Abstract
Osteoarthritis is one of the foremost disabling disorders in the world. There is no definitive treatment to prevent the progression of osteoarthritis. Hence, palliative treatment aims at minimizing pain, disability and improving function, performance and quality of life. Oral administration of nonsteroidal anti-inflammatory drug is associated with number of adverse effects and reduced therapeutic efficacy. Intra-articular injection has been the preferred route of drug administration. However, the clearance of drug from the arthritic site, risk of infections, cost and the pain associated with frequent injections make this route highly non-compliant to patients. Since osteoarthritis is a chronic condition which requires treatment for prolonged duration, there is an urgent need for another administration route which circumvents the hindrances linked with intra-articular route. Transdermal route across the skin locally at the osteoarthritis site could help in surpassing the disadvantages associated with intra-articular route. However, traversing skin barrier and reaching the chondrocytes with sufficient amount of the drug is extremely difficult. Nanocarrier-based approaches could hold an answer to the said shortcomings owing to their reduced size, targeting tunability and site specificity. In this article, we discuss the pathophysiology of osteoarthritis, molecular targets, and utilization of nanocarrier-based approaches to strategize the treatment of osteoarthritis in a new direction, i.e. topical delivery of nanocarriers in osteoarthritis.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The term ‘osteoarthritis (OA)’ was coined by Archibald Edward Garrod in 1890 [1]. It is one of the most disabling painful conditions in the world [2]. OA is a prolonged debilitating disorder which involves the migration of inflammatory mediators to the synovium. This leads to severe inflammation and gradual loss of the bone and joint cartilage. OA is prevalent in the elderly and the most frequent sites influenced include knees, hips, spine, feet and hands [3]. The incidence of OA upsurges with age; however, women are more prone compared to men [4]. The world witnessed about 303.1 million cases of hip and knee OA since 2017 [5]. The pooled global pervasiveness of knee OA was 16.0% (95% CI, 14.3%–17.8%) in individuals with age 15 and above and was 22.9% (95% CI, 19.8%–26.1%) in individuals aged 40 and above. Correspondingly, there are approximately 654.1 (95% CI, 565.6–745.6) million people (40 years and elder) with knee OA in 2020 [6, 7]. Figure 1 demonstrates the prevalence of OA across various continents throughout the globe [8, 9].
To date, there is no cure for OA; hence, symptomatic treatments are available to alleviate pain and inflammation. Symptomatic treatment alternatives used for OA include non-steroidal anti-inflammatory drugs (NSAIDs) like ibuprofen, diclofenac, and naproxen along with COX (cyclooxygenase) inhibitors like celecoxib, etoricoxib and parecoxib [10]. Acetaminophen is recognized as first-line therapy by the American College of Rheumatology owing to its minimal adverse effects. Intra-articular injections containing corticosteroids and hyaluronic acid (Hyalgan®) have also demonstrated efficacy in providing symptomatic relief [11]. Apart from chemotherapy, several non-medicinal and non-surgical interventions including physical therapy, acupuncture and chiropractic care are available to improve joint function [12, 13]. Intra-articular injection of disease-modifying osteoarthritic drugs and macromolecules into the joint could improve bioavailability locally and attenuate systemic toxicity. However, they are rapidly cleared from the joint owing to the microvasculature surrounding the joint resulting in diminished drug action at the bioactive site resulting in poor therapeutic efficacy. Additionally, intra-articular injections succumb extreme pain during weekly dosing intervals and could lead to sepsis if appropriate precaution is not taken [14]. Table 1 shows the current treatment options for OA. Since the therapy for OA continues for a prolonged duration, intra-articular injections prove to be highly patient non-compliant. Topical drug delivery could prove to be a safer option for patients since unwanted gastrointestinal, cardiovascular and renal adverse effects associated with oral delivery could be evaded [15]. However, the drug permeation across the transdermal route becomes questionable. Highly lipophilic and hydrophilic molecules are not amenable to be delivered across the skin layers to the synovium. Additionally, the cytochrome P450 (CYP450) enzymes residing in the skin actively metabolize the drug moieties which diminishes their efficacy. To circumvent these pitfalls, incorporation of therapeutic moieties within the nanoparticulate matrix could lead to superior therapeutic efficacy when administered via the topical route. Nanotechnology-based medicine could hold the key to traversing the skin barrier, targeting the synovium and surpassing the obstacles of the current therapy [16]. Through this article, we emphasize the pathophysiology and different molecular signalling pathways controlling OA, drug repurposing and topical nano-medicine-based strategies as a new direction in OA therapy. Figure 2 depicts the physiological changes in the joints of OA patients.
Pathophysiology and molecular signalling cascades
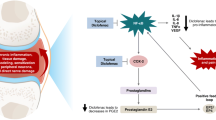
OA is a biomechanical chronic insidious disorder residing within the cartilage and subchondral bone which influences the entire joint function [20]. Complex pathological changes within the chondrocyte matrix are a major hallmark in OA [21]. OA could be divided into primary and secondary OA. Primary OA is idiopathic in nature, whereas secondary OA possesses numerous reasons such as congenital abnormalities, trauma and inflammatory arthropathies [22]. During the initial stages, chondrocyte injury occurs attributed to pre-disposing genetic and biochemical factors and ageing. leading to mechanical wear and tear. In early OA, enhanced proliferation of chondrocytes results in higher levels of matrix metalloproteinase (MMP) and inflammatory mediators such as collagenases, proteases and proteoglycans (PG) [23]. This results in matrix restructuring and secondary inflammatory changes in synovial tissues and sub-chondral bone. Once mild OA occurs, its progression is inevitable. During the later stages of OA, chronic injury and inflammation lead to chondrocyte dropout [24]. Chondrocyte proliferation causes swelling and cleaves collagen-2 fibres, fissures and clefts at the articular surface (cartilage) which finally leads to chondrocytes death. When this cartilage sloughs off, sub-chondral bone transforms into the new articular surface. This causes increased friction resulting in sclerosis of cancellous bone and forms bone spurs called osteophytes [25]. Tissue macrophages are activated which further promotes stimulation of inflammatory cytokines like interleukin (IL)-1B and tumour necrosis factor (TNF)-ɑ, IL-6 [26, 27]. IL-1 and IL-6 trigger mononuclear precursor differentiation in osteoclasts and stimulate bone resorption in osteoclasts via the receptor-activator of NF-κβ ligand (RANKL), which has a catabolic effect on bone. By enhancing the surface expression of TNF receptor (TNFR) in chondrocytes, IL-1 has a significant role in the onset and progression of OA [28]. In bone remodelling, TNF induces indirect osteoclast activation through RANKL. RANKL is the most important cytokine in transforming osteoclasts into mature multinucleated osteoclasts [29]. During inflammation, bone macrophages fuse and proliferate into multinucleated cells. M-CSF (macrophage colony-stimulating factor) has been shown to promote macrophage proliferation and differentiation. RANKL stimulates osteoclast proliferation after being stimulated by M-CSF. Multinucleated giant cells originating from RANKL-induced fusion-competent osteoclasts develop to form multinucleated osteoclasts or giant cell. NF-κβ signalling pathway is stimulated when catabolic factors like TNF-α or IL-1 are activated. The transcription factor NF-κβ is associated with joint inflammation and tissue degradation. NF-κβ signalling pathway downregulates chondrocyte anabolism concomitantly triggering metalloproteinases (specifically MMP-1, MMP-3 and MMP-13 in OA) and a dis-integrin and metalloproteinase with thrombospondin motifs (ADAMTS) specifically ADAMTS4 and ADAMTS5, which results in destruction of articular cartilage. Matrix metalloproteases (MMPs), also known as matrixins, are zinc-dependent endopeptidases which control the composition of the cell matrix and are thought to be the main proteases responsible for the degradation of all extracellular matrix (ECM) components [30]. Since they are rate-limiting in the phase of collagen annihilation, MMP-1 and MMP-13 play a major role in OA. Synovial cells that line the joints produce MMP-1 while chondrocytes in the cartilage produce MMP-13. MMP-13 also destroys proteoglycans and thus serves a dual function in matrix degradation. Other MMP enzymes like MMP-2, MMP-3 and MMP-9 deteriorate non-collagen matrix components which worsen the osteoarthritic condition of the joint [31]. Transcriptional activity of nuclear factor (NF)-κβ results in inducible nitric oxide synthase (iNOS) overexpression and enhanced NO release along with the expression of matrix-degrading proteinases (MMP) [32]. NO produced by iNOS adds to the progression of OA by altering ECM homeostasis by enhancing MMP activity and inhibiting aggrecan and collagen biosynthesis [32]. Vascular endothelial growth factor (VEGF) is also linked with OA. VEGF and E-selectin together with inflammatory cytokines will promote inflammatory process. Mitochondrial failure in chondrocytes which is associated with decreased activated protein kinase (AMPKα), peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), sirtuin-1 (SIRT-1), nuclear respiratory factor-1 (NRF-1), nuclear respiratory factor-2 (NRF-2) and mitochondrial transcriptional factor A (TFAM) activity also plays a prominent role in OA progression [33].
OA is regulated by several other pathways which include Wnt/β-catenin signalling, PI3K/Akt/mTOR pathway, notch signalling pathway, SIRT1/AMPK pathway, hippo pathway-YAP/TAZ signalling and disruption of telomeric silencing 1-like (DOTIL). The involvement of these pathways in induction and development of OA is depicted in Fig. 3.
Recent advancements in nanocarrier-based topical delivery for the management of OA
The attributes and pitfalls associated with the intra-articular route have been discussed in detail in the previous sections. Certain attributes like target specificity and drug availability at the arthritic site make this route desirable for patient therapy. However, the pain associated with the need for frequent administration, rapid clearance of drugs, cost, high probability of sepsis, etc. reduces its popularity during long-term treatment of OA patients. Therefore, there is a need to find an alternative route as well as dosage form to circumvent the pitfalls associated with the intra-articular route. This is achievable by the employment of a topical route in delivering the drug across the skin layers to reach the chondrocytes. However, adequate penetration of drugs into the chondrocytes traversing various layers of the skin is another challenge altogether. Nanocarrier-based topical delivery systems could be tuned to possess target specificity, prolong drug action, limit drug clearance, circumvent the pain associated with intra-articular injections, etc. [34]. Figure 4 depicts various topical nano-medicine-based strategies for OA treatment.
Microemulsion
Microemulsions are translucent ternary systems consisting of oil, surfactant and cosurfactant with droplet diameters ranging from 10 to 100 nm [35]. Microemulsions have several advantages like enhanced solubility and dissolution rate, thermodynamic stability, increased skin permeation and biocompatibility [36]. One of the strategies to administer the drug topically at the targeted site, i.e. chondrocytes, includes the incorporation of microemulsion within a hydrogel matrix. To impart site specificity, stimuli-responsive hydrogel matrices which release the entrapped microemulsion in response to pH, temperature, redox, etc. at the OA site could be used. Compared to traditional systems, it is thermodynamically more stable and hence possesses prolonged stability and shelf life [37]. These attributes could harness the clinical translation potential of microemulsions [38]. Hu and co-workers found that the amount of 3,5,4′-trimethoxy-trans-stilbene (BTM) permeation across skin layers was substantially increased by both microemulsion and microemulsion-based hydrogel formulations compared to free drug. For microemulsion and microemulsion-based hydrogel formulations, the total amount of BTM permeated after 12 h was 3.25 and 1.96 times greater than that of emulsion gel (EG). In a rabbit model of papain-induced OA, topical delivery of BTM microemulsion-based hydrogel (BTM-MBH) displayed a significant anti-OA effect, with reduced levels of pro-inflammatory cytokines. After treatment with BTM-MBH for 4 weeks, 1% BTM-MBH showed maximum reduction in TNF-α and IL-1β compared to 0.5% BTM-MBH, 1% BTM-EG, and 1% diclofenac gel treatment groups. Owing to the higher skin permeability of the microemulsion, symptomatic relief was better in the case of BTM microemulsion-based hydrogel than BTM emulsion-loaded gel [39]. Ibuprofen, a NSAID, is highly efficient in the management of OA. To decrease the adverse effects and circumvent its extensive first-pass metabolism, ibuprofen was incorporated into various microemulsions and the ability of different microemulsions to deliver ibuprofen across the skin was investigated. In vitro permeation study revealed that microemulsions improved the permeation rate of ibuprofen from 5.72–30.0-fold compared to plain drug saturated solution. However, further studies are required to prove its efficacy [40]. Goindi and co-workers formulated tenoxicam (TNX) microemulsion-based hydrogel for the treatment of arthritis. Ex vivo permeation study indicated superior skin permeation of tenoxicam microemulsion (64.647 ± 0.97% (TNX 03) and 70.829 ± 0.84% (TNX 04)) compared to conventional cream (27.972 ± 0.81% (TNX 01)) and aqueous suspension (7.31 ± 0.63% (TNX 02)). Amongst the two microemulsions, TNX 04 showed better permeation which might be associated with the combined action of oleic acid and ethanol. TNX 03 retention was found to be 11.429 ± 0.399%, which was 4.6-fold greater than TNX 01 (2.469 ± 0.24%) and 11.5-fold greater than TNX 02 (0.988 ± 0.18%). TNX04, on the other hand, showed skin retention of 13.551 ± 0.25%, which was 5.5 times that of the traditional cream TNX 01 and 13.7 times that of TNX 02. Skin retention was also remarkably higher in microemulsions as compared to conventional cream and suspension. Additionally, anti-inflammatory efficacy using air pouch model was studied. Microemulsions TNX 03 and TNX 04 were found to be more effective than traditional topical formulations, with potency equal to that of oral dosage forms. In carrageenan-induced hind paw oedema in rats, TNX 03 and TNX 04, inhibited oedema significantly, with 64.54% and 67.89% inhibition compared to 21.57% and 6.31% inhibition with TNX 01 and TNX02, respectively. Amelioration in skin permeation could lead to greater drug accumulation within the chondrocytes leading to superior therapeutic activity circumventing the pain- and sepsis-associated adverse effects of the intra-articular route [41]. Self-emulsifying systems based on topical hydrogels have shown promising activity in topical disorders like cutaneous leishmaniasis [42] and antiaging [43] but have not been explored in osteoarthritis. Researchers could extrapolate and claim an advantage over this approach as a novel futuristic avenue in osteoarthritic drug delivery.
Liposomes
Liposomes consist of vesicles consisting of one or more concentrically arranged lipid bilayers containing an aqueous core. Hydrophilic entities could be incorporated in the aqueous core while hydrophobic entities are localized within the phospholipid bilayer [44]. Liposomes are preferred in topical administration as they are easily absorbed into the skin for local action, thus preventing side effects [45]. Concentration of lipid content is responsible for modifying permeability of liposomes via topical route. Liposome acts as reservoir and thus can be used for controlled and sustained release of drug in topical therapies [44]. In one of the studies performed by Frisbie, arthroscopically induced OA in horses was treated with diclofenac-loaded liposomal cream. Results showed that diclofenac liposomal cream had disease-modifying effects. Treatment of horses with diclofenac liposomal cream exhibited improved articular glycosaminoglycan content, decreased carpal bone sclerosis and overall gross cartilage erosion. This formulation offered locally improved topical delivery without reaching a plasma concentration susceptible to systemic adverse effects. In the USA, a topically applied diclofenac liposomal cream has been approved for the treatment of horses with OA [46, 47]. In a randomized clinical trial, 30-day therapy with Leech saliva extract (LSE) liposomal gel relieved the pain by up to 50% and attenuated joint inflammation and stiffness which could be potentially translated to humans for the management of OA [48]. A randomized, placebo-controlled, double-blind clinical trial of diclofenac lipogel in patients with signs and symptoms of OA showed significant improvement in the treatment of OA compared to the marketed product Voveran® Emulgel®. The diclofenac lipogel showed an improved Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) compared to the marketed formulation. Along with efficacy, the diclofenac lipogel also showed improved safety due to the better tolerability of the formulation in comparison to marketed as well as placebo formulation [49].
Transfersomes
Transfersomes are ultra-deformable lipid vesicles consisting of an aqueous core surrounded by a lipid bilayer introduced by Gregor Cevc in 1990. Transfersomes are more amenable to the transport of therapeutic agents across the skin owing to their elasticity compared to traditional liposomal vesicles [50]. Enhanced flexibility imparted by edge activators, render relative ease to squeeze out through the pores in the stratum corneum leading to improved penetration of intact vesicles. The deformability prevented rupturing of transfersomal vesicles while traversing the skin barriers [51]. Edge activator/surfactant provides elasticity and permeability to lipid bilayer structure [52]. The ability of transfersomes to deform and penetrate through deeper layers of skin makes them carriers of choice over liposomes and niosomes [53]. Rother and co-workers compared ketoprofen-loaded transfersomal gel, placebo and oral celecoxib in treating patients with OA. Ketoprofen transfersomes were found to be comparable to the oral dose of celecoxib and superior to placebo in all efficacy outcomes for knee OA. Gastrointestinal adverse effects were averted by ketoprofen transfersomal gel. In addition, systemic exposure to ketoprofen incorporated within the transfersomal gel was significantly lower than oral administration. Drug molecules are prone to cutaneous clearance through the microvasculature once they have crossed the stratum corneum layer. Incorporation of ketoprofen within transfersomal vesicles shields the drugs and prevents their metabolism and clearance by cutaneous cytochrome enzymes. Thus, transferosomes loaded gels are versatile carriers in significantly improving drug concentration at the target site [54]. A clinical trial of ketoprofen transfersomal gel (IDEA-033) was carried out in OA patients. The results of the clinical trial have shown promising results as compared to the marketed conventional gel in terms of alleviation of symptoms of OA. The transfersomal gel formulation was withdrawn from the market due to the higher cost associated with this formulation as compared to the marketed conventional gel [55].
Cubosomes
Cubosomes are bi-continuous cubic bilayer structured vesicles that may contain hydrophobic, hydrophilic and amphiphilic molecules for drug delivery [56]. Amphiphilic bilayer has the ability to self-assemble in water to form cubosomes in presence of stabiliser by crosslinking of hydrophobic domain [57]. Cubosomes are thermodynamically stable cubic vesicles with a constrained liquid crystalline phase resembling a cavernous (cave-like) structure [58]. Stratum corneum provides high resistance for topical delivery, but cubosomes, due to its bio adhesive property, improve skin permeation thus effectively delivering the drug with less irritation [59]. These are versatile formulations that can be administered percutaneously with the key benefit of supplying and solubilising poor water-soluble drugs effectively [60]. Puglia et al. formulated curcumin cubosomes and evaluated them for different parameters. Topical administration of curcumin in diseases such as OA may not be used due to the low solubility and chemical instability of curcumin, although it has anti-inflammatory efficacy. Hence, curcumin was formulated into two different cubosomes using different emulsifiers, viz, pluronic F-127 (MAD-A) (mono-olein aqueous dispersion) and sodium cholate-sodium caseinate (MAD-B). Curcumin cubosomes showed more than 98% entrapment efficiency using the emulsifier Poloxamer-407. Curcumin-loaded cubosomes containing pluronic F-127 were found to be stable and retarded degradation of curcumin over time. MAD-B which consisted of sodium cholate-sodium caseinate as an emulsifier was comparatively less stable and possessed lower shelf life due to presence of mixture of different vesicles and hexosomes. T1/2 values for MAD-A and MAD-B are approximately 2 years and nearly 10 months, respectively. However, both MAD-A and MAD-B showed similar AAPH (2,2′-azobis (2-methyl propionamide) dihydrochloride) scavenging activity as well as iNOS (inducible nitric oxide synthase) and COX-2 inhibition activity than curcumin alone. This is linked to ability of MAD-A and MAD-B to react more rapidly with AAPH to form dimers. Incorporation of curcumin into cubosomes also extended its anti-inflammatory activity and is capable of controlled drug diffusion via skin [61]. Elakkad et al. prepared tenoxicm-loaded hyalcubosomes. The formulation showed improved anti-inflammatory activity in rats. The preliminary clinical study in patients with knee osteoarthritis showed the safety and efficacy of the developed formulation through the treatment period of eight weeks [62]. Based on the key findings above, cubosomes can be used as a highly promising nanocarrier for the treatment of OA.
Sequessome
Sequessome is a phospholipid bilayer vesicle free of drugs with an ultra-deformable functionality [63]. They are capable of entering synovial space via topical route. TDT-064 is a phospholipid bilayer aqueous gel which is used in the management of OA topically [63]. Sequessome possesses bio-lubricant activity; hence, its use in topical therapy for management of OA has led to interest in these nanocarriers. Due to their comparatively large size, these ultra-deformable phospholipid vesicular systems cannot be cleared by cutaneous blood microcirculation [64]. They are transferred and penetrated into deeper tissues below the application site with the interstitial fluid [65]. For patients with comorbidities like GI disorder, cardiovascular diseases, diabetes, hypertension, renal diseases and hepatic diseases, which make them liable to the adverse effects of NSAIDs and put them at risk, TDT-064 is a possible drug-free therapy of choice promoting its possible use for a large group of OA patients [66]. In a randomized clinical trial, the WOMAC subscales for pain, work and stiffness demonstrated progressive and clinically meaningful changes in patients treated with 2.2 g or 4.4 g of TDT-064 for 12 weeks, equivalent in magnitude to those found in patients receiving topical IDEA-033 (ketoprofen transferosomal gel) and oral celecoxib [67, 68].
Solid lipid nanoparticles
Solid lipid nanoparticles (SLN) are colloidal nanocarriers that incorporate solid biodegradable lipid within the matrix surrounded by stabilising surfactant [69]. SLN offer controlled drug release and enhanced stability by preventing degradation of active moiety incorporated [70]. The excipients used in the preparation of SLN comply with generally regarded as safe (GRAS) status [71]. Film formation on the skin imparts an occlusive character to the skin by preventing transdermal water loss and enhancing skin hydration. High loading drug capacity, good patient compliance and local action enhancing efficacy are the main advantages of SLN topical drug delivery systems [72]. Piroxicam is a widely used NSAID which may be used in the treatment of OA, but it is known to have GI side effects and cardiovascular risks when taken orally and intravenously [73]. Mohammadi and colleagues studied the percutaneous penetration of piroxicam-loaded SLN gel in comparison with the commercial gel. In vitro permeation study revealed that piroxicam-loaded SLN incorporated into gel formulation displayed about 148% greater permeation than commercial gel. But enhancement in in vivo anti-OA activity was not explored. The potential application of SLN as a versatile carrier for OA treatment is yet to be explored.
Nanostructured lipid carriers
Nanostructured lipid carriers (NLC) are second generation of lipid nanoparticles comprising a mixture of solid and liquid lipids integrated into a surfactant stabilised aqueous solution and has been identified lately in 1990s [74]. The lipid matrices utilized in NLC preparation are derived from the human body and possess GRAS suggesting augmented biocompatibility [75]. They inherit certain attributes like sustained release profile, versatile carrier for hydrophilic drugs to enhance their transdermal permeation, biocompatibility, etc. The presence of liquid lipid increases membrane fluidity and improves skin permeation compared to SLN [76]. When the liquid lipid is incorporated into the nanoparticulate matrix, amount of imperfections in the solid core matrix increase, leading to reduction in crystallinity thereby promoting enhanced drug loading and preventing drug leakage [77]. NLC modify intercellular packaging and reduce the packaging between the corneocytes, leading to an increased inter-corneocyte space that enables deeper drug penetration into the skin. On topical application, NLC forms a monolayer film responsible for the occlusion effect, preventing water from evaporating from the skin, increasing skin hydration, resulting in better penetration. At 35% crystallinity, an efficient occlusive effect is observed [78]. Solid to liquid lipid proportion ratio, concentration and stabiliser composition affect the NLC particle size and surface charge. Kaur et al. formulated diflunisal-loaded lipid-based nanocarriers (DIF SLN) and evaluated its anti-inflammatory action in three different in vivo models. Compared with traditional cream, the findings of all three models showed substantially improved anti-inflammatory efficacy with DIF SLN gel. The anti-inflammatory activity was 2.3-fold greater in the carrageenan-induced paw oedema model compared to the conventional cream. In contrast with the traditional oral dosage, higher therapeutic efficacy was observed at a much lower dose. The unique nano-scaled architect of NLC as well as the occlusive effect on the skin increases its permeation into deeper layers of the skin,justifying better efficacy by the topical route [79]. Thus, the results indicate superior therapeutic potential for NLC with enhanced efficacy, safety and compliance projecting its use in the near future.
Safety and tolerability of topical nanocarriers
Several studies have been reported on the toxicity of nanomaterials over the past few years. Topical nanocarriers have been investigated over a decade and proven to offer several advantages over other drug delivery platforms [80]. However, tremendous increase in the use of nanoformulations requires the establishment of safety for their clinical use. Toxicity of nanoformulations differs for different nanomaterials depending upon their origin, i.e. lipid, polymer or metal-based [81]. Nanometrology becomes more complex for dynamic nano-scale systems owing to their reduced size which renders alterations in physicochemical attributes [82]. Topical route is advantageous in diminishing the systemic adverse effects. Employment of GRAS excipients could help in circumventing the safety considerations. Safety and tolerability could be established by investigating the presence of signs of skin irritation after 4 h of application of the formulation [83]. Aspects influencing the skin irritation potential include the amount of drug, skin permeation rate, residence time and frequency of administration [84]. For topical delivery, non-ionic surfactants are relatively a safer choice owing to their reduced sensitization potential. Draize test is widely used to assess skin irritation potential. The formulation is applied on shaved rabbit skin checked for oedema/erythema after 1, 24, 48 and 78 h with the help of scoring [78]. Cell lines like human keratinocytes (HaCaT) [85], human foreskin fibroblast (HFF) cell line [86] and L-929 cells (mouse fibroblasts) could also be used to determine the safety and tolerability of the formulation. Repeat insult patch tests for 24 h and 48 h or cumulative 21-day irritation examination could also be used to prove safety of the formulation topically [87]. The reconstructed human epithelium models (RhE) have been found to be the most popular model for the evaluation of in vitro skin irritation potential of topical formulations. There are various RhE models which have been accepted by OECD TG 439 including EpiDerm™, EpiSkin™, Keraskin™, LabCyte EPI-MODEL24 SIT and SkinEthic™ [88].
Current clinical trials for topical delivery
The clinical significance of the application of topical route in OA management has attracted researchers’ attention across the globe. Currently, only one nanocarrier-based clinical trial comprising of establishing safety and efficacy of treatment with 3% Diclofenac nanoemulsion cream versus placebo in patients with knee OA has been carried out (NCT00484120). This study included a total of 126 subjects randomized to receive either a 3% diclofenac nanoemulsion cream or a placebo cream respectively. This step could pose as a dawn of application of nanotechnology as carriers in topical delivery for OA. Various other ongoing trials on topical delivery platforms have been displayed in Table 2.
Barriers to clinical translation of nanocarrier-based topical delivery
The advantages associated with the nanocarrier-based drug delivery systems include nanometric size and site-specific delivery with programmed release have made their path to the market. However, these drug delivery systems still face certain hindrances to get into the marketplace and reach the patients in need. The challenges are mainly associated with stability considerations, complex structural characterizations, circumvention of physiological barriers, safety concerns, scalability and technology transfer obstacles accompanied by regulatory expectations which decelerate the clinical translation of nanocarriers. These can be divided into four categories namely formulation and scale-up challenges, regulatory challenges, poor understanding of permeation mechanisms and safety concerns.
The primary challenge faced by nanocarrier formulation is reproducibility followed by another bigger challenge as scalability at the industrial level under good manufacturing practice (GMP) environment [89]. Critical quality attributes (CQAs) which include particle size, shape, drug loading, crystallinity, release and surface functionality (charge, hydrophobicity, hydrophilicity) significantly influence the quality target product profile attributed to its susceptibility towards diminutive changes in the formulation parameters thereby affecting its efficacy. Apart from reproducibility, scale-up is one of the biggest challenges faced by the commercial development of nanocarrier-based formulations [90]. The laboratory-level development and optimization of these formulations can be achieved relatively easily while large-scale development faces several issues. As compared to already established conventional dosage forms, nanocarrier drug delivery systems have less probability of technology transfer from lab to pilot to large scale.
The majority of the approaches followed for the manufacturing of nanocarriers include bottom-up and top-down approaches. In bottom-up approach, the formulation begins with the dissolution of molecules and then precipitation of nanocarriers while the top-down approach involves size reduction of large drug molecules into smaller ones [91]. The bottom-up approach involves the use of organic solvent for the nanoprecipitation and traces of the removal of these organic solvents is a challenging process owing to its time consumption and elevated cost by introduction of solvent removal step. Therefore, this method is less popular on the industrial scale [92]. Very few studies have been reported for development of nanocarriers from lab scale to scale up with the use of a bottom-up approach. One of the examples includes the preparation of ibuprofen nanoparticles from lab scale to pilot scale development (batch size from 6 mL to 1.5 L). Ibuprofen nanoparticles were prepared with the help of salting out, emulsification-diffusion and nanoprecipitation methods. The nanoparticles were prepared by using Eudragit® L100-55 as a polymer while poly (vinyl alcohol) as an emulsifying agent. The nanoparticles have been found to be reproduced at both lab as well as pilot scales but there was a slight decrease in the particle size and drug loading at the pilot scale [93].
The manufacturing as well as characterization of nanocarriers are difficult to predict during scale-up as these are affected by properties of raw material attributes and processing conditions. The process analytical technology (PAT) can be a useful technique in order to monitor the quality of the product [94]. The application of PAT techniques for the manufacturing process has been encouraged by FDA in order to obtain real-time data and build quality assurance [95]. PAT can provide guidance regarding the optimization and scale-up of the manufacturing processes along with the information about CQAs in order to improve the finished product quality. Along with PAT, QbD and multi-variate statistical tools could help overcome scalability-associated challenges. Knowledge of the design space, desirability, predictability and efficient control over the experimental factors and interrelationships could be beneficial in lab to large-scale production transition [96].
Due to the benefits associated with nanocarrier-based drug delivery systems, the amount of research is going on in this field and some of the products are also entering clinical trials [97]. But these products need to meet the criteria assigned by regulatory bodies with respect to their safety and efficacy. According to regulatory guidelines, the active pharmaceutical ingredient present in nanocarrier represents specifications that need to be analysed for the regulatory approval process [98]. In the case of biological-based active molecules such as antibodies, proteins and peptides, the innovator has to follow the regulations specified for the biological medicinal products along with regulations mentioned for new chemical entities [99]. The United States Food and Drug Administration (US-FDA) and the European Medical Agency (EMA) have approved various nanocarrier-based drug products to date. However, a proper regulatory guidance related to the characterization of nanocarrier-based drug delivery systems is still lacking [100]. Currently, these products are getting approved based on a conventional basis. Because of the complex nature of these drug delivery systems, it is necessary to evaluate their safety and toxicity and this becomes one of the hurdles in the regulatory approval process [99]. In our previous article, we have emphasized the applications of the 505(b)(2) pathway and various ways to overcome the regulatory hurdles with the help of nanotechnology-based approaches [101].
Apart from lacunae associated with the current guidance, certain steps taken by regulatory authorities such as the definition of nanomaterials given by FDA guidance, “considering whether an FDA-regulated product involves the application of nanotechnology” [102], along with collaboration of FDA and the European Technology Platform on Nanocarrier (ETPN) with the Nanotechnology Characterization Laboratory (NCL) and European Nano-Characterization Laboratory (EUNCL) respectively in order to encourage the regulatory review and in-depth characterization of nanocarrier products represents the positive side of it [103].
Another concern is the cost associated with the development and manufacturing of nanocarriers. The additional cost is required for the development of nanocarriers including raw materials and regulations which is about 15% higher than the conventional medication [104]. However, cost–benefit analysis is another criterion which is required to be taken into consideration towards the patient. The balance of cost and benefit inclines towards the benefit for disabling and fatal diseases like osteoarthritis and cancer. Hence, nanomedicine-based strategies could prove to be fruitful even with 15% greater costs. The research carried out by Tufts Center for the study of drug development has reported that for the profit of nanocarriers without considering advertising and marketing expenses there is a need for around 2870 million US$. The cost for the development of an approved product is estimated at $1395 million out of which the cost for a clinical trial estimates at about $1012–1744 million. As the product development requires a long time until it gets marketed therefore by reducing the cost due to the patent expiration, inflation and discount rates, then the total costs are found to be around $2558 million [105].
Before entering the market pharmaceutical products have to go through rigorous trials including preclinical and clinical to prove their safety and efficacy. Along with safety and efficacy, it also requires a proper understanding of pharmacokinetic parameters of the products. These in vivo evaluations of pharmacokinetics, efficacy and toxicity are expensive. Even though there are in vitro alternatives available for in vivo experimentation, it is very difficult to recreate in vivo conditions due to complexities. For example, in vitro cell culture models do not involve biological fluid flow which influences adsorption and endocytosis dynamics compared to newer models which further alter activity [105]. Therefore, it is necessary to evaluate pharmacokinetic, efficacy and toxicity studies by developing various animal models which can mimic human body conditions. Apart from in vivo experimentation, microfluidic technology also mimics fluid flow conditions and has much potential in the clinical translation of nanocarriers from lab scale [106]. The physicochemical properties of nanocarriers are affected by small changes in formulation parameters thereby altering their absorption, distribution, metabolism and excretion (ADME), safety and efficacy profile. These properties include particle size distribution, shape and surface charge are primarily important. The nanocarriers are reactive due to their particle size in nano-range and high surface-to-volume ratio and can alter the therapeutic effect due to their interaction with the biological surfaces. Small-size nanocarriers are eliminated by renal excretion while larger sized nanocarriers are taken up by mononuclear phagocytic cells [107].
One of the concerns for the topical delivery of nanocarriers for OA is the amount of drug reaching the required site of action. The permeability of nanocarriers to reach the deeper layers of skin through topical route still remain a challenge. Certain authors have suggested the use of microneedles before applying topical formulation. They have found the increase in permeation of drug through the skin for the first hour due to the formation of micropores into the skin. But it was observed to decrease during an extended duration of time [108]. The reason behind the decrease in the permeation in the deeper layers of skin is due to the resealing of the pores within 2 h after the application of microneedles [109].
It is necessary to evaluate the efficacy of the formulations either in vivo or in vitro models which mimic the bone microenvironment in order to warrant the clinical translation of these nanocarriers. To mimic the bone microenvironment is itself a challenging task due to the complexity of structural features. The widely used tool to mimic the bone microenvironment is use of 2D cell culture models such as traditional cell culture models, sandwich culture, micro-patterning and altering substrate stiffness. The growing knowledge in the field of bone and tissue engineering showed that 2D cell culture models fail to mimic in vivo conditions due to the presence of complex matrix surrounding the bone cells. In contrast to 2D models, 3D cell culture models can mimic more complex structural features of bone cells. They also have certain advantages compared with 2D cell cultures such as high stability, long lifespan, less altered genotype and maintenance of their original shape. The 3D models include spheroids, cell sheets, scaffolds, hydrogels, bioreactors and microfluidics [110].
Another reason for the immune response involves the formation of protein corona on the surface of nanocarriers due to the interaction of proteins with surface of nanocarriers. The nanocarrier properties such as particle size, shape and surface characteristics affect the protein corona formation. The formation of protein corona not only alters the immune response but also affects properties of nanocarrier, cell uptake, biodegradation, targeting capabilities, toxicity and clearance. Therefore, a complete understanding of protein corona formation is necessary for the development of nanocarriers in order to obtain desired therapeutic measures [111]. Ways to reduce the protein corona over the surface of nanocarriers include charge neutralization [112], surface hydrophilicity [113] and recruitment of specific proteins by surface coating over the nanocarriers [114].
Some studies have shown that nanocarriers can stimulate certain cellular organelles or biomolecules such as enzymes, proteins and polysaccharides which [112] further lead to unfavourable biological interactions. One of the concerns while delivering the nanocarriers through the skin is the associated skin reactions/irritations when these nanocarriers come in contact with the skin. These skin irritation tests of nanocarriers are generally involved in academic research [115]. But there is still lack of data associated with skin irritation after the long-term usage of these nanocarriers [116].
In recent years, nanotoxicology is a growing field of research which is leading towards a large amount of data generation related to toxicity aspects of nanocarriers [117]. However, it is a difficult task to determine the toxicity of nanocarriers including in vivo and long-term toxicity. The basic understanding of toxicity is mainly achieved by performing preclinical toxicity studies in animal models but it lacks the accuracy as well as the data obtained cannot always be extrapolated to humans [118]. Apart from in vivo models, in vitro toxicity studies are performed in 2D cell culture models. These models have become obsolete for evaluating the toxicity and efficacy of nanocarriers. Nowadays, co-culture and 3D spheroids and osteochondral organoids are being employed for this purpose owing to their better simulating ability towards the cell microenvironment in vitro [119]. Recently various skin-on-a-chip platforms have been developed as an alternative to the animal and cell culture models in order to evaluate various drug delivery systems. Jeon et al. developed a skin-on-chip model which simulates physiological skin irritation. The model consisted of Transwell membranes which are sandwiched between patterned polydimethylsiloxane (PDMS) chips. The model mimics three layers of the skin including epidermis, dermis and endothelial compartments. The model was used to assay the tight junction ratio in order to evaluate the skin irritation potential of a test substance [120]. Kim et al. studied the immune response mechanism occurring in the skin with the help of a skin-on-a-chip platform. In this study, they evaluated the neutrophil migration behaviour in response to bacterial infection [121].
For toxicity associated with the skin, normal human skin primary cell lines could be used to check the biocompatibility of the proposed nanocarriers. The newer models for in vitro toxicity assessment could be used to evaluate target cell specificity sparing the normal cells implying high selectivity and enhanced biocompatibility.
As the nanocarriers interact with the cell surface, receptor-mediated endocytosis occurs leading to cell internalization. However, when nanocarriers form aggregates, their size increases leading to reduced uptake and an altered pharmacokinetic-pharmacodynamic (PK-PD) profile attributed to reticuloendothelial system uptake and diminished pharmacodynamic effect. For instance, the agglomeration of nanocarriers increases the particle size and diminishes the permeation across the skin layers. This makes dose estimation difficult [122].
Nanocarriers lead to the production of reactive oxygen species thereby causing cytotoxicity and genotoxicity due to the small particle size and higher reactive surface area. The large surface area causes adsorption of heavy metals onto the surface leading to the generation of hydroxyl radicals due to catalyzation of various reactions [123]. Moreover, nanocarriers are also associated with environmental safety issues during the manufacturing process. Due to the nano-size range, these nanocarriers require protection as these carriers can permeate through the skin barrier and could additionally be responsible for pulmonary and associated systemic toxicities [124]. Figure 5 depicts the barriers to the clinical translation of nanocarrier-based topical drug delivery.
Conclusion
OA is a chronic condition associated with prolonged pain and inflammation at the arthritic site. Since there exists no cure for OA, palliative treatment is the only viable alternative. Various molecular signalling cascades regulating OA have been highlighted in the article. We also discuss disease-modifying anti-OA drugs along with the advent of drug repurposing in OA. Different routes of administration like oral, intravenous and intra-articular are available; however, they have their own virtues and vices. Out of all these routes, intra-articular route is the most widely used route followed by the oral route. However, the pain associated with administration, risk of sepsis, cost and need for hospitalization, etc. diminish its patient compliance. Topical route across the skin layers to the inflamed chondrocyte tissue offers an alternate route devoid of the vices of the intra-articular route. The transport of plain drugs across various skin layers to the inflamed tissue is highly variable and at times inadequate. The efficacy of drug delivery across the topical route could be enhanced with the help of nanocarrier-based approaches as drug carriers to the bioactive site. Nanocarrier-based approaches improve PK-PD properties of drugs, prevent their clearance, circumvent the pain associated with intra-articular route, etc. However, limited research has been done in this field yet. Through this review, we would like to draw the attention of researchers to work in the topical nanocarrier-based delivery in OA, a novel avenue, and bring new drugs for the molecular cascades and clinically translatable nanocarriers for topical products advancing the current delivery systems. As there is lack of evidence-based reports regarding the nanocarrier-based topical osteoarthritis therapy having potential for tissue repair and regeneration, this represents the futuristic approach for researchers.
Availability of data and materials
Not applicable.
References
Greco A, Lorengo V, Malfatti N. ICR-like and osteoarthritis in geriatric patients: pilot study at an RCH facility. J Orthop Rheumatism. 2018;2(1):33–46.
Lo J, Chan L, Flynn S. A systematic review of the incidence, prevalence, costs, and activity and work limitations of amputation, osteoarthritis, rheumatoid arthritis, back pain, multiple sclerosis, spinal cord injury, stroke, and traumatic brain injury in the United States: a 2019 update. Arch Phys Med Rehabil. 2021;102(1):115–31.
Heidari B. Knee osteoarthritis prevalence, risk factors, pathogenesis and features: Part I. Caspian J Intern Med. 2011;2(2):205.
Verbrugge LM. Women, men, and osteoarthritis. Arthritis & Rheumatism: Official Journal of the American College of Rheumatology. 1995;8(4):212–20.
Kloppenburg M, Berenbaum F. Osteoarthritis year in review 2019: epidemiology and therapy. Osteoarthr Cartil. 2020;28(3):242–8.
Cui A, et al. Global, regional prevalence, incidence and risk factors of knee osteoarthritis in population-based studies. EClinicalMedicine. 2020;29: 100587.
Quicke J, Osteoarthritis year in review, et al. epidemiology & therapy. Osteoarthr Cartil. 2021;2021:180–9.
Long H, Prevalence trends of site-specific osteoarthritis from, et al. to 2019: findings from the Global Burden of Disease Study 2019. Arthritis & Rheumatology. 1990;2022:1–22.
Centers for Disease Control and Prevention. Arthritis related statistics,. 2021 [cited 2021, October 12,]; Available from: https://www.cdc.gov/arthritis/data_statistics/arthritis-related-stats.htm.
Puljak L, et al. Celecoxib for osteoarthritis. Cochrane Database Syst Rev. 2017;5:1–199.
Wieland HA, et al. Osteoarthritis—an untreatable disease? Nat Rev Drug Discovery. 2005;4(4):331–44.
Kan H, et al. Non-surgical treatment of knee osteoarthritis. Hong Kong Med J. 2019:127–133.
Puett DW, Griffin MR. Published trials of nonmedicinal and noninvasive therapies for hip and knee osteoarthritis. Ann Intern Med. 1994;121(2):133–40.
Brown S, Kumar S, Sharma B. Intra-articular targeting of nanomaterials for the treatment of osteoarthritis. Acta Biomater. 2019;93:239–57.
Meng Z, Huang R. Topical treatment of degenerative knee osteoarthritis. Am J Med Sci. 2018;355(1):6–12.
Zhang Z, Huang G. Micro-and nano-carrier mediated intra-articular drug delivery systems for the treatment of osteoarthritis. J Nanotechnol. 2012;2012:1–11.
Rodriguez-Merchan E. Topical therapies for knee osteoarthritis. Postgrad Med. 2018;130(7):607–12.
Smith SR, et al. Comparative pain reduction of oral non-steroidal anti-inflammatory drugs and opioids for knee osteoarthritis: systematic analytic review. Osteoarthr Cartil. 2016;24(6):962–72.
Zhang Y, et al. Development and prospect of intra-articular injection in the treatment of osteoarthritis: a review. J Pain Res. 2020;13:1941.
Harst MRvd, et al. Biochemical analysis of the articular cartilage and subchondral and trabecular bone of the metacarpophalangeal joint of horses with early osteoarthritis. Am J Vet Res. 2005;66(7):1238–1246.
Loeser RF. Aging and osteoarthritis: the role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr Cartil. 2009;17(8):971–9.
Yang Y, et al. Comparison of early-stage changes of osteoarthritis in cartilage and subchondral bone between two different rat models. PeerJ. 2020;8:e8934.
Goldring MB. The role of cytokines as inflammatory mediators in osteoarthritis: lessons from animal models. Connect Tissue Res. 1999;40(1):1–11.
Lin C, et al. Activation of mTORC1 in subchondral bone preosteoblasts promotes osteoarthritis by stimulating bone sclerosis and secretion of CXCL12. Bone Res. 2019;7(1):1–13.
Messent E, et al. Osteophytes, juxta-articular radiolucencies and cancellous bone changes in the proximal tibia of patients with knee osteoarthritis. Osteoarthr Cartil. 2007;15(2):179–86.
Chou C-H, et al. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci Rep. 2020;10(1):1–14.
Woodell-May JE, Sommerfeld SD. Role of inflammation and the immune system in the progression of osteoarthritis. J Orthop Res®. 2020;38(2):253–257.
Novack DV. Role of NF-κB in the skeleton. Cell Res. 2011;21(1):169–82.
Chow YY, Chin K-Y. The role of inflammation in the pathogenesis of osteoarthritis. Mediators Inflamm. 2020;2020:1–19.
Mehana E-SE, Khafaga AF, El-Blehi SS. The role of matrix metalloproteinases in osteoarthritis pathogenesis: an updated review. Life Sci. 2019;234: 116786.
Afifah E, et al. Induction of matrix metalloproteinases in chondrocytes by interleukin IL-1β as an osteoarthritis model. J Math Fundam Sci. 2019;51(2):103–11.
Lepetsos P, Papavassiliou KA, Papavassiliou AG. Redox and NF-κB signaling in osteoarthritis. Free Radical Biol Med. 2019;132:90–100.
Wang Y, et al. Mitochondrial biogenesis is impaired in osteoarthritis chondrocytes but reversible via peroxisome proliferator–activated receptor γ coactivator 1α. Arthritis Rheumatol. 2015;67(8):2141–53.
Geiger BC, et al. Cartilage-penetrating nanocarriers improve delivery and efficacy of growth factor treatment of osteoarthritis. Sci Transl Med. 2018;10(469):1–12.
Callender SP, et al. Microemulsion utility in pharmaceuticals: implications for multi-drug delivery. Int J Pharm. 2017;526(1–2):425–42.
Shukla T, et al. Biomedical applications of microemulsion through dermal and transdermal route. Biomed Pharmacother. 2018;108:1477–94.
Date AA, Patravale VB. Microemulsions: applications in transdermal and dermal delivery. Critical Reviews™ in Therapeutic Drug Carrier Systems. 2007;24(6):547–596.
Kale SN, Deore SL. Emulsion microemulsion and nano emulsion: a review. Syst Rev Pharm. 2017;8(1):39.
Hu X-B, et al. Topical delivery of 3, 5, 4′-trimethoxy-trans-stilbene-loaded microemulsion-based hydrogel for the treatment of osteoarthritis in a rabbit model. Drug Deliv Transl Res. 2019;9(1):357–65.
Chen H, et al. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm. 2006;315(1–2):52–8.
Goindi S, Narula M, Kalra A. Microemulsion-based topical hydrogels of tenoxicam for treatment of arthritis. AAPS PharmSciTech. 2016;17(3):597–606.
Lalatsa A, et al. Topical buparvaquone nano-enabled hydrogels for cutaneous leishmaniasis. Int J Pharm. 2020;588: 119734.
Ponto T, et al. Novel self-nano-emulsifying drug delivery systems containing astaxanthin for topical skin delivery. Pharmaceutics. 2021;13(5):649.
Egbaria K, Weiner N. Liposomes as a topical drug delivery system. Adv Drug Deliv Rev. 1990;5(3):287–300.
Storm G, Crommelin DJ. Liposomes: quo vadis? Pharm Sci Technol Today. 1998;1(1):19–31.
Frisbie DD, et al. Evaluation of topically administered diclofenac liposomal cream for treatment of horses with experimentally induced osteoarthritis. Am J Vet Res. 2009;70(2):210–5.
McIlwraith CW. Management of joint disease in the sport horse. in Proceedings of the 2010 Kentucky Equine Research Nutrition Conference, Feeding and Veterinary Management Of The Sport Horse, Lexington, KY, April. 2010.
Shakouri A, et al. Effectiveness of topical gel of medical leech (Hirudo medicinalis) saliva extract on patients with knee osteoarthritis: a randomized clinical trial. Complement Ther Clin Pract. 2018;31:352–9.
Bhatia A, et al. Evaluation of efficacy and safety of a novel lipogel containing diclofenac: a randomized, placebo controlled, double-blind clinical trial in patients with signs and symptoms of osteoarthritis. Contemp Clin Trials Commun. 2020;20:100664.
Tiwari G, et al. Ultra-deformable liposomes as flexible nanovesicular carrier to penetrate versatile drugs transdermally. Nanosci Nanotechnol-Asia. 2020;10(1):12–20.
Bhardwaj V, et al. Transfersomes ultra flexible vesicles for transdermal delivery. Int J Pharm Sci Res. 2010;1(3):12–20.
Kumar A. Transferosome: a recent approach for transdermal drug delivery. J Drug Deliv Ther. 2018;8(5-s):100–104.
Pawar AY. Transfersome: a novel technique which improves transdermal permeability. Asian Journal of Pharmaceutics (AJP): Free full text articles from Asian J Pharm. 2016;10(04):S424-S436.
Rother M, et al. Efficacy and safety of epicutaneous ketoprofen in transfersome (IDEA-033) versus oral celecoxib and placebo in osteoarthritis of the knee: multicentre randomised controlled trial. Ann Rheum Dis. 2007;66(9):1178–83.
Fernández-García R, et al. Transferosomes as nanocarriers for drugs across the skin: quality by design from lab to industrial scale. Int J Pharm. 2020;573: 118817.
He Z, et al. White light emission from a single organic molecule with dual phosphorescence at room temperature. Nat Commun. 2017;8(1):1–8.
Ha S, La Y, Kim KT. Polymer cubosomes: infinite cubic mazes and possibilities. Acc Chem Res. 2020;53(3):620–31.
Sharma P, Dhawan S, Nanda S. Cubosome: a potential liquid crystalline carrier system. Curr Pharm Des. 2020;26(27):3300–16.
Gaballa SA, El Garhy OH, Abdelkader H. Cubosomes: composition, preparation, and drug delivery applications. Journal of advanced Biomedical and Pharmaceutical Sciences. 2020;3(1):1–9.
Azmi ID, Moghimi SM, Yaghmur A. Cubosomes and hexosomes as versatile platforms for drug delivery. Ther Deliv. 2015;6(12):1347–64.
Puglia C, et al. Evaluation of monooleine aqueous dispersions as tools for topical administration of curcumin: characterization, in vitro and ex-vivo studies. J Pharm Sci. 2013;102(7):2349–61.
Elakkad YE, et al. Tenoxicam loaded hyalcubosomes for osteoarthritis. Int J Pharm. 2021;601: 120483.
Collins J, Rother M. 10 Ultra-deformable drug-free sequessome™ vesicles (TDT 064) for the treatment of joint pain following exercise: a case report and clinical data. BMJ Publishing Group Ltd and British Association of Sport and Exercise Medicine. 2015;A3-A4.
Cevc G, Vierl U. Nanotechnology and the transdermal route: a state of the art review and critical appraisal. J Control Release. 2010;141(3):277–99.
Cevc G, Vierl U, Mazgareanu S. Functional characterisation of novel analgesic product based on self-regulating drug carriers. Int J Pharm. 2008;360(1–2):18–28.
Lanas A, et al. Prescription patterns and appropriateness of NSAID therapy according to gastrointestinal risk and cardiovascular history in patients with diagnoses of osteoarthritis. BMC Med. 2011;9(1):38.
Conaghan PG, et al. A multicentre, randomized, placebo-and active-controlled trial comparing the efficacy and safety of topical ketoprofen in Transfersome gel (IDEA-033) with ketoprofen-free vehicle (TDT 064) and oral celecoxib for knee pain associated with osteoarthritis. Rheumatology. 2013;52(7):1303–12.
Rother M, Conaghan PG. A randomized, double-blind, phase III trial in moderate osteoarthritis knee pain comparing topical ketoprofen gel with ketoprofen-free gel. J Rheumatol. 2013;40(10):1742–8.
Lingayat VJ, Zarekar NS, Shendge RS. Solid lipid nanoparticles: a review. Nanoscience and Nanotechnology Research. 2017;2:67–72.
Iqbal B, Ali J, Baboota S. Recent advances and development in epidermal and dermal drug deposition enhancement technology. Int J Dermatol. 2018;57(6):646–60.
Paliwal R, et al. Solid lipid nanoparticles: a review on recent perspectives and patents. Expert Opin Ther Pat. 2020;30(3):179–94.
Mu H, Holm R. Solid lipid nanocarriers in drug delivery: characterization and design. Expert Opin Drug Deliv. 2018;15(8):771–85.
Shah S, Mehta V. Controversies and advances in non-steroidal anti-inflammatory drug (NSAID) analgesia in chronic pain management. Postgrad Med J. 2012;88(1036):73–8.
Jijie R, et al. Nanomaterials for transdermal drug delivery: beyond the state of the art of liposomal structures. J Mater Chem B. 2017;5(44):8653–75.
Sharma G, et al. Nanostructured lipid carriers: a new paradigm in topical delivery for dermal and transdermal applications. Crit Rev™ Ther Drug Carr Syst. 2017;34(4):355–386.
Müller R, et al. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59(6):522–30.
Dudhipala N, Janga KY, Gorre T. Comparative study of nisoldipine-loaded nanostructured lipid carriers and solid lipid nanoparticles for oral delivery: preparation, characterization, permeation and pharmacokinetic evaluation. Artificial cells, nanomedicine, and biotechnology. 2018;46(sup2):616–25.
Czajkowska-Kośnik A, Szekalska M, Winnicka K. Nanostructured lipid carriers: a potential use for skin drug delivery systems. Pharmacol Rep. 2019;71(1):156–66.
Kaur A, Goindi S, Katare OP. Formulation, characterisation and in vivo evaluation of lipid-based nanocarrier for topical delivery of diflunisal. J Microencapsul. 2016;33(5):475–86.
Müller-Goymann C. Physicochemical characterization of colloidal drug delivery systems such as reverse micelles, vesicles, liquid crystals and nanoparticles for topical administration. Eur J Pharm Biopharm. 2004;58(2):343–56.
Nene S, et al. Lipid based nanocarriers: a novel paradigm for topical antifungal therapy. J Drug Deliv Sci Technol. 2021;102397.
Nyström AM, Fadeel B. Safety assessment of nanomaterials: implications for nanomedicine. J Control Release. 2012;161(2):403–8.
United Nations. Economic Commission for Europe. Secretariat, Globally harmonized system of classification and labelling of chemicals (GHS). 2015. Copyright Law of the United St.
Doktorovova S, et al. Preclinical safety of solid lipid nanoparticles and nanostructured lipid carriers: current evidence from in vitro and in vivo evaluation. Eur J Pharm Biopharm. 2016;108:235–52.
Sanchez L, et al. Potential irritation of lysine derivative surfactants by hemolysis and HaCaT cell viability. Toxicol Lett. 2006;161(1):53–60.
Lee JK, et al. In vitro cytotoxicity tests on cultured human skin fibroblasts to predict skin irritation potential of surfactants. Toxicol In Vitro. 2000;14(4):345–9.
Lazzarini R, Duarte I, Ferreira AL. Patch tests. An Bras Dermatol. 2013;88(6):879–88.
Lee M, Hwang J-H, Lim K-M. Alternatives to in vivo Draize rabbit eye and skin irritation tests with a focus on 3D reconstructed human cornea-like epithelium and epidermis models. Toxicological research. 2017;33(3):191–203.
Ragelle H, et al. Nanoparticle-based drug delivery systems: a commercial and regulatory outlook as the field matures. Expert Opin Drug Deliv. 2017;14(7):851–64.
Tinkle S, et al. Nanomedicines: addressing the scientific and regulatory gap. Ann N Y Acad Sci. 2014;1313(1):35–56.
Patel DM, Patel NN, Patel JK. Nanomedicine scale-up technologies: feasibilities and challenges. In: emerging technologies for nanoparticle manufacturing. Springer; 2021. p. 511–39.
Junghanns J-UA, Müller RH. Nanocrystal technology, drug delivery and clinical applications. Int J Nanomed. 2008;3(3):295.
Galindo-Rodríguez SA, et al. Comparative scale-up of three methods for producing ibuprofen-loaded nanoparticles. Eur J Pharm Sci. 2005;25(4–5):357–67.
Agrahari V, Agrahari V. Facilitating the translation of nanomedicines to a clinical product: challenges and opportunities. Drug Discovery Today. 2018;23(5):974–91.
U.S. Department of Health and Human Services Food and Drug Administration. PAT — A framework for innovative pharmaceutical development, manufacturing, and quality assurance. 2004. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/pat-framework-innovative-pharmaceutical-development-manufacturing-and-quality-assurance.
Rangaraj N, et al. QbD aided development of ibrutinib-loaded nanostructured lipid carriers aimed for lymphatic targeting: evaluation using chylomicron flow blocking approach. Drug Deliv Transl Res. 2020;10(5):1476–94.
Gadekar V, et al. Nanomedicines accessible in the market for clinical interventions. J Control Release. 2020;372–397.
Nene S, et al. Lipid based nanocarriers: a novel paradigm for topical antifungal therapy. J Drug Deliv Sci Technol. 2021;62:102397.
Sainz V, et al. Regulatory aspects on nanomedicines. Biochem Biophys Res Commun. 2015;468(3):504–10.
Kaur IP, et al. Issues and concerns in nanotech product development and its commercialization. J Control Release. 2014;193:51–62.
Shah S, et al. Bridging the gap: academia, industry and FDA convergence for nanomaterials. Drug Dev Ind Pharm. 2020;46(11):1735–46.
Guidance D. Considering whether an FDA-regulated product involves the application of nanotechnology. FDA. 2011 Jun.
Hafner A, et al. Nanotherapeutics in the EU: an overview on current state and future directions. Int J Nanomed. 2014;9:1005.
Hare JI, et al. Challenges and strategies in anti-cancer nanomedicine development: an industry perspective. Adv Drug Deliv Rev. 2017;108:25–38.
DiMasi JA, Grabowski HG, Hansen RW. Innovation in the pharmaceutical industry: new estimates of R&D costs. J Health Econ. 2016;47:20–33.
Sato K, et al. Microfluidics for nano-pathophysiology. Adv Drug Deliv Rev. 2014;74:115–21.
Gaumet M, et al. Nanoparticles for drug delivery: the need for precision in reporting particle size parameters. Eur J Pharm Biopharm. 2008;69(1):1–9.
Fernández-García R, et al. Ultradeformable lipid vesicles localize amphotericin B in the dermis for the treatment of infectious skin diseases. ACS Infectious Diseases. 2020;6(10):2647–60.
Kalluri H, Kolli CS, Banga AK. Characterization of microchannels created by metal microneedles: formation and closure. AAPS J. 2011;13(3):473–81.
Yuste I, et al. Mimicking bone microenvironment: 2D and 3D in vitro models of human osteoblasts. Pharmacol Res. 2021;169: 105626.
Corbo C, et al. The impact of nanoparticle protein corona on cytotoxicity, immunotoxicity and target drug delivery. Nanomedicine. 2016;11(1):81–100.
Cao X, et al. Impact of protein-nanoparticle interactions on gastrointestinal fate of ingested nanoparticles: not just simple protein corona effects. NanoImpact. 2019;13:37–43.
Palchetti S, et al. The protein corona of circulating PEGylated liposomes. Biochimica et Biophysica Acta (BBA)-Biomembranes. 2016;1858(2):189–196.
Mirshafiee V, et al. Impact of protein pre-coating on the protein corona composition and nanoparticle cellular uptake. Biomaterials. 2016;75:295–304.
Kianvash N, et al. Evaluation of propylene glycol nanoliposomes containing curcumin on burn wound model in rat: biocompatibility, wound healing, and anti-bacterial effects. Drug Deliv Transl Res. 2017;7(5):654–63.
Tiwari N, et al. Nanocarriers for skin applications: where do we stand? Angewandte Chemie International Edition. 2021;1–26.
Zhao J, Castranova V. Toxicology of nanomaterials used in nanomedicine. Journal of Toxicology and Environmental Health, Part B. 2011;14(8):593–632.
Agrahari V, Hiremath P. Challenges associated and approaches for successful translation of nanomedicines into commercial products. Nanomedicine. 2017;12(8):819–23.
Gupta N, et al. Microfluidics-based 3D cell culture models: utility in novel drug discovery and delivery research. Bioengineering & Translational Medicine. 2016;1(1):63–81.
Jeon B, et al. Enhanced predictive capacity using dual-parameter chip model that simulates physiological skin irritation. Toxicol In Vitro. 2020;68: 104955.
Kim JJ, et al. A microscale, full-thickness, human skin on a chip assay simulating neutrophil responses to skin infection and antibiotic treatments. Lab Chip. 2019;19(18):3094–103.
Behzadi S, et al. Cellular uptake of nanoparticles: journey inside the cell. Chem Soc Rev. 2017;46(14):4218–44.
Fu PP, et al. Mechanisms of nanotoxicity: generation of reactive oxygen species. J Food Drug Anal. 2014;22(1):64–75.
Desai N. Challenges in development of nanoparticle-based therapeutics. AAPS J. 2012;14(2):282–95.
Acknowledgements
The authors would like to acknowledge the research funding support by the Department of Pharmaceuticals, Ministry of Chemicals and Fertilizers, Govt. of India.
Author information
Authors and Affiliations
Contributions
Pratiksha Patil: ideation, writing—original draft preparation, literature search; Shweta Nene:—writing—original draft preparation, literature search; Saurabh Shah: literature search, drafting, writing—review and editing; Dr. Shashi Bala Singh: critical revision of the manuscript; Dr. Saurabh Srivastava:—critical revision of the manuscript, supervision.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors approve for publication.
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Key summary points
• Osteoarthritis is a physically debilitating disease that predominantly affect around one in every 30 people and a total of 190 million worldwide.
• It is a degenerative joint disorder primarily affecting cartilage due to an imbalance in biomechanical and metabolic factors.
• Current pharmacologic treatment options primarily consist of non-steroidal anti-inflammatory drugs with associated undesirable side effects and poor patient compliance which have led physicians to search for alternative treatment modalities.
• Disease-modifying drugs, drug repurposing, and topical nano-carriers present potential therapeutic avenues to bypass the loopholes associated with the conventional therapies for the treatment of osteoarthritis.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Patil, P., Nene, S., Shah, S. et al. Exploration of novel drug delivery systems in topical management of osteoarthritis. Drug Deliv. and Transl. Res. 13, 531–546 (2023). https://doi.org/10.1007/s13346-022-01229-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-022-01229-z