Abstract
The wandering glider dragonfly, Pantala flavescens (Fabricius), arrives in Japan from tropical regions every spring. The offspring colonize areas throughout Japan, with rapid increases in populations in the autumn, but all individuals die in the winter, suggesting low tolerance to low temperatures. However, few quantitative data on egg development and water temperature have been reported for this species. Females at the reproductive stage were collected from fields throughout the flying season and their eggs released using an artificial oviposition technique. Almost all of the eggs were fertilized. Egg size was stable throughout the seasons. Most eggs hatched within a period of 5 days at high water temperatures (35 and 30 °C), which were recorded in the shallow ponds and rice paddy fields from summer to early autumn. However, the egg-stage duration increased with declining water temperature. All eggs in water at 15 °C had failed to hatch by 90 days. The calculated critical temperature of water was determined to be approximately 14.3 °C; the total effective temperature for the egg stage was about 80 degree-days. Thus, low water temperatures in winter may prevent P. flavescens overwintering in Japan.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Egg development is an important ontogenetic stage of an odonate’s life history (Lutz and Rogers 1991). Odonates have many types of oviposition behavior, such as laying eggs in water (Schenk et al. 2004) and mud (Watanabe 2015), on the stems of emergent plants (Bennett and Mill 1995), and whilst flying (Watanabe and Kato 2012). In general, the eggs of most odonate species start to develop in water. Consequently, the hatching success and the speed of egg development are directly influenced by environmental conditions, particularly water quality. Punzo (1988) reported that a low pH of water disturbed egg development in Anax junius (Drury). In Calopteryx splendens xanthostoma (Charpentier), eggs enveloped by algae showed high mortality, probably due to a lack of oxygen (Siva-Jothy et al. 1995). However, the most important factor affecting the eggs’ development is water temperature (Corbet 1999). Pilon et al. (1989) reported that the duration of the egg stage in Leucorrhinia glacialis Hagen was about 7 days at 32.5 °C water temperature, whereas the eggs needed more than 35 days to hatch in water at 17.5 °C. In the case of Leucorrhinia rubicunda L., eggs did not hatch below 6 °C (Soeffing 1986).
Odonate species living in temperate regions must adapt to low temperatures because water temperatures drop substantially in the winter. Many odonate species can overwinter in the egg stage due to a high tolerance for low water temperatures. These species have been called “summer species” by Corbet (1999). Inoue and Tani (2010) reported that Sympetrum frequens Sélys females oviposit in rice paddy fields in autumn, and their eggs diapause during winter. The eggs hatch simultaneously in the spring when the rice paddy fields are flooded for planting. However, eggs of the wandering glider dragonfly, Pantala flavescens (Fabricius), are assumed not to overwinter in temperate regions (Arai 2007).
P. flavescens is a remarkable migrating dragonfly that lives in tropical and temperate regions of the world. It travels to temperate regions every spring and spreads extensively throughout the world (Troast et al. 2016). Inoue and Tani (1999) observed many P. flavescens adults on the Nansei Islands in southern Japan in early spring; their offspring move northwards, arriving in Hokkaido in the autumn. They show oviposition behavior in various types of open water, such as shallow small ponds, rice paddy fields, swimming pools, and puddles, stopping over during their migration flight (Arai 2012; Hawking and Ingram 1994; Hirake 2012; Watanabe 1999). Ichikawa and Watanabe (2014) showed that P. flavescens females, just after sexual maturation, can lay more than 1,000 mature eggs/day when they find suitable oviposition sites. In laboratory experiments, females could produce over 800 mature eggs within 24 h when they fed sufficiently (Ichikawa and Watanabe 2015, 2016). The eggs and larvae develop very rapidly at high water temperatures, completing development within 50 days in Japan (Inoue and Tani 2010). Consequently, the adult population rapidly increases, frequently forming foraging swarms in summer and autumn (Ishizawa 2007). However, P. flavescens seems not to overwinter, irrespective of developmental stage (Arai 2007).
Arai (2012) reported that the number of flying P. flavescens adults suddenly decreases in October in the Kanto district, central Japan, and that they disappear by November. Watanabe (1999) surveyed the community structure of odonate larvae in outdoor swimming pools in the Mie Prefecture, and found many carcasses of P. flavescens larvae in the winter. Although P. flavescens seems to be intolerant of low temperatures, little information about the relationship between egg development and water temperature has been reported except for tentative data in Iwata et al. (2009). Arai (1991) found live larvae of P. flavescens in small puddles in the Kanto district in winter, suggesting that P. flavescens could overwinter if the water temperature was adequate for egg and larval development on the mainland of Japan. Therefore, detailed studies of the critical water temperature for the development of the eggs and larvae of P. flavescens are essential to clarify the conditions in which generations of this dragonfly migrate over long distances. In the present study, we conducted experiments to clarify the egg developmental speed of P. flavescens. As a first step, egg developmental traits such as egg size and egg fertilization rate were examined. Then, the duration of the egg stages at various water temperatures was measured.
Materials and methods
Females were captured from the foraging masses in fields on sunny and windless days (total of 23 days), irrespective of time of day, in 2013 and 2014. Females were captured using a net (at about 2 m above the ground) in Ise, Mie Prefecture (early July), Choshi, Chiba Prefecture (late July), and Tsukuba, Ibaraki Prefecture (August and September) when foraging swarms appeared at a low height above grasslands. The former two survey fields were near the sea, and the latter field was far from the sea (about 45 km). From the females captured, we used those that were in reproductive stage M, just after sexual maturation, as identified by their worn wing condition, and partly brownish wings without visible damage (Ichikawa and Watanabe 2014). Females in the pre-reproductive and those in older stages were released without taking measurements.
Immediately after capture, the artificial oviposition technique (Ichikawa and Watanabe 2014) was applied to each P. flavescens female at stage M in the survey fields. Females were gently held by the wings, and the abdomen tips were repeatedly dipped vertically into a water-filled vial once per second, to allow the female to release her mature eggs. The water temperature in the vial was equal to the air temperature. Even if a female released no eggs, the dipping procedure was conducted for 1 min, after which the female was considered unable to oviposit. Since females released almost all of their mature eggs, irrespective of the number of mature eggs loaded in their ovaries (Ichikawa and Watanabe 2014; Watanabe et al. 2011), the number of eggs actually released by the females might vary according to their oviposition experience before capture. The Anisoptera, to which P. flavescens belongs, lay several hundred eggs per day (Corbet 1999). When females release a few eggs by the artificial oviposition technique they have finished their daily oviposition activity. The mature eggs remaining in the ovaries might be reserved for oviposition on the following day. Thus, in the present study, females that released more than 150 eggs (the minimum number for the egg-rearing experiment) were considered no longer able to lay more eggs within 1 day. The time required to release eggs was also measured.
The color of fertilized eggs changes slightly, from white to yellow, soon after they are released, whereas unfertilized eggs remain white for at least several weeks. To detect fertility, the color change of each egg was examined under a binocular microscope when eggs were taken from the survey field to the laboratory. In addition, the hind wing length of each female was measured with electronic calipers (accuracy, 0.01 mm).
The egg-rearing experiment was conducted using the fertilized eggs released by females captured in Tsukuba from early August to early September 2014. Five fertilized eggs were randomly chosen from each clutch to measure egg length (accuracy, 0.01 mm) and width (accuracy, 0.01 mm) under a binocular microscope. To calculate egg volume, eggs were assumed to be spheroid, and volume (V) was calculated as:
where a and b represent the length and width, respectively. Then, 150 eggs were facultatively taken from each clutch for rearing. They were divided into five groups, with 30 eggs in each group. Using a syringe, eggs in each group were gently placed in a plastic cup (diameter 12 cm) with water (depth 5 cm). A total of 125 plastic cups were placed in the incubator at 35, 30, 25, 20, and 15 °C under a 14-h/10-h light/dark photoperiod. About half of the water in each cup was renewed once every 3 days.
Eggs in each cup were observed every day to confirm the number of hatched eggs. To avoid the negative effect of larvae on the development of unhatched eggs, they were immediately removed from the cups. The rearing experiment was continued for 90 days after the eggs were released. The eggs that did not hatch by 90 days were observed under a binocular microscope to confirm their developmental condition. After 90 days, several eggs which had not hatched at 15 °C were transferred to water at temperatures of 20, 25, 30, and 35 °C, and then reared for 1 month.
In addition, the critical degree of the water temperature (t 0) was calculated by measuring the developmental speed (V = 1/D) of eggs at each water temperature, where D is egg-stage duration (days). The total effective temperature for the egg stage (K; degree-days) was calculated as:
where T represents the rearing water temperature.
All statistical analyses were performed with JMP IN 11 (SAS Institute). Hind-wing length of females captured in the various seasons, numbers of eggs released, duration of egg release, and egg-release rate were compared by one-way ANOVA. Regression analysis was used to investigate the relationship between water temperature and the developmental speed of eggs for the females collected in early August, late August, and early September. To test whether developmental speed is different among season, an analysis of covariance was used that included water temperature as an independent factor and sampling season as a covariate.
Results
In July 2013, foraging swarms of P. flavescens in Ise and Choshi were easily observed above grasslands near the sea. Each foraging swarm we observed seemed to consist of several dozen adults comprising both sexes at various stages of sexual maturation. We observed four and three females at reproductive stage M, collected in early July at Ise and in late July at Choshi, respectively, each of which released more than 150 eggs.
From August to early September, many foraging swarms containing large numbers of females in reproductive stage M were observed in Tsukuba. The numbers of females that released more than 150 eggs in early August, late August, and early September were ten, 19, and nine, respectively. Although foraging swarms were also observed in late September in Tsukuba, most swarms included both sexes at pre-reproductive stages. Thus, only two females at reproductive stage M were collected in late September at Tsukuba.
There was no significant difference among the seasons for mean hind-wing length of females collected in each season (ANOVA, df = 5, F = 1.48, p = 0.22; Table 1). There was also little variation in body size of P. flavescens females, regardless of season and survey field.
The numbers of eggs released by females that released more than 150 eggs were not significantly different among the seasons (ANOVA; df = 5, F = 0.43, p = 0.82; Fig. 1). The number of eggs released by the artificial oviposition technique was highly variable across seasons, and the median for each season was 1,000 eggs. The only exception was for females in late July at Choshi, suggesting that these females laid mature eggs loaded before capture. Thus, it was deduced that females at reproductive stage M flying during July to September may have about 1,000 mature eggs in their ovaries, except immediately after they oviposit.
The number of eggs released by Pantala flavescens females captured in each season. The boxes show upper and lower quartiles, and the center lines the median. Bars indicate the range of eggs released. The numerals above each box show the numbers of females collected. Filled boxes indicate the seasons when ca. 150 eggs were used for the egg-rearing experiment
The duration of egg release was not significantly different among flying seasons (ANOVA, df = 5, F = 1.11, p = 0.37; Fig. 2). Although the egg-release speeds of females collected in late July and late September were relatively low, the speeds were not significantly different among the seasons (ANOVA, df = 5, F = 1.24, p = 0.31; Table 1). In addition, almost all eggs released by females appeared to be fertilized regardless of sampling season.
The duration of egg release by P. flavescens females collected in each season. The boxes show upper and lower quartiles, and the center lines the median. Bars indicate the range of duration. The numerals above the boxes show the numbers of females collected. Filled boxes indicate the seasons when ca. 150 eggs were used for the egg-rearing experiment
Fertilized eggs collected from ten (early August), nine (late August), and six (early September) females were used for the egg-rearing experiment. All fertilized eggs collected in early August, late August, and early September were oval, and approximately 0.50 mm in length and 0.33 mm in width. The egg volume was 0.028 mm3 regardless of the female’s size or the season (range, 0.44–0.54 mm in length, 0.30–0.40 mm in width).
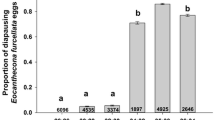
Except for eggs reared at 15 °C, almost all eggs hatched successfully during 90 days irrespective of the rearing temperature. Figure 3 shows changes in the duration of the egg stage of eggs released by females collected in early August, late August, and early September, reared at 15, 20, 25, 30, and 35 °C. At high temperatures (35 and 30 °C), most eggs hatched within a period of 5 days. However, the duration of the egg stage was prolonged by decreasing the water temperature. At a water temperature of 25 °C, about 8 days were needed for the eggs to hatch. Eggs at a water temperature of 20 °C required more than 2 weeks to hatch. Simultaneous hatching in water temperatures of 20, 25, 30, and 35 °C resulted in very small SEs (Fig. 3). No eggs reared at 15 °C hatched until 90 days, irrespective of the females that released the eggs and the collecting season. Several eggs left unhatched at 15 °C were moved to water temperatures of 20, 25, 30, and 35 °C after 90 days. However, no eyespots were observed in these eggs, indicating that they did not start development.
Although there was some variation in the developmental periods of eggs among the females, the developmental speed of eggs was stable at each water temperature and in each season. Figure 4 shows the regressions between water temperature and developmental speed. These relationships were not significantly different among seasons. However, the developmental speed of egg was positively correlated with water temperature (Table 2). The critical water temperatures were 14.5 (early August), 14.3 (late August), and 14.2 °C (early September). The total effective temperatures for the egg stages in early August, late August, and early September were 78.2, 75.2, and 80.6 degree-days, respectively.
Discussion
P. flavescens eggs showed high developmental speed at high water temperatures, requiring about 4 and 5 days at 35 and 30 °C, respectively. Iwata et al. (2009) reported that P. flavescens eggs hatched within 5 days at 30 °C. In central Japan, the water temperatures of ponds and rice paddy fields that served as oviposition sites for P. flavescens increased from summer to early autumn, and exceeded 30 °C (Douma et al. 2010; Kanao et al. 2009). Tachibana and Yakushiji (2012) reported a water temperature of more than 35 °C in a shallow pond artificially established in an urban area in the summer. Even in northern Japan, including Hokkaido, the water temperature of a fish farm pond rose to 25 °C in the summer (Kudou 2012). Therefore, P. flavescens eggs must be able to hatch during the summer to early autumn, even in north Japan. The high egg developmental speed at a high water temperature must facilitate the quick emergence of P. flavescens adults, resulting in a rapid increase in their population in Japan during the summer up to the early autumn. Lutz and Rogers (1991) reported that water temperatures of 25–35 °C were suitable for Erythemis simplicicollis (Say) egg development, and that all its eggs died at 40 °C. On the other hand, the duration of the egg stage in P. flavescens was prolonged with decreasing water temperature (Fig. 3). Eggs required about 8 days or 2 weeks to hatch at respective water temperatures of 25 and 20 °C.
Corbet (1999) pointed out that odonate species need a large quantity of thermal energy for egg development to occur at a low temperature. All P. flavescens eggs reared at 15 °C failed to hatch in the present study, suggesting that eggs might not start to develop immediately after a drop in the water temperature. After 90 days, several eggs unhatched at 15 °C were moved to water at 20, 25, 30, and 35 °C. However, none of these eggs hatched, and eyespots did not appear. Therefore, the critical water temperature for P. flavescens eggs must be 14.2–14.5 °C. Lutz and Rogers (1991) showed that the mortalities of eggs of four libellulid species in the laboratory were nearly 100 % at 15 °C, except for those of Libellula cyanea Fabricius. In general, the critical temperature for Lepidoptera, Coleoptera, and Thysanoptera is about 10 °C (Kiritani 1997), suggesting that insects living in the tropics must have a higher critical temperature than those living in temperate regions.
Sternberg (1995) reported that the relationship between temperature and the developmental speed of Somatochlora arctica (Zetterstedt) eggs varies among seasons, probably due to adaptation to low water temperatures in winter. Corbet (1999) reported that several odonate species could lay both diapause and non-diapause eggs, and that the proportion of diapause eggs increased with the season. However, in P. flavescens, the relationship between water temperature and egg development did not change among seasons (Fig. 4; Table 2). The size of eggs was also similar throughout the flying season (early August to early September). On the other hand, Gribbin and Thompson (1990) stated that the egg size of Pyrrhosoma nymphla (Sulzer) decreased in response to the body size of the female.
Watanabe and Adachi (1987) revealed that the size of mature eggs in Copera annulata (Sélys) decreased with female age. However, the hatching qualities of P. flavescens eggs in the present study were similar among females and seasons, probably because the females sampled were mostly at reproductive stage M. In Japan, the temperature of open waters usually drops below 15 °C during the winter, except in southern Okinawa, which is in a sub-tropical zone (Iwata et al. 2009; Douma et al. 2010). The low water temperature in winter may prevent the overwinter and colonizing of P. flavescens in Japan. On the other hand, gene flow occurs on a global scale among P. flavescens populations (Troast et al. 2016). Part of the summer populations observed in Japan might move to other ideal places as a result of P. flavescens’ high ability to migrate, which would be beneficial to the sharing of genes of this species across the globe. Tolerance to low temperatures in P. flavescens adults and larvae needs to be examined to clarify the basic ecology of this dragonfly, which migrates from tropical to temperate regions.
References
Arai Y (1991) On the drought and cold resistance of Pantala flavescens larvae. Gracile 45:15–22 (in Japanese)
Arai Y (2007) The mystery of red dragonflies. Doubutsu-sha, Tokyo (in Japanese)
Arai Y (2012) Some ecological observations of Pantala flavescens (Fabricius) in Kanto District. Gracile 72:32–39 (in Japanese with English summary)
Bennett S, Mill PJ (1995) Lifetime egg production and egg mortality in the damselfly Pyrrhosoma nymphula (Sulzer) (Zygoptera: Coenagrionidae). Hydrobiologia 310:71–78
Corbet PS (1999) Dragonflies: bhavior and ecology of Odonata. Cornell University Press, Ithaca
Douma A, Harada M, Hiramatsu K, Marui A (2010) Dynamic analysis of chlorophyll-a and nutrients in eutrophic reservoirs by using a water quality model based on species composition of algae. Sci B Fac Agric Kyushu 65:73–89 (in Japanese with English summary)
Gribbin SD, Thompson DJ (1990) Egg size and clutch size in Pyrrhosoma nymphula (Sulzer) (Zygoptera: Coenagrionidae). Odonatologica 19:347–357
Hawking JH, Ingram BA (1994) Rate of larval development of Pantala flavescens (Fabricius) at its southern limit of range in Australia (Anisoptera: Libellulidae). Odonatologica 23:63–68
Hirake T (2012) Marking and flight observation of Pantala flavescens. Gracile 72:42–47 (in Japanese)
Ichikawa Y, Watanabe M (2014) Changes in the number of eggs loaded in Pantala flavescens females with age from mass flights (Odonata: Libellulidae). Zool Sci 31:721–724
Ichikawa Y, Watanabe M (2015) The daily food intake of Pantala flavescens females from foraging swarms estimated by the faeces excreted (Odonata: Libellulidae). Odonatologica 44:375–389
Ichikawa Y, Watanabe M (2016) Daily egg production in Pantala flavescens in relation to food intake (Odonata: Libellulidae). Odonatologica 45:107–116
Inoue K, Tani K (1999) All about dragonflies. TOMBOW, Osaka (in Japanese)
Inoue K, Tani K (2010) All about red dragonflies. TOMBOW, Osaka (in Japanese)
Ishizawa N (2007) Morphological variations in relation to maturation in Pantala flavescens (Fabricius) in central Japan (Anisoptera: Libellulidae). Odonatologica 36:147–157
Iwata N, Akieda N, Hirai N, Ishii M (2009) Seasonal prevalence of the migratory dragonfly, Pantala flavescens (Anisoptera, Libellulidae), in Sakai City, Osaka Prefecture, central Japan. Tombo 51:29–37 (in Japanese with English summary)
Kanao S, Ohtsuka T, Maehata M, Suzuki N, Sawada H (2009) Effectiveness of paddy fields as an initial growth environment for larval and Juvenile nigorobuna Carassius auratus grandoculis. Nippon Suisan Gakkaishi 75:191–197 (in Japanese with English summary)
Kiritani K (1997) The low development threshold temperature and the thermal constant in insects, mites and nematodes in Japan. Misc Publ NIAS 21 (in Japanese with English summary)
Kudou S (2012) Alien fish problems in Hokkaido (largemouth, smallmouth bass and bluegill as invasive alien species). Nippon Suisan Gakkaishi 78:983–987 (in Japanese)
Lutz PE, Rogers A (1991) Thermal effects on embryonic development in four summer species of Libellulidae (Anisoptera). Odonatologica 20:281–292
Pilon J-G, Pilon L, Lagacé D (1989) Notes on the effect of temperature on egg development of Leucorrhinia glacialis Hagen (Anisoptera: Libellulidae). Odonatologica 18:293–296
Punzo F (1988) Effects of low environmental pH and temperature on hatching and metabolic rates in embryos of Anax junius Drury (Odonata: Aeshnidae) and the role of hypoxia in the hatching process. Comp Biochem Phys 91:333–336
Schenk K, Suhling F, Martens A (2004) Egg distribution, mate-guarding intensity and offspring characteristics in dragonflies (Odonata). Anim Behav 68:599–606
Siva-Jothy MT, Gibbons DW, Pain D (1995) Female oviposition-site preference and egg hatching success in the damselfly Calopteryx splendens xanthostoma. Behav Ecol Sociobiol 37:39–44
Soeffing K (1986) Ecological studies on eggs and larvae of Leucorrhinia rubicunda (L.) (Odonata, Libellulidae). Jber ForschInst Borstel 1986:234–237
Sternberg K (1995) Influence of oviposition date and temperature upon embryonic development in Somatochlora alpestris and S. arctica (Odonata: Corduliidae). J Zool 235:163–174
Tachibana D, Yakushiji K (2012) Influence of a biotope built in the metropolitan on the animals which appeared, and problems which the biotope holds now. AIJ J Technol Des 18:391–396 (in Japanese with English summary)
Troast D, Suhling F, Jinguji H, Sahlén G, Ware J (2016) A global population genetic study of Pantala flavescens. PLoS One 11:e0148949. doi:10.1371/journal.pone.0148949
Watanabe M (1999) Basic research on the Odonata larvae as a teaching material from the swimming pool of elementary and junior high schools. Jpn J Biol Educ 39:65–76 (in Japanese with English summary)
Watanabe M (2015) Ecology of Odonata. University of Tokyo Press (in Japanese)
Watanabe M, Adachi Y (1987) Fecundity and oviposition pattern in the damselfly Copera annulata (Selys) (Zygoptera: Platycnemididae). Odonatologica 16:85–92
Watanabe M, Kato K (2012) Oviposition behaviour in the dragonfly Sympetrum infuscatum (Selys) mistaking dried-up rice paddy fields as suitable oviposition sites (Anisoptera: Libellulidae). Odonatologica 41:151–160
Watanabe M, Suda D, Iwasaki H (2011) The number of eggs developed in the ovaries of the dragonfly Sympetrum infuscatum (Selys) in relation to daily food intake in forest gaps (Anisoptera: Libellulidae). Odonatologica 40:317–325
Acknowledgments
We would like to thank Mr. T. Konagaya and Mr. G. Takahashi for helping to rear the eggs.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ichikawa, Y., Yokoi, T. & Watanabe, M. Thermal factors affecting egg development in the wandering glider dragonfly, Pantala flavescens (Odonata: Libellulidae). Appl Entomol Zool 52, 89–95 (2017). https://doi.org/10.1007/s13355-016-0457-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13355-016-0457-9