Abstract
Cancer is a multidimensional and challenging disease to handle. Current statistics reveal that we are far from satisfying cancer treatment. Taking advantage of different therapeutic agents that affect multiple pathways has been established as highly productive. Nevertheless, owing to several hindrances to conventional combination therapy, such as lack of tumor targeting, non-uniform pharmacokinetic of the combined drugs, and off-target side effects, it is well documented that this treatment approach is unlikely to address all the difficulties observed in monotherapy. Co-delivery systems could enhance the therapeutic efficacy of the combination therapy by targeting cancer cells and improving the pharmacokinetic and physicochemical properties of the therapeutic agents. Nevertheless, it seems that present knowledge in responding to the challenges in cancer treatment is still inadequate and far from optimal treatment, which highlights the urgent need for systematic studies direct to identify various aspects of co-delivery systems. Accordingly, to gather informative data, save time, and achieve superior results, the following steps are necessary: (1) implementing computational methods to predict drug-drug interactions (DDIs) in vitro and in vivo, (2) meticulous cancer studies at the cellular and molecular levels to obtain specific criteria for selecting preclinical and clinical models, (3) extensive physiological and pharmacokinetic study of nanocarriers behavior in preclinical models, and (4) finding the optimal formulation and analyzing its behavior in cellular and animal models facilitates bridging in vivo models to clinical trials. This review aims to deliver an overview of co-delivery systems, rationales, and suggestions for further studies in this field.
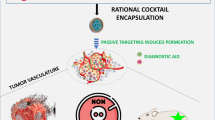
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Despite new methods of diagnosing and treating cancer, a recent WHO forecast shows that by 2040, more than 16 million cancer deaths are expected each year [1]. The high mortality rate of cancer indicates that the current treatment approach is questionable to bring about promising tumor inhibition. The primary cancer treatment in the current practice is limited to surgical resection, radiotherapy, immunotherapy, and predominantly chemotherapy. The first option is mostly workable in the primary stages of cancer treatment. Despite the fact that surgical resection is still used in the treatment and control of most solid cancers, it has long been reported that surgery may accelerate tumor recurrence, the concept states that tumor resection may increase tumor recurrence [2]. To sum up, a growing body of evidence suggests that surgery may provide an appropriate environment for tumor progression. According to the in vivo studies, sites of injury provide desired site for tumor growth and that surgical trauma increases local metastases [3]. Besides, several experimental findings showed the tumor growth acceleration after surgery in distant locations [4]. On top, some studies show that open cancer resections are associated with shorter survival rate for patient compared to minimally invasive resections, a concept that is strongly corroborated by experimental data [5].
The unsuccessfulness of current therapies can be attributed to pharmacological and formulations issues [6]. Nonselective therapeutics specially in the case of chemotherapy and radiation [7,8,9], intrinsic/acquired resistance [10, 11], and mutations in the cancer cells [12, 13] have been observed in most of the conventional treatments. Moreover, the complexity of the tumor microenvironment (TME) limits drug delivery to the target site [14]. The poor solubility of chemotherapeutics, inappropriate and toxic excipients [15], short blood circulation time [16], and short half-life [17] of some therapeutic agents can decrease drug delivery to the tumor site. The findings of the long-term study in the cancer field confirm that the current treatments are not well-armed [18].
Combination therapy has been represented as simultaneous/sequential administration of the therapeutic agents that enhance the overall therapeutic outcome compared with the administration of the individual therapeutic agents. Combination therapy has shown advantages and merits over monotherapy. In the case of cancer, this hypothesis is somewhat plausible as combination therapies are used in the clinic frequently [19,20,21].
In general, combination therapy includes the following approaches: firstly, targeting multiple signaling pathways to overcome the cascade of mechanisms employed by cancer cells to counter therapeutics [22]; secondly, drug repositioning by reusing existing generic drugs for the alternate indication, which is an excellent approach for cancer treatment that can reduce the required dose of both individual drugs and shorten clinical translation and post-marketing process [23]; and thirdly, medicine personalization by developing new drugs based on the bimolecular characteristics of the tumor and genetic distinctions in humans can help to patient selection for a specific treatment [24]. Medicine personalization tailored to the tumor molecular characteristics is one of the topics of interest in current cancer treatment, which aims to target specific signaling pathways based on the individuals’ genetic profile [25].
Despite recent achievements in this field, there are few numbers of targeting therapeutic agents such as small molecules designed for targeting HER2 on breast cancer (BC) cells [26], BRCA mutations in breast and ovarian cancer [27], BRAF mutations in melanoma [28], and EGFR mutations in lung cancer (LC) cells [29]. The findings of the clinical studies indicated the low efficacy of these therapeutic agents to bring about favorable outcomes [30]. It was reported that some of the targeting agents do not just act on the cancer cells; in other words, the targets of these therapeutic agents can be found in healthy tissues [31]. However, combination therapy presented noteworthy benefits over monotherapy; disappointing results usually accompany it due to the dissimilarities in the physicochemical and pharmacokinetics of drugs and distinctive action sites of the therapeutics [32].
In light of the necessity for optimal treatments, drug delivery systems (DDSs) are based on the unique characteristics of the TME, such as enhanced permeability and retention (EPR), hypoxia, and overexpressed ligands in the tumor have been designed [33,34,35,36]. DDSs present benefits by protecting drugs, increasing drug blood circulation [37], reducing side effects [38], and drug targeting [39, 40]. Co-delivery of anticancer agents exhibited superiority over the cocktails of a combination of anticancer [41, 42]. Co-delivery system design is extremely challenging cause besides adjusting the dose of therapeutic agents and their ratio; it is essential to determine the location [43], sequence [44], and release rate of both therapeutics [45]. In recent years, a variety of co-delivery systems have been designed in preclinical studies, while few of them exist on the market. The first part of this review aims to provide a brief overview of the reasons for the importance of co-delivery systems, followed by discussion over benefits, challenges, and opportunities in this field.
Combination therapy considerations
Therapeutics combination ratio: there is no general principle
In theory, when two therapeutics are administrated simultaneously, the combination effectiveness may be less (antagonistic), equal (additive), or higher (synergism) than the summed effect of the individual drugs [46]. Nevertheless, the difference between drug interactions, e.g., synergism and additive, is practically indistinguishable in pharmacotherapy. In other words, the principle to the best choice of regimen and drug combinations in the clinic is not well-known, and there is no mathematical formula to find the exact relationship between therapeutic agents in practice [18]. According to a recent analysis of human clinical trials (phases II and III) over a simulated population that implicated all possible combination therapies from patient-derived tumor xenograft models (PDX) data [47], < 5% of potential combination therapies presented superior outcomes and improved progression-free survival, compared to monotherapy of individual drugs [48]. This unexpected report proved that most of the preclinical studies fail to show promising effects in the clinical trials, and there is an urgent need to employ a suitable strategy to match appropriate therapeutic agents.
Pharmacokinetic considerations
Drug-drug interactions (DDIs) are concerned to be one of the critical factors in designing combination therapies. These interactions affect not only the pharmacodynamics but also the pharmacokinetics (absorption, distribution, metabolism, and excretion) of the individual drugs. The combination of the free form of therapeutic agents does not show satisfying outcomes due to the distinctive properties (solubility, permeability, stability, half-life, distribution) and non-uniform distribution [49]. Several databases have valuable information for checking DDIs such as Drug-Bank and the databases, which are beneficial to check adverse effects such as FDA Adverse Event Reporting System (FAERS) [50]. The accurate computational prediction of cancer patients’ responses is necessary to find the optimal combination therapy. Computational models are widely used to facilitate finding appropriate candidates for combination therapy [51]. These models utilize bio/chemo informatics information (physicochemical, pharmacodynamics, and pharmacokinetics) and clinical information (indications, safety information, drug interactions) from a variety of sources and make it possible to predict the behavior of drugs in combination [23]. Therefore, novel promising combinations can be recognized through high throughput and systematic approaches [52].
Cell and animal models
A fundamental issue in developing practical combination therapy is the mismatch between in vitro and in vivo test outcomes. The fact of the matter is that the simple methods of examining the effect of therapeutic agents in combination, which usually take place in a culture medium, do not provide accurate and reliable results [53]. Numerous reasons are associated with this consequence. The complex physiopathological condition in cancer which cannot be studied in the culture medium [54, 55], genetic differences in individuals [56], and the insufficient animal models to simulate genetic variations and carcinogenesis in humans [57] result in difficulties in the toxicity–efficacy studies of combinations therapies.
Recent findings have documented that TME in human tumor tissues contains dense stroma remarkably higher than the stroma observed in the tumor xenograft models. Besides, animal models cannot simulate drug pharmacodynamics accurately due to the lack of molecular characteristic simulation [58,59,60,61]. Preclinical outcomes are often meaningfully far from the clinical studies [62], and just about 8% of animal studies are translated into clinical trials [57]. Ultimately, roughly one-third of clinical trials successfully pass phase I [63]. These surprising results showed that the main reason for the poor translation of animal studies to clinical trials is the biological differences between models, which highlights the importance of developing reliable preclinical models.
Numerous studies conducted to determine if PDX mouse models could serve as a reliable model to simulate treatment response in individual cancer patients. These tumor models are developed by implantation of tumor cells or parts of a patient’s tumors into the host mice [64]. One of the advantages of patient-derived models is the facilitation of molecular mechanism studies such as resistance of the tumor to the therapeutic agents. In a recent study, a set of PDX ovarian cancer models were generated from patients under the clinical procedure. These models simulate patients’ responses to the standard chemotherapy treatment which is beneficial to a better understanding of the molecular-level mechanism of the therapeutic agents in ovarian cancer tumors [61]. In a new research on the mechanism of alkylating agents and PARP inhibitors, a triple-negative BC patient xenograft model was generated which uncovers a new resistance mechanism in BRCA1-methylated models [65]. One of the approaches to examine this is to treat PDX models with the therapeutic agents. For examples, Zhang et al. designed patient-derived human BC xenograft models to achieve in vivo models that reflect the complexity of BC pathophysiology particularly in the primary stages. This research showed strong correlation between PDX models and clinical response [66]. In this regard, a number of prospective studies used PDX models to guide clinical treatment decisions in a small number of patients. There are studies that have shown the strong correlation between PDX and clinical results [67]; for example, the research by Stebbing’s et al. showed that 20.1% (6/29) of the patients with advanced, metastatic sarcoma received direct clinical benefits in from PDX-guided therapy [68]. Taken together, these models can mimic molecular characteristics of cancer cells and the TME, which present a great platform for drug development studies.
Why co-delivery systems?
Rational
Even though valuable, traditional combination therapy faces several limitations. For instance, differences in pharmacological fate and pharmacokinetic profile of individual therapeutic agents may cause serious side effects and systemic toxicity. When the therapeutic agent is loaded in a DDS, its pharmacokinetics are enclosed by the carrier; thus, the physicochemical properties of the delivery system determine the biological fate of the therapeutic agent [35, 39]. Co-delivery systems can unify the pharmacokinetic behavior of the therapeutic agents, improve physicochemical properties, increase biodistribution time, and enhance selectivity to the tumor [69]. The remarkable advantage of nanosystems is the ability to release therapeutic agents in a controlled manner in terms of location, time, amount, and sequence [33, 41, 42, 70,71,72,73]. Therefore, co-delivery systems can be considered potential candidates to maximize treatment efficiency, minimize side effects, and improve the pharmacokinetic profile of combined therapeutic agents (Fig. 1). Design and fabrication of the co-delivery system for cancer treatment are extremely complicated process. There are three major strategies to co-deliver therapeutics which combine chemotherapy with immunotherapy and/or gene therapy. Table 1 summarizes the rationales of designing co-delivery systems.
Targeting delivery
The pathophysiological condition in solid tumors is very complicated. Since cancer cells need high energy to multiply actively, their demand for oxygen and nutrients is excessive [33]. These features contribute to an abnormal condition that significantly differs from healthy tissues. Extreme angiogenesis, hypoxia, and acidity eventually lead to a condition in which the vessels and the lymphatic drainage are impaired, which contributes to the interstitial fluid pressure elevation [54, 86].
The concept, EPR, which is known to exist to some degrees in solid tumors, is the main positive point about the tumors that explains why DDSs can penetrate the tumors efficiently [87]. Passive targeting, the primary evidence-based targeting delivery mechanism in the clinic [34, 88], relies on the EPR effect and facilitates tumor delivery of particles with specific features [89]. Another way to target tumors is by taking advantage of ligands that are overexpressed in the tumor cells or TME. This strategy, active targeting, is implemented in several clinical studies and seems to be in collaboration with passive targeting [35, 40, 90]. Here, some of the examples of targeting delivery are elaborated.
Anthracyclines are known to be the core stone of several cancer treatments [91]. Despite the usefulness of these therapeutic agents in cancer, several side effects limit their use in the clinic [92, 93]. According to the previous studies, doxorubicin administration as the first-line therapy in BC [94] showed irreversible cardiac dysfunction, which is exacerbated in combination with HER2-suppressing agents administrated in HER2-positive BC [95]. Even though advantageous, the doxorubicin and trastuzumab (HER2 targeting agent) combination failed to get FDA approval.
Various studies have surveyed the efficacy of receptor targeting liposomes in comparison to conventional therapies [96, 97]. Research studies have shown that liposomes can increase the therapeutic effect of highly potent drugs, specially chemotherapeutics [98, 99]. PEGylated liposomal formulation improved doxorubicin pharmacokinetics and showed less cardiac dysfunction compared with conventional doxorubicin or its combination with trastuzumab [100]. The relying mechanism of this result can be attributed to the particular characteristics of TME, such as abnormal vasculature that support nanosystems delivery into the tumor through the EPR effect [91]. However, there is some consideration in the combination of liposomal doxorubicin and trastuzumab, as trastuzumab may cause cardiac dysfunction by targeting HER2 receptors expressed in cardiomyocytes [31]. To take the most benefit and the fewer side effects of doxorubicin and trastuzumab-like agents, HER2-targeted liposomal doxorubicin was designed. Several preclinical models exhibited HER2-targeted liposomal doxorubicin superiority to non-targeted ones [90, 101]. The mechanism of this targeting doxorubicin-loaded liposome has shown in Fig. 2 [90].
Schematic illustration of improved drug delivery of doxorubicin by targeting anti-HER2 peptide in murine breast tumor model [90]. Reprinted with permission from Elsevier, June 2021
Despite these reports, it was necessary to evaluate whether HER2-targeted liposomal doxorubicin is uptaken by cardiomyocytes or not. The findings of a recent study indicated that the uptake of HER2-targeted liposomal doxorubicin is negligible [102]. According to this research, slight or no cardiomyocyte cell death or dysfunction was observed. As HER2-targeted liposomal doxorubicin showed a promising effect in preclinical studies, it was entered into clinical studies. In a clinical dose-escalation study performed on patients who had metastatic BC, no cardiac adverse event in HER2-targeted liposomal doxorubicin monotherapy was reported, and progression-free survival increased about 3 months compared with conventional treatment regime [103]. These findings highlighted the potential of targeting co-delivery system application to reduce off-target side effects and increase selective toxicity in the target site.
Improving pharmacokinetic profile
There are various methods for co-delivery of the chemotherapeutics; some of them are easy to implement, such as coencapsulation into the polymeric core, which suffers from poor release kinetics of the individual drugs and inefficiency in loading hydrophilic therapeutic agents [104]. In contrast, this method showed promising results in the case of lapatinib and paclitaxel combination, which are hydrophobic chemotherapeutics [105]. There are alternative methods to deliver therapeutics with distinctive physicochemical prosperities such as polymer-drug conjugation [106]. The pH-responsive supramolecular hydrogel was designed, which could deliver NPOD, a hydrophobic molecule, by conjugation with PEG and doxorubicin as a hydrophilic molecule [107]; this co-delivery system showed a higher cytotoxicity effect in comparison with monotherapy.
The efficacy of co-delivery systems has been long-established in several clinical trials (Table 3). Vyxeos® is a liposomal formulation that combines daunorubicin and cytarabine (1:5 molar ratio), which received approval for acute myeloid leukemia (AML) [108]. The traditional regimes for AML consisted of cytarabine infusion for 1 week and doxorubicin bolus administration in the first 3 days. In contrast, this delivery system is infused in 1, 3, and 5 days [109]. As the clinical benefit of this co-delivery system was remarkable, which exhibited superior activity compared to the combination of bared drugs and patient compliance improvement, Vyxeos® was approved by the FDA for AML treatment in 2017 [110]. Switching genes on and off is one of the promising tools for cancer treatment as it enables treatment at the molecular level. However, using these strategies is challenging due to systemic toxicity, stability issues, low circulation time, and low cellular uptake of genetic materials [111]. Therefore, choosing the appropriate delivery system to take advantage of gene therapy is vital [112]. The first dual-targeted siRNA drug (ALN-VSP02) is a lipoplex that loads two different siRNA, one for kinesin spindle protein (KSP) targeting, which is responsible for cell proliferation, and the other one for vascular endothelial growth factor (VEGF) targeting. The clinical trial showed antitumor activity in patients with solid tumors [79]. It was observed that hypoxia-induced factor 1α (HIF-1α) is overexpressed in cancer cells. Several studies confirmed the correlation between hypoxia and cancer development and progression [113, 114]. Metformin is an antidiabetic medicine that showed potential anticancer effects by intervention in the hypoxic process [115, 116]. However, short blood circulation time, low tumor accumulation, poor bioavailability, and short half-life limit metformin application in the clinic as evidence-based researches are indicating low tumor accumulation of metformin [117]. The findings of a study that designed a liposomal co-delivery system for metformin and doxorubicin indicated the faster release of the metformin from the liposome, which could increase doxorubicin cytotoxicity in drug-resistant BC cells. This result is in accordance with the hypoxia improvement caused by doxorubicin and metformin coloaded liposome [76].
Controlling sequential/site-specific co-delivery systems
Individual therapeutic agents present diverse pharmacodynamics, so it is necessary to control the release sequence, site, and rate of each therapeutic. As mentioned in the previous section, the sequence of drug administration is an important factor in the combination of therapy regimens. To achieve this goal, sequential and site-specific DDSs are designed. Generally, site-specific DDSs target tumor tissue and TME entities, such as vascular endothelial cells [118], tumor associated fibroblasts, tumor associated macrophage [119], and cancer stem cells [120] or affect cellular/subcellular mechanisms such as mitochondria [121, 122] and nuclei [123] related pathways. To the purpose, various DDSs are designed which can be triggered to release load in response to defined pH, temperature, enzyme activity, redox potential, and the external triggers such as light irradiation, magnetic, and electric fields [124]. There are mainly three strategies in this area:
Increasing NC penetration into the tumor
Recent studies aim to target TME components included but are not limited to vascular endothelial cells [125], immune cells [126], and cancer stem cells [127]. Studies have confirmed that dexamethasone showed TME modulating effect, which is attributed to interstitial fluid pressure reduction [14]. Docetaxel and dexamethasone liposomal co-delivery system showed promising effect in BC mice. It was reported that dexamethasone release before docetaxel release increased docetaxel delivery to the tumor, which resulted in an enhanced therapeutic effect in vivo [44].
Increasing therapeutic efficacy by targeting extracellular and intracellular cell death pathways
Doxorubicin and TNF-related apoptosis-inducing ligand (TRAIL) hybrid NP was designed in which TRAIL was crossed linked to the outer shell of the NP and doxorubicin was encapsulated in the liposome core [128]. The extracellular release of TRAIL-induced cell death by targeting cell death ligand expressed in the cancer cell membrane beside increased doxorubicin uptake through cell-penetrating peptide modification on liposome showed promising cytotoxicity effect in the PDX model.
Reducing side effects
Most cancer treatment regimens include protocols to prevent or reduce complications which are not specified to prevent off-target site effects in some of the cancer cases [129, 130]. It seems that the use of new methods such as simultaneous released co-delivery system may reduce the drugs side effects administrated in the cancer therapy protocols. In a recent study, a disulfide cross-linked low-generation peptide dendrimer-based nano polymer conjugate was designed (Fig. 3) [131]. This delivery system could increase doxorubicin release in the colon cancer cells due to the high concentration of glutathione in the cancer cells, while the presence of nattokinase, a thrombolytic drug [132], could reduce thrombolytic side effects in vitro [131].
Schematic illustration of the doxorubicin and nattokinase co-loaded DDS action on cancer cells [131]. Reprinted with permission from Elsevier, June 2021
Challenges in co-delivery systems: are we doing right?
Despite extensive studies at the academic centers and pharmaceutical companies, few DDSs enter clinical trials and many of which fail in the early phases. There is a considerable gap between enormous articles published in this field and nanomedicine on the market. As it can be seen (Table 2), there are few co-delivery DDSs in the market. Finally, the question arises as to what is the promising procedure for developing a co-delivery system which will be discussed in the following section.
DDS design
Carrier exploration in the body
Physicochemical properties of components of delivery systems including compatibility between all the materials and their ratio, shape, size, and surface charge of the NP should be optimized to achieve an ideal DDS that can inhibit cancer cell proliferation noticeably. In reality, DDSs do no show promising results in the clinic. It was reported that only about 0.7% of the injected NPs delivered into the tumor [144].
Size
Further, a recent quantitative study showed that a tiny portion (< 0.0014%) of active targeting NPs could target cancer cells [145]. In addition, a recent study from 2015 to 2018 revealed that only 2.23% (mean) and 0.76% (medium) of the injected dose could be delivered to the tumor site. According to the analysis of this study, the NPs having hydrodynamic sizes lower than 10 nm showed higher tumor delivery efficacy than the NPs with a size smaller than 10 nm [146]. This report is in agreement with the recent study, which showed that the 12-nm size NPs target tumors more selectively than the larger NPs, which were found in the off-target sites (liver, lung, and pancreas) [147]. Since nanosystems are known to be alien by the body’s immune system, the other fraction of these particles taken up by the macrophages or filtered by the kidney, especially if the hydrodynamic diameter of the NP is lower than 5 nm, the other extraction way for NPs is through the liver in which NPs over ≈ 10–20-nm hydrodynamic diameter is eliminated [148].
Shape
In terms of particle shape, several studies showed that nonspherical NPs could penetrate tumors more effectively than spherical NPs, as a study suggested that rod NPs are more promising for drug delivery purposes than the disc and sphere form NPs [149]. The results of a study indicated that nanorod gold NP tumor accumulation was higher than gold nanospheres [150]. Findings of another study which compared tumor penetration of nanorod and nanosphere particles showed that nanorods could penetrate tumor about 1.7 times more than nanospheres with the same hydrodynamic diameter [151].
Surface charge
The other factor that controls NP delivery to the tumors is the surface charge, which plays a critical role in the absorption of biomolecules. Positive charge NPs have higher tumor delivery efficacy in comparison to negative charged NPs, which can be associated with the electrostatic interactions between the negative charge surface of cells and the NPs. During DDSs’ circulation in the bloodstream, they are covered with a layer called “biocorona” that may lead to the rapid elimination of NPs [79]. The composition of the biocorona is significantly linked to the NP physicochemical properties (size, shape, charge, …). Studies have shown that the size of the NPs affects the composition and thickness of the biocrona layer [152]. The DDS type can affect the amount of biocorona composition and the toxicity of the DDS to the body. For example, the results of a study suggest that regardless of physicochemical properties, the adsorption of biocorona by AUNP is significantly influenced the biological effect of NP through the reduction of sole NP-negative hemotoxic [153]. Other findings suggest that regardless of NP chemical composition, proteins interact differently with NP, and some proteins, such as HSA, interact more strongly with the NP. The HAS-NP complex showed higher cellular uptake in comparison to fibrinogen-bound NP [154].
Safety
Despite numerous studies in nanotechnology, achieving effective and safe DDS remains a major challenge. The specific physical and chemical properties of NPs can lead to serious and unpredictable side effects in the human body [155]. Safety of a novel DDS must be strictly analyzed to prevent development of side effects in clinic which maybe the first step toward developing a successful DDS from the discovery phase to its entry into clinical trials [156]. Inorganic DDSs are amazing DDS of choice as they can overcome some of the inherent drawbacks of conventional organic DDSs [157], such as multidrug resistance due to the compositional properties such as high stability. In a recent study, a mesoporous silica-based targeting nanocarriers could circumstance multidrug resistance efficiently [158]; however, there was some unsolved matters such as toxicity and side effects which are the major drawback of inorganic DDS which may be lessen by some modifications in their structure by application of biocompatible polymers [156]. Polymer-based NPs can be consisting of biodegradable and nonbiodegradable parts. Due to the chronic toxicity and high immunological response of nonbiodegradable polymeric NPs, biodegradable NPs are more promising for application as DDSs. [159]. The results of a recent study showed the efficacy of biodegradable thermo-sensitive copolymer hydrogel for the co-delivery of gemcitabine and cis-platinum to inhibit cellular proliferation synergistically and promote apoptosis in pancreatic cancer Bxpc-3 cell [159]. Extracellular vesicle DDSs have shown unique properties which make them promising DDSs. Extracellular vesicle carriers participate in important physiological and pathological processes such as of intercellular communication, cell maintenance, tissue repair, immune modulation, and tumor growth [159]. The results of Pei et al. on the tumor immune microenvironment after administration of cRGD-modified coloaded siFGL1 and siTGF-β1 showed tumor infiltration CD8 + T cells increase and immunosuppressive cells decrease, which can be conclude as the promoted antitumor immunity in the TME [160]. Although effective, the complex manufacturing process of this DDSs limits application of these DDSs in the clinic [161]. Most of the DDSs of interest in the clinical trials and also in the clinic are lipid-based NPs. Lipid-based DDSs that present interesting benefits include but not limited to biocompatibility, simple to synthesis, and capable to deliver various therapeutic agents (chemotherapeutics, siRNA, polyphenols, peptides) [39].
Co-delivery systems in the clinical trials
As mentioned above, to deliver drugs simultaneously, several DDSs are employed, including liposomes [42], micelles [162], dendrimers [163], hydrogels [164], and antibody–drug conjugates (ADCs). Regarding trends in the delivery systems for cancer therapy (Table 3), it can be found that liposome has received more attention [100]. These spherical structures composed of phospholipid and cholesterol are highly compatible and safe with the human body [165]; also, the structure of these DDSs allows the simultaneous transport of drugs with different physicochemical properties [166].
Liposomes can be called as the simplest forms of DDSs that can protect loaded drugs from external media, which can reduce systematic toxicity and loss of drugs in off-target tissues [171]. Despite several advantages of liposomes, several studies showed high liver and spleen accumulation of these DDSs, which results in poor delivery into the target site [172]. Besides, it was found that the reticuloendothelial system eliminates liposomes rapidly [173]; to solve this problem, PEGylated liposome was introduced; however, PEGylation results in lower cellular uptake [174]. To address these issues, the natural carriers are introduced based on cell compositions. These carriers are safe as they are body-originated; they can cross physiological barriers and induce particular responses in target cells. Despite their superiority to synthetic delivery systems, no vesicle-based co-delivery system has been passed clinical trials, and more meticulous studies are needed to take advantage of these promising carriers. Elsharkasy et al. have discussed about extracellular vesicles in detail recently [175]. Conjugation of the drug molecule with an antibody, called “antibody–drug conjugates” (ADC), combines the characteristics of antibodies and drugs [176]. The presence of antibodies in these DDSs reduces the nonselective effect of the drug, minimizing side effects, and increasing tolerability and efficacy compared to the free drugs [36]. These appropriate characteristics make them interesting for combination therapy. Today, there are nine approve ADCs for cancer therapy in the clinic [177]. Between several co-delivery Emtansine is a potent antimicrotubule derivative of maytansine, and trastuzumab is a HER2 binding monoclonal antibody, which enables ADC uptake through receptor-mediated endocytosis. Kadcyla® is a coupling-based combination therapy consisting of trastuzumab and Emtansine. Clinical trials have shown the superior supporting effect of Kadcyla® compared to trastuzumab in HER2- positive BC patients [178]. Thus, it has been approved for the treatment of advanced BCs, mainly positive for HER2 [143].
Manufacturability and clinical translation: suggestions
Although nanotechnology has provided a worthy platform for the design of DDSs, due to technological limitations, such as loading method of the therapeutics (hydrophobic [179], electrostatic interaction [180] and chemical conjugation [181]), and DDIs, it is very difficult and challenging to predict nano-biological interactions in such a complex field [182]. Experimental and computational studies have enriched our knowledge of physicochemical properties of DDSs and the loaded therapeutic agent, the mechanism of action between them and nano-biological interactions including interactions with biological membranes and other biological molecules generally [183]. Theoretically, these models are based on primary screening of variables to predict appropriate conditions for successive experiments. Besides, these methods generally examine variables in detail are precious tools for high efficiency DDSs investigation [184]. Consequently, application of computational and experimental methods may be one of the best ways to proceed in the experimental process in the shorter time. Various types of computational models are presented to facilitate achieving DDS of interest considering size and continuum, which are generally employed to predict kinetics nano-biointeractions, biodistribution, and DDS penetration into target-site. Shamsia et al. discussed the recent advancements in computational modeling for nano-engineered DDSs [185]. Taking advantage of the experimental method, it is imaginable to determine the interaction effects of variables on response simultaneously [184]. In previous studies, the use of this method has been effective in accomplishing the optimal ratio and amount of formulation components to achieve the maximum drug load in the paclitaxel and lapatinib in the co-delivery system [105, 186]. Notwithstanding clear guidelines for nano medicine approved by the FDA, the growing transfer of DDSs from paper to the trials is increasing the application of nanomedicine in the clinic [187], the pharmaceutical industry is a potential barrier to combination therapy implementation. Many pharmaceutical companies are hesitant to cooperate in the clinical trials and marketing of combination therapies, which can be attributed to financial and insurance issues. On the other hand, the use of combination therapies requires special care and consideration because there are always concerns about controlling therapeutics dose in a combination and avoid side effects by such co-delivery systems [188, 189]. These studies are time-consuming due to the complicated process needed to prove the superiority of co-delivery systems to the bare form combination therapies. Therefore, combination therapy by co-delivery systems seems more expensive than the traditional treatment approaches. On the other hand, recent findings suggest that the extraordinary advantages of co-delivery systems are worth the short-term cost of these studies [190,191,192]. From this point of view, designing co-delivery systems for cancer treatment is of utmost prominence.
Conclusions and suggestions
An expanding understanding of cancer physiopathology offers considerable advances and accomplishments in cancer treatment. In light of various studies, the superiority of combination therapy is concluded. Co-delivery systems hold countless promises in improving the pharmacokinetics of the therapeutics and control release properties. Far too frequently, the wide gap separating preclinical research and approved co-delivery systems indicated that in vitro and in vivo studies fail to simulate human physiopathology. Despite the variety of DDSs, few co-delivery systems enter clinical trials, and a negligible number of them successfully pass the phases of clinical trials. Thus, the design of DDSs in this area requires careful and original studies. First of all, the choice of the appropriate drug combination should be made by computational designs: Furthermore, basic research is needed to achieve the optimal combination index based on the DDIs and pharmacokinetics of each drug. Additionally, in vitro models should be harmonious with animal models, and the selected animal models should be able to simulate human tumor histopathology to bridge preclinical and clinical models. Predictive biomarkers can facilitate patient preselection for delivering specific and personalized treatment. In the formulation part, DDS design should be based on the computational studies to find the optimal formulations regarding physicochemical properties, pharmacokinetics, loading capacity, targeting the ability, and controlled release profile of the NP. Most importantly, experimental designs should be employed to ensure the proper drug ratio, pharmacokinetics, biological distribution, and adequate drug concentration at the tumor site to fulfill enhanced therapeutic efficacy. In the end, it should be noted that manufacturability, a limiting factor in developing co-delivery systems, should be considered by implementing simple, straightforward, and affordable preparation methods to translate lab studies to industrial-scale fabrication.
References
Norouzi M, Amerian M, Amerian M, Atyabi F. Clinical applications of nanomedicine in cancer therapy. Drug Discov Today. 2020 Jan;25(1):107–25.
Halsted WS. The results of radical operations for the cure of carcinoma of the breast. Ann Surg. 1907;46:1–19.
Murthy SM, Goldschmidt RA, Rao LN, Ammirati M, Buchmann T, Scanlon EF. The influence of surgical trauma on experimental metastasis. Cancer. 1989;64:2035–44.
Demicheli R, Retsky MW, Hrushesky WJM, Baum M, Gukas ID. The effects of surgery on tumor growth: a century of investigations. Ann Oncol. 2008;19(11):1821–8.
Coffey JC, Smith MJF, Wang JH, Bouchier-Hayes D, Cotter TG, Redmond HP. Cancer surgery: risks and opportunities. BioEssays. 2006;28(4):433–7.
Zhou Z, D’Emanuele A, Attwood D. Solubility enhancement of paclitaxel using a linear-dendritic block copolymer. Int J Pharm. 2013;452(1–2):173–9.
Williams PA, Cao S, Yang D, Jennelle RL. Patient-reported outcomes of the relative severity of side effects from cancer radiotherapy. Support Care Cancer. 2020;28:309–16.
Lammers T, Kiessling F, Hennink WE, Storm G. Drug targeting to tumors: principles pitfalls and (pre-) clinical progress. J Control Release. 2012;161(2):175–87.
Aluise CD, Sultana R, Tangpong J, Vore M, St Clair D, Moscow JA, et al. Chemo Brain (Chemo Fog) as a potential side effect of doxorubicin administration: role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv Exp Med Biol. 2010;678:147–56.
Song IS, Savaraj N, Siddik ZH, Liu P, Wei Y, Wu CJ, Kuo MT. Role of human copper transporter Ctr1 in the transport of platinum-based antitumor agents in cisplatin-sensitive and cisplatin-resistant cells. Mol Cancer Ther. 2004;3(12):1543–9.
Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–74.
van Helden EJ, Angus L, Menke-van der Houven van Oordt CW, Heideman DAM, Boon E, van Es SC, et al. RAS and BRAF mutations in cell-free DNA are predictive for outcome of cetuximab monotherapy in patients with tissue-tested RAS wild-type advanced colorectal cancer. Mol Oncol. 2019;13:2361–74.
Smith CC, Wang Q, Chin CS, Salerno S, Damon LE, Levis MJ, Perl AE, Travers KJ, Wang S, Hunt JP, Zarrinkar PP. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485(7397):260–3.
Popilski H, Abtew E, Schwendeman S, Domb A, Stepensky D. Efficacy of paclitaxel/dexamethasone intra-tumoral delivery in treating orthotopic mouse breast cancer. J Control Release. 2018;279:1–7.
O’Connor TL, Kossoff E. Delayed seizure associated with paclitaxel-Cremophor EL in a patient with early-stage breast cancer. Pharmacotherapy. 2009;29:993–6.
Shi C, Zhang Z, Shi J, Wang F, Luan Y. Co-delivery of docetaxel and chloroquine via PEO-PPO-PCL/TPGS micelles for overcoming multidrug resistance. Int J Pharm. 2015;495:932–9.
Zinutti C, Barberi-Heyob M, Hoffman M, Maincent P. In-vivo evaluation of sustained release microspheres of 5-FU in rabbits. Int J Pharm. 1998;166(2):231–4.
Zanardi E, Bregni G, De Braud F, Di Cosimo S. Better together: targeted combination therapies in breast cancer. Semin Oncol. 2015;42(6):887–95.
Socinski MA, Bondarenko I, Karaseva NA, Makhson AM, Vynnychenko I, Okamoto I, et al. Weekly nab-paclitaxel in combination with carboplatin versus solvent-based paclitaxel plus carboplatin as first-line therapy in patients with advanced non-small-cell lung cancer: final results of a phase III trial. J Clin Oncol. 2012;30(17):2055–62.
Cardoso F, Bedard PL, Winer EP, Pagani O, Senkus-Konefka E, Fallowfield LJ, Kyriakides S, Costa A, Cufer T, Albain KS, ESO-MBC Task Force. International guidelines for management of metastatic breast cancer: combination vs sequential single-agent chemotherapy. J Natl Cancer Inst. 2009;101(17):1174–81.
Van Cutsem E, Nordlinger B, Cervantes A, ESMO Guidelines Working Group. Advanced colorectal cancer: ESMO Clinical Practice Guidelines for treatment. Ann Oncol. 2010;21:93–7.
Jonker D, Rumble RB, Maroun J, Gastrointestinal Cancer Disease Site Group of Cancer Care Ontario’s Program in Evidence-Based Care. Role of oxaliplatin combined with 5-fluorouracil and folinic acid in the first-and second-line treatment of advanced colorectal cancer. Curr Oncol. 2006;13(5):173–84.
Shameer K, Readhead B, T Dudley J. Computational and experimental advances in drug repositioning for accelerated therapeutic stratification. Curr Top Med Chem. 2015;15(1):5–20.
Melero I, Berman DM, Aznar MA, Korman AJ, Gracia JL, Haanen J. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15(8):457–72.
Whirl-Carrillo M, McDonagh EM, Hebert JM, Gong L, Sangkuhl K, Thorn CF, et al. Pharmacogenomics knowledge for personalized medicine. Clin Pharmacol Ther. 2012;92(4):414–7.
Baselga J, Bradbury I, Eidtmann H, Di Cosimo S, De Azambuja E, Aura C, Gómez H, Dinh P, Fauria K, Van Dooren V, Aktan G. Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised open-label multicentre phase 3 trial. Lancet. 2012;379(9816):633–40.
Wang Y, Du C, Zhao Y, Nie G, Yang Y. Trap and kill strategy for non-BRCA mutant pancreatic cancer by co-delivery of olaparib and JQ1 with plectin-1 targeting peptide nanoparticles. Nano Today. 2020;33:100877.
Zaman A, Wu W, Bivona TG. Targeting oncogenic BRAF: Past present and future. Cancer. 2019;11(8):1197.
Raoof S, Mulford IJ, Frisco-Cabanos H, Nangia V, Timonina D, Labrot E, Hafeez N, Bilton SJ, Drier Y, Ji F, Greenberg M. Targeting FGFR overcomes EMT-mediated resistance in EGFR mutant non-small cell lung cancer. Oncogene. 2019;38(37):6399–413.
Lopez JS, Banerji U. Combine and conquer: challenges for targeted therapy combinations in early phase trials. Nat Rev Clin Oncol. 2017;14(1):57–66.
Durand JB, Valero V, Lenihan DJ, Ewer MS, Vooletich MT, Woods ML, et al. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment predictive value of circulating tumor cells view project reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. Artic J Clin Oncol. 2005;23:7820–6.
Chowdhary M, Patel KR, Danish HH, Lawson DH, Khan MK. BRAF inhibitors and radiotherapy for melanoma brain metastases: potential advantages and disadvantages of combination therapy. OncoTargets Ther. 2016;9:7149.
Zhang Y, Ho SH, Li B, Nie G, Li S. Modulating the tumor microenvironment with new therapeutic nanoparticles: A promising paradigm for tumor treatment. Med Res Rev. 2020;40(3):1084–102.
Ehling J, Theek B, Gremse F, Baetke S, Möckel D, Maynard J, Ricketts SA, Grüll H, Neeman M, Knuechel R, Lederle W. Micro-CT imaging of tumor angiogenesis: quantitative measures describing micromorphology and vascularization. Am J Pathol. 2014;184(2):431–41.
Handali S, Moghimipour E, Rezaei M, Ramezani Z, Kouchak M, Amini M, et al. A novel 5-Fluorouracil targeted delivery to colon cancer using folic acid conjugated liposomes. Biomed Pharmacother. 2018;108:1259–73.
Ulbrich K, Etrych T, Chytil P, Jelínková M, Říhová B. Antibody-targeted polymer-doxorubicin conjugates with pH-controlled activation. J Drug Target. 2004;12:477–89.
Cheng T, Zhang Y, Liu J, Ding Y, Ou H, Huang F, et al. Ligand-switchable micellar nanocarriers for prolonging circulation time and enhancing targeting efficiency. ACS Appl Mater Interfaces. 2018;10:5296–304.
Zang X, Lee JB, Deshpande K, Garbuzenko OB, Minko T, Kagan L. Prevention of paclitaxel-induced neuropathy by formulation approach. J Control Release. 2019;303:109–16.
Ravar F, Saadat E, Gholami M, Dehghankelishadi P, Mahdavi M, Azami S, Dorkoosh FA. Hyaluronic acid-coated liposomes for targeted delivery of paclitaxel in-vitro characterization and in-vivo evaluation. J Control Release. 2016;229:10–22.
Saadat E, Amini M, Khoshayand MR, Dinarvand R, Dorkoosh FA. Synthesis and optimization of a novel polymeric micelle based on hyaluronic acid and phospholipids for delivery of paclitaxel in vitro and in-vivo evaluation. Int J Pharm. 2014;475:163–73.
Wu J, Zhang H, Hu X, Liu R, Jiang W, Li Z, Luan Y. Reduction-sensitive mixed micelles assembled from amphiphilic prodrugs for self-codelivery of DOX and DTX with synergistic cancer therapy. Colloids Surf B Biointerfaces. 2018;161:449–56.
Yu J, Chen H, Jiang L, Wang J, Dai J, Wang J. Codelivery of adriamycin and P-gp inhibitor quercetin using PEGylated liposomes to overcome cancer drug resistance. J Pharm Sci. 2019;108:1788–99.
Naderinezhad S, Amoabediny G, Haghiralsadat F. Co-delivery of hydrophilic and hydrophobic anticancer drugs using biocompatible pH-sensitive lipid-based nano-carriers for multidrug-resistant cancers. RSC Adv. 2017;7(48):30008–19.
Zhang L, Su H, Liu Y, Pang N, Li J, Qi XR. Enhancing solid tumor therapy with sequential delivery of dexamethasone and docetaxel engineered in a single carrier to overcome stromal resistance to drug delivery. J Control Release. 2019;294:1–6.
Meng F, Wang J, Ping Q, Yeo Y. Camouflaging nanoparticles for ratiometric delivery of therapeutic combinations. Nano Lett. 2019;19(3):1479–87.
Zhang RX, Wong HL, Xue HY, Eoh JY, Wu XY. Nanomedicine of synergistic drug combinations for cancer therapy–Strategies and perspectives. J Control Release. 2016;240:489–503.
Clohessy JG, Pandolfi PP. Mouse hospital and co-clinical trial project—from bench to bedside. Nat Rev Clin Oncol. 2015;12:491.
Palmer AC, Sorger PK. Combination cancer therapy can confer benefit via patient-to-patient variability without drug additivity or synergy. Cell. 2017;171(7):1678–91.
Chakrabarti S, Michor F. Pharmacokinetics and drug interactions determine optimum combination strategies in computational models of cancer evolution. Cancer Res. 2017;77:3908–21.
Sun W, Sanderson PE, Zheng W. Drug combination therapy increases successful drug repositioning. Drug Discov Today. 2016;21(7):1189–95.
Chen J, Weihs D, Vermolen FJ. Computational modeling of therapy on pancreatic cancer in its early stages. 2020;19:427–44.
Al-Lazikani B, Banerji U, Workman P. Combinatorial drug therapy for cancer in the post-genomic era. Nat Biotechnol. 2012;30(7):679–92.
Hirsch C, Roesslein M, Krug HF, Wick P. Nanomaterial cell interactions: are current in vitro tests reliable? Nanomedicine. 2011;6:837–47.
Chen F, Zhuang X, Lin L, Yu P, Wang Y, Shi Y, Hu G, Sun Y. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13(1):1–4.
Köbel M, Kalloger SE, Huntsman DG, Santos JL, Swenerton KD, Seidman JD, Gilks CB. Differences in tumor type in low-stage versus high-stage ovarian carcinomas. Int J Gynecol Pathol. 2010;29(3):203–11.
Bedard PL, Hansen AR, Ratain MJ, Siu LL. Tumour heterogeneity in the clinic. Nature. 2013;501(7467):355–64.
Tsai S, McOlash L, Palen K, Johnson B, Duris C, Yang Q, et al. Development of primary human pancreatic cancer organoids matched stromal and immune cells and 3D tumor microenvironment models. BMC Cancer. 2018;18(1):1–3.
Matsumura Y. Cancer stromal targeting (CAST) therapy. Adv Drug Deliv Rev. 2012;64(8):710–9.
Bradford JR, Wappett M, Beran G, Logie A, Delpuech O, Brown H, Boros J, Camp NJ, McEwen R, Mazzola AM, D’Cruz C. Whole transcriptome profiling of patient-derived xenograft models as a tool to identify both tumor and stromal specific biomarkers. Oncotarget. 2016;7(15):20773.
Meehan TF, Conte N, Goldstein T, Inghirami G, Murakami MA, Brabetz S, et al. Focus on computer resources PDX-MI: minimal information for patient-derived tumor xenograft models. Cancer Res. 2017;77(21):62–6.
Ricci F, Guffanti F, Affatato R, Brunelli L, Roberta P, Fruscio R, Perego P, Bani MR, Chiorino G, Rinaldi A, Bertoni F. Establishment of patient-derived tumor xenograft models of mucinous ovarian cancer. Am J Cancer Res. 2020;10(2):572.
Matthews RAJ. Medical progress depends on animal models-doesn’t it? J R Soc Med. 2008;101(2):95–8.
Mak IW, Evaniew N, Ghert M. Lost in translation: animal models and clinical trials in cancer treatment. Am J Transl Res. 2014;6(2):114.
Bhimani J, Ball K, Stebbing J. Patient-derived xenograft models—the future of personalised cancer treatment. Br J Cancer. 2020;122:601–2.
Ter Brugge P, Kristel P, Van Der Burg E, Boon U, De Maaker M, Lips E, Mulder L, De Ruiter J, Moutinho C, Gevensleben H, Marangoni E. Mechanisms of therapy resistance in patient-derived xenograft models of BRCA1-deficient breast cancer. J Natl Cancer Inst. 2016;108(11).
Byrne AT, Alférez DG, Amant F, Annibali D, Arribas J, Biankin AV, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254.
Zhang X, Claerhout S, Prat A, Dobrolecki LE, Petrovic I, Lai Q, et al. A renewable tissue resource of phenotypically stable biologically and ethnically diverse patient-derived human breast cancer xenograft models. Cancer Res. 2013;73:4885–97.
Stebbing J, Paz K, Schwartz GK, Wexler LH, Maki R, Pollock RE, et al. Patient-derived xenografts for individualized care in advanced sarcoma. Cancer. 2014;120:2006–15.
Wan X, Beaudoin JJ, Vinod N, Min Y, Makita N, Bludau H, Jordan R, Wang A, Sokolsky M, Kabanov AV. Co-delivery of paclitaxel and cisplatin in poly (2-oxazoline) polymeric micelles: Implications for drug loading release pharmacokinetics and outcome of ovarian and breast cancer treatments. Biomaterials. 2019;192:1–4.
Shim G, Kim MG, Kim D, Park JY, Oh YK. Nanoformulation-based sequential combination cancer therapy. Adv Drug Deliv Rev. 2017;115:57–81.
Salvioni L, Rizzuto MA, Bertolini JA, Pandolfi L, Colombo M, Prosperi D. Thirty years of cancer nanomedicine: success frustration and hope. Cancer. 2019;11(12):1855.
Mohammad IS, Teng C, Chaurasiya B, Yin L, Wu C, He W. Drug-delivering-drug approach-based codelivery of paclitaxel and disulfiram for treating multidrug-resistant cancer. Int J Pharm. 2019;557:304–13.
Wacker M. Nanocarriers for intravenous injection—the long hard road to the market. Int J Pharm. 2013;457(1):50–62.
Dehghankelishadi P, Saadat E, Ravar F, Safavi M, Pordeli M, Gholami M, et al. In vitro and in vivo evaluation of paclitaxel–lapatinib-loaded F127 pluronic micelles. Drug Dev Ind Pharm. 2017;43:390–8.
Peng H, Chen B, Huang W, Tang Y, Jiang Y, Zhang W, et al. Reprogramming tumor-associated macrophages to reverse EGFRT790M resistance by dual-targeting codelivery of Gefitinib/Vorinostat. Nano Lett. 2017;17:7684–90.
Li Y, Luo J, Lin MT, Zhi P, Guo WW, Han M, et al. Co-delivery of metformin enhances the antimultidrug resistant tumor effect of doxorubicin by improving hypoxic tumor microenvironment. Mol Pharm. 2019;16(7):2966–79.
Du C, Qi Y, Zhang Y, Wang Y, Zhao X, Min H, et al. Epidermal growth factor receptor-targeting peptide nanoparticles simultaneously deliver gemcitabine and olaparib to treat pancreatic cancer with breast cancer 2 (BRCA2) mutation. ACS Nano. 2018;12:10785–96.
Wang H, Wu J, Xie K, Fang T, Chen C, Xie H, et al. Precise engineering of prodrug cocktails into single polymeric nanoparticles for combination cancer therapy: extended and sequentially controllable drug release. ACS Appl Mater Interfaces. 2017;9:10567–76.
Cervantes A, Alsina M, Tabernero J, Infante JR, LoRusso P, Shapiro G, et al. Phase I dose-escalation study of ALN-VSP02 a novel RNAi therapeutic for solid tumors with liver involvement. J Clin Oncol. 2011;29:3025–3025.
Chen W, Shi K, Chu B, Wei X, Qian Z. Mitochondrial surface engineering for multidrug resistance reversal. Nano Lett. 2019;19:2905–13.
Xie J, Lu Y, Yu B, Wu J, Liu J. Galactose-modified enzymatic synthesis of poly(amino-co-ester) micelles for co-delivery miR122 and sorafenib to inhibit hepatocellular carcinoma development. Chinese Chem Lett. 2020;31:1173–7.
Wang T, Mu W, Li F, Zhang J, Hou T, Pang X, et al. “Layer peeling” co-delivery system for enhanced RNA interference-based tumor associated macrophages-specific chemoimmunotherapy. Nanoscale. 2020;12:16851–63.
Sun J, Chen Y, Huang Y, Zhao W, Liu Y, Venkataramanan R, et al. Programmable co-delivery of the immune checkpoint inhibitor NLG919 and chemotherapeutic doxorubicin via a redox-responsive immunostimulatory polymeric prodrug carrier. Acta Pharmacol Sin. 2017;38:823–34.
Mei KC, Liao YP, Jiang J, Chiang M, Khazaieli M, Liu X, et al. Liposomal delivery of mitoxantrone and a cholesteryl indoximod prodrug provides effective chemo-immunotherapy in multiple solid tumors. ACS Nano. 2020;14:13343–66.
Yin Y, Hu Q, Xu C, Qiao Q, Qin X, Song Q, et al. Co-delivery of doxorubicin and interferon-γ by thermosensitive nanoparticles for cancer immunochemotherapy. Mol Pharm. 2018;15:4161–72.
Gao H. Shaping tumor microenvironment for improving nanoparticle delivery. Curr Drug Metab. 2016;17(8):731–6.
Maeda H. Toward a full understanding of the EPR effect in primary and metastatic tumors as well as issues related to its heterogeneity. Adv Drug Deliv Rev. 2015;91:3–6.
Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JS. Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res. 2001;7(2):243–54.
Wang Y, Wang Z, Xu C, Tian H, Chen X. A disassembling strategy overcomes the EPR effect and renal clearance dilemma of the multifunctional theranostic nanoparticles for cancer therapy. Biomaterials. 2019;197:284–93.
Zahmatkeshan M, Gheybi F, Rezayat SM, Jaafari MR. Improved drug delivery and therapeutic efficacy of PEgylated liposomal doxorubicin by targeting anti-HER2 peptide in murine breast tumor model. Eur J Pharm Sci. 2016;86:125–35.
Barenholz YC. Doxil®—the first FDA-approved nano-drug: lessons learned. J Control Release. 2012;160(2):117–34.
Dadson K, Calvillo-Argüelles O, Thavendiranathan P, Billia F. Anthracycline-induced cardiomyopathy: cellular and molecular mechanisms. Clin Sci. 2020;134(13):1859–85.
Sala V, Della Sala A, Hirsch E, Ghigo A. Signaling pathways underlying anthracycline cardiotoxicity. Antioxid Redox Signal. 2020;32(15):1098–114.
Cagel M, Moretton MA, Bernabeu E, Zubillaga M, Lagomarsino E, Vanzulli S, Nicoud MB, Medina VA, Salgueiro MJ, Chiappetta DA. Antitumor efficacy and cardiotoxic effect of doxorubicin-loaded mixed micelles in 4T1 murine breast cancer model: Comparative studies using Doxil® and free doxorubicin. J Drug Del Sci Technol. 2020;56:101506.
Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–92.
Lakkadwala S, dos Santos RB, Sun C, Singh J. Dual functionalized liposomes for efficient co-delivery of anti-cancer chemotherapeutics for the treatment of glioblastoma. J Control Release. 2019;307:247–60.
Batist G, Sawyer M, Gabrail N, Christiansen N, Marshall JL, Spigel DR, et al. A multicenter phase II study of CPX-1 liposome injection in patients (pts) with advanced colorectal cancer (CRC). J Clin Oncol. 2008;26:4108–4108.
Ağardan NM, Değim Z, Yılmaz Ş, Altıntaş L, Topal T. Tamoxifen/raloxifene loaded liposomes for oral treatment of breast cancer. J Drug Deliv Sci Technol. 2020;57:101612.
Okamoto Y, Taguchi K, Imoto S, Chuang VT, Yamasaki K, Otagiri M. Cell uptake and anti-tumor effect of liposomes containing encapsulated paclitaxel-bound albumin against breast cancer cells in 2D and 3D cultured models. J Drug Deliv Sci Technol. 2020;55:101381.
Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, Catane R, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil®) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15:440–9.
Espelin CW, Leonard SC, Geretti E, Wickham TJ, Hendriks BS. Dual HER2 targeting with trastuzumab and liposomal-encapsulated doxorubicin (MM-302) demonstrates synergistic antitumor activity in breast and gastric cancer. Cancer Res. 2016;76(6):1517–27.
Reynolds JG, Geretti E, Hendriks BS, Lee H, Leonard SC, Klinz SG, Noble CO, Lücker PB, Zandstra PW, Drummond DC, Olivier KJ Jr. HER2-targeted liposomal doxorubicin displays enhanced anti-tumorigenic effects without associated cardiotoxicity. Toxicol Appl Pharmacol. 2012;262(1):1.
Munster P, Krop IE, LoRusso P, Ma C, Siegel BA, Shields AF, Molnár I, Wickham TJ, Reynolds J, Campbell K, Hendriks BS. Safety and pharmacokinetics of MM-302 a HER2-targeted antibody–liposomal doxorubicin conjugate in patients with advanced HER2-positive breast cancer: a phase 1 dose-escalation study. Br J Cancer. 2018;119(9):1086–93.
Hu CMJ, Aryal S, Zhang L. Nanoparticle-assisted combination therapies for effective cancer treatment. Ther Deliv. 2010;1:323–34.
Kelishady PD, Saadat E, Ravar F, Akbari H, Dorkoosh F. Pluronic F127 polymeric micelles for co-delivery of paclitaxel and lapatinib against metastatic breast cancer: preparation optimization and in vitro evaluation. Pharm Dev Technol. 2015;20:1009–17.
Sheu MT, Jhan HJ, Su CY, Chen LC, Chang CE, Liu DZ, Ho HO. Codelivery of doxorubicin-containing thermosensitive hydrogels incorporated with docetaxel-loaded mixed micelles enhances local cancer therapy. Colloids Surf B Biointerfaces. 2016;143:260–70.
Yu J, Ha W, Chen J, Shi YP. pH-Responsive supramolecular hydrogels for codelivery of hydrophobic and hydrophilic anticancer drugs. RSC Adv. 2014;4(103):58982–9.
Lin T, Ryan DH, Ritchie EK, Strickland SA, Hogge DE, Solomon SR, Kolitz JE, Schiller GJ, Wieduwilt MJ, Ryan RJ, Faderl S. Efficacy and Safety of CPX-351 Versus 7+ 3 in a Phase 3 Exploratory Analysis in Patients with High-Risk/Secondary Acute Myeloid Leukemia (sAML) with Prior Hypomethylating Agent (HMA) Exposure Who Achieved Remission. Biol Blood Marrow Transplant. 2020;26(3):S102.
Feldman EJ, Lancet JE, Kolitz JE, Ritchie EK, Roboz GJ, List AF, Allen SL, Asatiani E, Mayer LD, Swenson C, Louie AC. First-in-man study of CPX-351: a liposomal carrier containing cytarabine and daunorubicin in a fixed 5: 1 molar ratio for the treatment of relapsed and refractory acute myeloid leukemia. J Clin Oncol. 2011;29(8):979.
Krauss AC, Gao X, Li L, Manning ML, Patel P, Fu W, Janoria KG, Gieser G, Bateman DA, Przepiorka D, Shen YL. FDA approval summary:(daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019 May 1;25(9):2685-90.
Xin Y, Huang M, Guo WW, Huang Q, Zhen Zhang L, Jiang G. Nano-based delivery of RNAi in cancer therapy. Mol Cancer. 2017;16(1):1–9.
Degors IMS, Wang C, Rehman ZU, Zuhorn IS. Carriers break barriers in drug delivery: endocytosis and endosomal escape of gene delivery vectors. Acc Chem Res. 2019;52:1750–60.
Salem A, Asselin MC, Reymen B, Jackson A, Lambin P, West CM, et al. Targeting hypoxia to improve non–small cell lung cancer outcome. J Natl Cancer Inst. 2018;110(1):14–30.
Qin J, Liu Y, Lu Y, Liu M, Li M, Li J, Wu L. Hypoxia-inducible factor 1 alpha promotes cancer stem cells-like properties in human ovarian cancer cells by upregulating SIRT1 expression. Sci Rep. 2017;7(1):1–2.
Dulskas A, Patasius A, Linkeviciute-Ulinskiene D, Zabuliene L, Urbonas V, Smailyte G. Metformin increases cancer specific survival in colorectal cancer patients—National cohort study. Cancer Epidemiol. 2019;62:101587.
Sesen J, Dahan P, Scotland SJ, Saland E, Dang VT, Lemarié A, et al. Metformin inhibits growth of human glioblastoma cells and enhances therapeutic response. PLoS One. 2015;10(4):e0123721.
Dowling RJ, Lam S, Bassi C, Mouaaz S, Aman A, Kiyota T, Al-Awar R, Goodwin PJ, Stambolic V. Metformin pharmacokinetics in mouse tumors: implications for human therapy. Cell Metab. 2016;23(4):567–8.
Zhou Z. Co-drug delivery of regorafenib and cisplatin with amphiphilic copolymer nanoparticles: enhanced in vivo antitumor cancer therapy in nursing care. Drug Deliv. 2020;27:1319–28.
Zhao P, Yin W, Wu A, Tang Y, Wang J, Pan Z, et al. Dual-targeting to cancer cells and M2 macrophages via biomimetic delivery of mannosylated albumin nanoparticles for drug-resistant cancer therapy. Adv Funct Mater. 2017;27:1–15.
Zhu YH, Sun CY, Shen S, Khan MIU, Zhao YY, Liu Y, et al. A micellar cisplatin prodrug simultaneously eliminates both cancer cells and cancer stem cells in lung cancer. Biomater Sci. 2017;5:1612–21.
Li Y, Xu X, Zhang X, Li Y, Zhang Z, Gu Z. Tumor-specific multiple stimuli-activated dendrimeric nanoassemblies with metabolic blockade surmount chemotherapy resistance. ACS Nano. 2017;11:416–29.
Zhang BF, Xing L, Cui PF, Wang FZ, Xie RL, Zhang JL, et al. Mitochondria apoptosis pathway synergistically activated by hierarchical targeted nanoparticles co-delivering siRNA and lonidamine. Biomaterials. 2015;61:178–89.
Xu C, Tian H, Wang P, Wang Y, Chen X. The suppression of metastatic lung cancer by pulmonary administration of polymer nanoparticles for co-delivery of doxorubicin and Survivin siRNA. Biomater Sci. 2016;4:1646–54.
Ahmadi S, Rabiee N, Bagherzadeh M, Elmi F, Fatahi Y, Farjadian F, et al. Stimulus-responsive sequential release systems for drug and gene delivery. Nano Today. 2020;34:100914.
Guo S, Lin CM, Xu Z, Miao L, Wang Y, Huang L. Co-delivery of cisplatin and rapamycin for enhanced anticancer therapy through synergistic effects and microenvironment modulation. ACS Publ. 2014;8:4996–5009.
Ji T, Li S, Zhang Y, Lang J, Ding Y, Zhao X, et al. An MMP-2 responsive liposome integrating antifibrosis and chemotherapeutic drugs for enhanced drug perfusion and efficacy in pancreatic cancer. ACS Appl Mater Interfaces. 2016;8:3438–45.
Zhou S, Zhang T, Peng B, Luo X, Liu X, Hu L, Liu Y, Di D, Song Y, Deng Y. Targeted delivery of epirubicin to tumor-associated macrophages by sialic acid-cholesterol conjugate modified liposomes with improved antitumor activity. Int J Pharm. 2017;523(1):203–16.
Jiang T, Mo R, Bellotti A, Zhou J, Gu Z. Gel-liposome-mediated co-delivery of anticancer membrane-associated proteins and small-molecule drugs for enhanced therapeutic efficacy. Adv Funct Mater. 2014;24:2295–304.
Palesh O, Scheiber C, Kesler S, Mustian K, Koopman C, Schapira L. Management of side effects during and post-treatment in breast cancer survivors. Breast J. 2018;24:167–75.
de Pauli PM, Araújo ALD, Arboleda LPA, Palmier NR, Fonsêca JM, Gomes-Silva W, et al. Tumor safety and side effects of photobiomodulation therapy used for prevention and management of cancer treatment toxicities: A systematic review. Oral Oncol. 2019;93:21–8.
Huang M, Ji Y, Yan J, Qi T, Zhang SF, Li T, et al. A nano polymer conjugate for dual drugs sequential release and combined treatment of colon cancer and thrombotic complications. Mater Sci Eng C. 2020;110:110697.
Wu H, Wang Y, Zhang Y, Xu F, Chen J, Duan L, Zhang T, Wang J, Zhang F. Breaking the vicious loop between inflammation, oxidative stress and coagulation, a novel anti-thrombus insight of nattokinase by inhibiting LPS-induced inflammation and oxidative stress. Redox Biol. 2020 May 1;32:101500.
Petre CE, Dittmer DP. Liposomal daunorubicin as treatment for Kaposi’s sarcoma. Int J Nanomedicine. 2007;2(3):277–88.
Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66:3271–8.
Venkatakrishnan K, Liu Y, Noe D, Mertz J, Bargfrede M, Marbury T, et al. Pharmacokinetics and pharmacodynamics of liposomal mifamurtide in adult volunteers with mild or moderate hepatic impairment. Br J Clin Pharmacol. 2014;77:998–1010.
Ohlmann CH, Gross-Langenhoff M. Efficacy and tolerability of leuprorelin acetate (Eligard®) in daily practice in Germany: pooled data from 2 prospective non-interventional studies with 3-or 6-month depot formulations in patients with advanced prostate cancer. Urol Int. 2018;100(1):66–71.
Zhang WW, Li L, Li D, Liu J, Li X, Li W, et al. The first approved gene therapy product for cancer Ad-p53 (Gendicine): 12 years in the clinic. Hum Gene Ther. 2018;29(2):160–79.
Kim JY, Do YR, Song HS, Cho YY, Ryoo HM, Bae SH, Kim JG, Chae YS, Kang BW, Baek JH, Kim MK. Multicenter phase II clinical trial of Genexol-PM® with gemcitabine in advanced biliary tract cancer. Anticancer Res. 2017;37(3):1467–73.
Miele E, Spinelli GP, Miele E, Tomao F, Tomao S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int J Nanomedicine. 2009;4:99.
Silverman JA, Deitcher SR. Marqibo® (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemother Pharmacol. 2013;71(3):555–64.
Crain ML. Daunorubicin & Cytarabine liposome (vyxeos). Oncol Time. 2018;40(10):30.
Fenwarth L, Fournier E, Cheok M, Boyer T, Gonzales F, Castaigne S, et al. Biomarkers of Gemtuzumab Ozogamicin response for acute myeloid leukemia treatment. Int J Mol Sci. 2020;21:5626.
Guerin M, Sabatier R, Goncalves A. Trastuzumab emtansine (Kadcyla (®)) approval in HER2-positive metastatic breast cancers. Bull Cancer. 2015;102(4):390–7.
Wilhelm S, Tavares AJ, Dai Q, Ohta S, Audet J, Dvorak HF, Chan WC. Analysis of nanoparticle delivery to tumours. Nat Rev Mater. 2016;1(5):1–2.
Dai Q, Wilhelm S, Ding D, Syed AM, Sindhwani S, Zhang Y, et al. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano. 2018;12:8423–35.
Cheng YH, He C, Riviere JE, Monteiro-Riviere NA, Lin Z. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano. 2020;14:3075–95.
Kang H, Rho S, Stiles WR, Hu S, Baek Y, Hwang DW, et al. Size-dependent EPR effect of polymeric nanoparticles on tumor targeting. Adv Healthc Mater. 2020;9(1):1901223.
Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2.
Banerjee A, Qi J, Gogoi R, Wong J, Mitragotri S. Role of nanoparticle size shape and surface chemistry in oral drug delivery. J Control Release. 2016;238:176–85.
Arnida, Janát-Amsbury MM, Ray A, Peterson CM, Ghandehari H. Geometry and surface characteristics of gold nanoparticles influence their biodistribution and uptake by macrophages. Eur J Pharm Biopharm. 2011;77:417–23.
Chauhan VP, Popović Z, Chen O, Cui J, Fukumura D, Bawendi MG, et al. Fluorescent nanorods and nanospheres for real-time in vivo probing of nanoparticle shape-dependent tumor penetration. Angew Chemie. 2011;50:11417–20.
Lundqvist M, Stigler J, Cedervall T, Berggård T, Flanagan MB, Lynch I, et al. The evolution of the protein corona around nanoparticles: a test study. ACS Nano. 2011;5:7503–9.
Sasidharan A, Chandran P, Monteiro-Riviere NA. Biocorona bound gold nanoparticles augment their hematocompatibility irrespective of size or surface charge. ACS Biomater Sci Eng. 2016;2:1608–18.
Sasidharan A, Riviere JE, Monteiro-Riviere NA. Gold and silver nanoparticle interactions with human proteins: impact and implications in biocorona formation. J Mater Chem B. 2015;3(10):2075–82.
Nikolova M, Slavchov R, Nikolova G. Nanotechnology in medicine. Drug Discov Eval Methods Clin Pharmacol. 2020;533–46.
Khandekar SV, Kulkarni MG, Devarajan PV. Polyaspartic acid functionalized gold nanoparticles for tumor targeted doxorubicin delivery. J Biomed Nanotechnol. 2014;10:143–53.
Maier-Hauff K, Ulrich F, Nestler D, Niehoff H, Wust P, Thiesen B, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317–24.
Chen Y, Chen H, Shi J. Inorganic nanoparticle-based drug codelivery nanosystems to overcome the multidrug resistance of cancer cells. Mol Pharm. 2014;11:2495–510.
Marin E, Briceño MI, Caballero-George C. Critical evaluation of biodegradable polymers used in nanodrugs. Int J Nanomedicine. 2013;8:3071.
Pei X, Zhang X, Zhang L, Yuan M, Sun L, Yu F, et al. Targeted exosomes for co-delivery of siFGL1 and siTGF-β1 trigger combined cancer immunotherapy by remodeling immunosuppressive tumor microenvironment. Chem Eng J. 2021;421:129774.
Ng KS, Smith JA, McAteer MP, Mead BE, Ware J, Jackson FO, et al. Bioprocess decision support tool for scalable manufacture of extracellular vesicles. Biotechnol Bioeng. 2019;116:307–19.
Su Z, Xiao Z, Wang Y, Huang J, An Y, Wang X, Shuai X. Codelivery of anti-PD-1 antibody and paclitaxel with matrix metalloproteinase and pH dual-sensitive micelles for enhanced tumor chemoimmunotherapy. Small. 2020;16(7):1906832.
Pan J, Attia SA, Subhan MA, Filipczak N, Mendes LP, Li X, et al. Monoclonal antibody 2C5- modified mixed dendrimer micelles for tumor-targeted codelivery of chemotherapeutics and siRNA. Mol Pharm. 2020;17:1638–47.
Ahsan A, Farooq MA, Parveen A. Thermosensitive chitosan-based injectable hydrogel as an efficient anticancer drug carrier. ACS Omega. 2020;5:20450–60.
Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification preparation and applications. Nanoscale Res Lett. 2013;8:102.
Handali S, Moghimipour E, Rezaei M, Saremy S, Dorkoosh FA. Co-delivery of 5-fluorouracil and oxaliplatin in novel poly(3-hydroxybutyrate-co-3-hydroxyvalerate acid)/poly(lactic-co-glycolic acid) nanoparticles for colon cancer therapy. Int J Biol Macromol. 2019;124:1299–311.
van Zandwijk N, Reid G, Baas P. Emerging therapies for malignant mesothelioma. Front Oncol. 2020;10:939.
Mamot C, Ritschard R, Wicki A, Stehle G, Dieterle T, Bubendorf L, et al. Tolerability safety pharmacokinetics and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 2012;13(12):1234–41.
Berinstein NL, Karkada M, Morse MA, Nemunaitis JJ, Chatta G, Kaufman H, et al. First-in-man application of a novel therapeutic cancer vaccine formulation with the capacity to induce multi-functional T cell responses in ovarian breast and prostate cancer patients. J Transl Med. 2012;10:156.
Curigliano G, Romieu G, Campone M, Dorval T, Duck L, Canon JL, et al. A phase I/II trial of the safety and clinical activity of a HER2-protein based immunotherapeutic for treating women with HER2-positive metastatic breast cancer. Breast Cancer Res Treat. 2016;156:301–10.
Kulukian A, Taylor J, Olson D, Zaval M, Thurman R, Hengel S, et al. Abstract P1–18-09: Tucatinib a HER2-selective tyrosine kinase inhibitor increases the anti-tumor activity of trastuzumab antibody-drug conjugates in preclinical models of HER2+ breast cancer. Cancer Res. 2020;80(4):1809.
Sercombe L, Veerati T, Moheimani F, Wu SY, Sood AK, Hua S. Advances and challenges of liposome assisted drug delivery. Front Pharmacol. 2015;6:286.
Ong SG, Chitneni M, Lee KS, Ming LC, Yuen KH. Evaluation of extrusion technique for nanosizing liposomes. Pharmaceutics. 2016;8(4):36.
Otake K, Shimomura T, Goto T, Imura T, Furuya T, Yoda S, et al. Preparation of liposomes using an improved supercritical reverse phase evaporation method. Langmuir. 2006;22:2543–50.
Shen M, Shen Y, Fan X, Men R, Ye T, Yang L. Roles of macrophages and exosomes in liver diseases. Front Med. 2020;7:583691.
Amani N, Dorkoosh FA, Mobedi H. ADCs as novel revolutionary weapons for providing a step forward in targeted therapy of malignancies. Curr Drug Deliv. 2020;17(1):23–51.
Hafeez U, Parakh S, Gan HK, Scott AM. Antibody–drug conjugates for cancer therapy. Molecules. 2020;25:4764.
von Minckwitz G, Huang CS, Mano MS, Loibl S, Mamounas EP, Untch M, et al. Trastuzumab emtansine for residual invasive HER2-positive breast cancer. N Engl J Med. 2019;380:617–28.
Lee AL, Venkataraman S, Sirat SB, Gao S, Hedrick JL, Yang YY. The use of cholesterol-containing biodegradable block copolymers to exploit hydrophobic interactions for the delivery of anticancer drugs. Biomaterials. 2012;33(6):1921–8.
Cha EJ, Kim JE, Ahn CH. Stabilized polymeric micelles by electrostatic interactions for drug delivery system. Eur J Pharm Sci. 2009;38(4):341–6.
Kang L, Gao Z, Huang W, Jin M, Wang Q. Nanocarrier-mediated co-delivery of chemotherapeutic drugs and gene agents for cancer treatment. Acta Pharm Sin B. 2015;5(3):169–75.
Ramezanpour M, Leung SS, Delgado-Magnero KH, Bashe BY, Thewalt J, Tieleman DP. Computational and experimental approaches for investigating nanoparticle-based drug delivery systems. Biochim Biophys Acta Biomembr. 2016;1858(7):1688–709.
van Gunsteren WF, Bakowies D, Baron R, Chandrasekhar I, Christen M, Daura X, et al. Biomolecular modeling: goals problems perspectives. Angew Chemie Int Ed. 2006;45:4064–92.
Huynh L, Neale C, Pomès R, Allen C. Computational approaches to the rational design of nanoemulsions polymeric micelles and dendrimers for drug delivery. Nanomed Nanotechnol Biol Med. 2012;8:20–36.
Shamsi M, Mohammadi A, Manshadi MK, Sanati-Nezhad A. Mathematical and computational modeling of nano-engineered drug delivery systems. J Control Release. 2019;307:150–65.
Ravar F, Saadat E, Kelishadi PD, Dorkoosh FA. Liposomal formulation for co-delivery of paclitaxel and lapatinib preparation characterization and optimization. J Liposome Res. 2016;26(3):175–87.
Venditto VJ, Szoka FC Jr. Cancer nanomedicines: so many papers and so few drugs! Adv Drug Deliv Rev Elsevier. 2013;65:80–8.
Deng B. Approval may embolden industry to combine cancer therapies. Nat Med. 2015;21:105.
Nastiuk KL, Krolewski JJ. Opportunities and challenges in combination gene cancer therapy. Adv Drug Deliv Rev. 2016;98:35–40.
Fares J, Kanojia D, Rashidi A, Ulasov I, Lesniak MS. Landscape of combination therapy trials in breast cancer brain metastasis. Int J Cancer. 2020;147:1939–52.
Derakhshani A, Rezaei Z, Safarpour H, Sabri M, Mir A, Sanati MA, et al. Overcoming trastuzumab resistance in HER2-positive breast cancer using combination therapy. J Cell Physiol. 2020;235(4):3142–56.
Dubrovska A, Elliott J, Salamone RJ, Kim S, Aimone LJ, Walker JR, Watson J, Sauveur-Michel M, Garcia-Echeverria C, Cho CY, Reddy VA. Combination therapy targeting both tumor-initiating and differentiated cell populations in prostate carcinoma. Clin Cancer Res. 2010;16(23):5692–702.
Author information
Authors and Affiliations
Contributions
Writing: Sepideh Nezhadi; Reviewing: Farid Dorkoosh. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Nezhadi, S., Dorkoosh, F.A. Co-delivery systems: hope for clinical application?. Drug Deliv. and Transl. Res. 12, 1339–1354 (2022). https://doi.org/10.1007/s13346-021-01041-1
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01041-1