
MS3 peak intensities of protonated molecules of intact (MH+), monooxygenated (MOH+), and dioxygenated (MO2H+) 2CEES are plotted against ozone concentration under zero and a moderate (10 g/m3) humidity conditions in Figure 2. The vapor concentration of 2CEES in the sample air was 25 μg/m3 (5 ppb). Under both humidity conditions, as the ozone concentration increases, the peak intensity of MH+ decreases gradually, that of MOH+ increases once and then decreases, and that of MO2H+ significantly increases until it reaches a plateau. This result is evidence that the in-line ozonation promotes oxygenation of 2CEES. Moisture seems to affect the ozonation for two reasons: first, the peak intensity of MO2H+ reaches the plateau at higher ozone concentration under moderate humidity than under zero humidity; second, that of MOH+ at the 2CEES concentration range giving the plateau is stronger under the moderate humidity than under zero humidity despite that the signal intensity of MOH+ is affected by humidity increase [26]. Under moderate humidity, the peak intensity of MO2H+ at 16-ppm ozone concentration is, respectively, 40 times and 100 times higher than that of MH+ and MOH+ at zero ozone concentration. Under zero humidity, the peak intensity of MO2H+ at the plateau is nearly 1 × 106 cps, which is similar to that under moderate humidity. The peak intensity of MH+ at zero ozone concentration is also nearly 1 × 106 cps, but it decreases significantly with increasing humidity. As was previously reported [26], suppression of protonation of 2CEES by moisture is more probable cause of this decrease than hydrolysis of 2CEES. In this study, hydrolysis of 2CEES and oxidation of hydrolyzed 2CEES were not evident in mass spectra.
Calibration Curve
MS3 signal intensities of MOH+ and MO2H+ are plotted against concentration of 2CEES vapor in Figure 3. Time for ion-loading into the ion trap was set to 100 ms, and six successive detections, taking 1 s, were averaged to form a measurement signal. Other measurement functions (including MS3 for MO2H+ with ion-loading time of 10 ms and full mass analyses) were run one after the other. The sampling tube was 1 m long, ozone concentration was 20 ppm (corresponding to HV of ±6 kV and 200 Hz), and humidity was 10 g/m3. As the concentration of 2CEES vapor increases from zero, the signal intensity of MO2H+ increases proportionally to the concentration up to 60 μg/m3 (12 ppb), over which it shows a concave-down response. On the other hand, the signal intensity of MOH+ is less than one-tenth that of MO2H+ and shows a concave-up response with increasing concentration over the whole range of concentrations investigated. Using shorter sampling tubes resulted in higher signal-intensity ratio of MOH+ to MO2H+ (data not shown) and narrower concentration range in which the signal intensity of MO2H+ increases proportionally to concentration. Reduced ion-loading time (10 ms) did not affect signal intensity. These results indicate that the concave-down response is caused by limited ozonation.
(a) MS3 signal intensities of protonated molecules of monooxygenated-2CEES (MOH+) and dioxygenated-2CEES (MO2H+) as a function of 2CEES vapor concentration obtained at moderate humidity (10 g/m3). (b) Enlarged linear calibration curve obtained for a concentration range of 0−60 μg/m3 by monitoring the MO2H+ signal. Error bars show standard deviation. Inset shows background signals measured for laboratory air
Analytical Performance
As seen in Figure 3b, the calibration plots fall off the linear calibration curve in low concentration range below 3 μg/m3 (equal to the STEL). This might be caused by adsorption of intact and oxygenated 2CEES on the surface of sampling tube or incomplete infusion of 2CEES vapor attributable to tube resistance. Suppression of the adsorption or improvement of the infusion is expected to give better fitting of the calibration plots to the linear calibration curve. The calibration plot at the lowest concentration in Figure 3 has the net signal intensity equal to nine times σ BG (Figure 3b); therefore, the concentration (0.6 μg/m3) can be detected. The concentrations equal to the WPL (0.4 μg/m3) and the GPL (0.02 μg/m3) for HD are expected to be detected without averaging and by averaging about 100 measurements, which takes 2 min (and is sufficiently short for hygiene management), respectively.
Conclusions
A commercialized on-site APCI-MSn instrument and a simple ozone-generator were used to demonstrate that in-line ozonation followed by detecting the dioxygenated product enhances the sensitivity of APCI-MSn for 2CEES, which is used as a simulant for HD. The in-line ozonation seemed to promote thorough dioxygenation of 2CEES. When ozone concentration was appropriate, the signal intensity of protonated dioxygenated 2CEES was several-dozen times greater than that of protonated 2CEES and protonated monooxygenated-2CEES (both obtained without ozonation). Short ozonation time (1 s) and subtle ozone concentration (20 ppm) were adequate for achieving a linear dynamic range covering 20 times the STEL, which is typically sufficient for hygiene management and quantifying the STEL level. Quantifying the WPL and GPL levels of 2CEES and HD vapors remains a future challenge. Moreover, interferences of sensitivity by potential matrices must be investigated. Practically, the sensitivity must be corrected for the quantification of HD, for which the simulant 2CEES is expected to be used as the internal standard.
References
Stewart, C.E., Sullivan Jr., J.B.: Hazardous Materials Toxicology—Clinical Principles of Environmental Health, Chap. 100 pp. 986–1014. Williams and Wilkins, Baltimore. (1992)
Somani, S.M.: Chemical Warfare Agents. Academic Press, San Diego (1992)
Organization for the Prohibition of Chemical Weapons. Chemical weapon convention. Available at: http://www.opcw.org. Accessed 14 March 2014
Smith, J.N., Noll, R.J., Cooks, R.G.: Facility monitoring of chemical warfare agent simulants in air using an automated, field-deployable miniature mass spectrometer. Rapid Commun. Mass Spectrom. 25, 1437–1444 (2011)
O.I. Analytical. GC continuous monitoring systems. Available at: http://www.oico.com/default.aspx?id=productline&PLID=21. Accessed 1 Dec 2012
Sekiguchi, H., Matsushita, K., Yamashiro, S., Sano, Y., Seto, Y., Okuda, T., Sato, A.: On-site determination of nerve and mustard gases using a field-portable gas chromatograph-mass spectrometer. Forensic Toxicol. 24, 17–22 (2006)
Murphy, T., Roberts, G., Davies, G.: Chemical warfare agents and use of thermal desorption-GC-MS to achieve improved trace-level detection. Spectroscopy April 1 2009. Available at: http://www.markes.com/News/Chemical-Warfare-Agents-and-use-of-Thermal-DeorptionGCMS.aspx. Accessed 9 Feb 2015
Lindinger, W., Hansel, A., Jordan, A.: On-line monitoring of volatile organic compounds at ppt levels by means of proton-transfer-reaction mass spectrometry (PTR-MS) medical applications. Int. J. Mass Spectrom. Ion Process. 173, 191–241 (1998)
Blake, R.S., Whyte, C., Hughes, C.O., Ellis, A.M., Monks, P.S.: Demonstration of proton-transfer reaction time-of-flight mass spectrometry for real-time analysis of trace volatile organic compounds. Anal. Chem. 76, 3841–3845 (2004)
Smith, D., Spanel, P.: Selected ion flow tube mass spectrometry (SIFT-MS) for on-line trace gas analysis. Mass Spectrom. Rev. 24, 661–700 (2005)
Milligan, D.B., Francis, G.J., Prince, B.J., McEwan, M.J.: Demonstration of selected ion flow tube MS detection in the parts per trillion range. Anal. Chem. 79, 2537–2540 (2007)
Cordell, R.C., Willis, K.A., Wyche, K.P., Blake, R.S., Ellis, A.M., Monks, P.S.: Detection of chemical weapon agents and simulants using chemical ionization reaction time-of-flight mass spectrometry. Anal. Chem. 79, 8359–8366 (2007)
Francis, G.J., Milligan, D.B., McEwan, M.J.: Detection and quantification of chemical warfare agent precursors and surrogates by selected ion flow tube mass spectrometry. Anal. Chem. 81, 8892–8899 (2009)
Nilles, J.M., Connel, T.R., Durst, H.D.: Quantitation of chemical warfare agents using direct analysis in real time (DART) technique. Anal. Chem. 81, 6744–6749 (2009)
Kassebacher, T., Sulzer, P., Jürschik, S., Hartungen, E., Jordan, A., Edtbauer, A., Feil, S., Hanel, G., Jaksch, S., Märk, L., Mayhew, C.A., Märk, T.D.: Investigations of chemical warfare agents and toxic industrial compounds with proton-transfer-reaction mass spectrometry (PTR-MS) for a real-time threat monitoring scenario. Rapid Commun. Mass Spectrom. 27, 325–332 (2013)
Wolf, J.-C., Schaer, M., Siegenthaler, P., Zenobi, R.: Direct quantification of chemical warfare agents and related compounds at low ppt levels: comparing active capillary dielectric barrier discharge plasma ionization and secondary electrospray ionization mass spectrometry. Anal. Chem. 87, 723–729 (2015)
Kojima, K., Sakairi, M., Takada, Y., Nakamura, J.: Vapor detection of TNT and RDX using atmospheric pressure chemical ionization mass spectrometry with counter-flow introduction (CFI). J. Mass Spectrom. Soc. Jpn. 48, 360–362 (2000)
Seto, Y., Kanamori-Kataoka, M., Tsuge, K., Ohsawa, I., Iura, K., Itoi, T., Sekiguchi, H., Matsushita, K., Yamashiro, S., Sano, Y., Sekiguchi, H., Maruko, H., Takayama, Y., Sekioka, R., Okumura, A., Takada, Y., Nagano, H., Waki, I., Ezawa, N., Tanimoto, H., Honjo, S., Fukano, M., Okada, H.: Sensitive monitoring of volatile chemical warfare agents in air by atmospheric pressure chemical ionization mass spectrometry with counter-flow introduction. Anal. Chem. 85, 2659–2666 (2013)
Seto, Y., Sekiguchi, H., Maruko, H., Yamashiro, S., Sano, Y., Takayama, Y., Sekioka, R., Yamaguchi, S., Kishi, S., Satoh, T., Sekiguchi, H., Iura, K., Nagashima, H., Nagoya, T., Tsuge, K., Ohsawa, I., Okumura, A., Takada, Y., Ezawa, N., Watanabe, S., Hashimoto, H.: Sensitive and comprehensive detection of chemical warfare agents in air by atmospheric pressure chemical ionization ion trap tandem mass spectrometry with counterflow introduction. Anal. Chem. 86, 4316–4326 (2014)
Hanaoka, S., Nomura, K., Wada, T.J.: Determination of mustard and lewisite related compounds in abandoned chemical weapons (yellow shells) from sources in China and Japan. J. Chromatogr. A 1101, 268–277 (2006)
Government of Japan, Cabinet Office, Abandoned Chemical Weapons Office. Available at: http://wwwa.cao.go.jp/acw/index-e.html. Accessed 9 May 2014
Munro, N.B., Talmage, S.S., Griffin, G.D., Waters, L.C., Watson, A.P., King, J.F., Hauschild, V.: The sources, fate, and toxicity of chemical warfare agent degradation products. Environ. Health Perspect. 107, 933–974 (1999)
Yang, Y.C., Baker, J.A., Ward, J.R.: Decontamination of chemical warfare agents. Chem. Rev. 92, 1729–1743 (1992)
Ringenbach, C.R., Livingston, S.R., Kumar, D., Landry, C.C.: Vanadium-doped acid-prepared mesoporous silica: synthesis, characterization, and catalytic studies on the oxidation of a mustard gas analogue. Chem. Mater. 17, 5580–5586 (2005)
Zuo, G., Cheng, Z., Li, G., Wang, L., Miao, T.: Photo-assisted reaction of sulfur mustard under UV light irradiation. Environ. Sci. Technol. 39, 8742–8746 (2005)
Okumura, A., Takada, Y., Watanabe, S., Hashimoto, H., Ezawa, N., Seto, Y., Sekiguchi, H., Maruko, H., Takayama, Y., Sekioka, R., Yamaguchi, S., Kishi, S., Satoh, T., Kondo, T., Nagashima, H., Nagoya, T.: Real-time air monitoring of mustard gas and lewisite 1 by detecting their in-line reaction products by atmospheric pressure chemical ionization ion trap tandem mass spectrometry with counterflow ion introduction. Anal. Chem. 87, 1314–1322 (2015)
Centers for Disease Control and Prevention. The emergency response safety and health database. Available at: http://www.cdc.gov/niosh/ershdb/AgentListCategory.html. Accessed 10 Feb 2015
Livingston, S.R., Landry, C.C.: Oxidation of a mustard gas analogue using an aldehyde/O2 system catalyzed by V-Doped mesoporous silica. J. Am. Chem. Soc. 130, 13214–13215 (2008)
Midey, A.J., Miller, T.M., Viggiano, A.A.: Kinetics of ion–molecule reactions with 2-chloroethyl ethyl sulfide at 298 K: a search for CIMS schemes for mustard gas. J. Phys. Chem. A 112, 10250–10256 (2008)
Wagner, G.W., Procell, L.R., Yang, Y., Bunton, C.A.: Molybdate/peroxide oxidation of mustard in microemulsions. Langmuir 17, 4809–4811 (2001)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Okumura, A. In-Line Ozonation for Sensitive Air-Monitoring of a Mustard-Gas Simulant by Atmospheric Pressure Chemical Ionization Mass Spectrometry. J. Am. Soc. Mass Spectrom. 26, 1532–1537 (2015). https://doi.org/10.1007/s13361-015-1167-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13361-015-1167-1