Abstract
There is an urgent need for treatments for hydrofluoric acid (HF) burns and their derivative problems that prevent hydrogen ion dissociation and fluoride ion binding to tissues. This study evaluated the ability of chitosan-based hydrogels combined with a buffer solution containing either boric acid or Tris and calcium gluconate (CHS-BA-CG and CHS-Tris-CG) to repair HF burn wounds and prevent wound infections. We assessed calcium release rates and biocompatability and constructed a mouse HF burn model to assess the tissue repair effects of the hydrogels. Finally, we performed disc diffusion tests from burn tissue and quantified the bacterial counts to assess the anti-infection properties of the hydrogels. Calcium was gradually released in the CHS-BA-CG and CHS-Tris-CG groups (73% and 43%, respectively, after 48 h). The cell viabilities at 48 h after HF burn in these groups were significantly higher than those in the phosphate-buffered saline (PBS) and CG-treated groups. Histopathological evaluation showed a clear boundary between the epidermal and dermal layers in both CHS-BA-CG and CHS-Tris-CG-treated groups, indicating their effectiveness in tissue repair. In the disc diffusion test, CHS-BA-CG and CHS-Tris-CG exhibited larger inhibition zones against Acinetobacter baumannii than those for PBS and CG. The bacterial counts on HF burn wounds were significantly lower in the CHS-BA-CG and CHS-Tris-CG-treated groups than those in the PBS and CG-treated groups. The in vitro studies demonstrated the biocompatibility and antimicrobial effects of the CHS-BA-CG and CHS-Tris-CG hydrogels. Both gels also demonstrated tissue repair and anti-infection effects. Thus, chitosan-based hydrogels may be candidates for HF burn therapy.
Graphical abstract

Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hydrofluoric acid (HF) burns are a serious problem in emergency medicine [1]. HF enters the human body via the skin, mucous membranes, respiratory tract, and other surfaces to cause systemic toxicity, severe hypocalcemia, and even death. The mechanism of burn injury starts with the release of fluoride ions, which combine with calcium ions to cause changes in membrane permeability to potassium ions that lead to progressive tissue necrosis. Additionally, the dissociation of hydrogen ions leads to the necrosis of clotting proteins. Thus, preventing HF absorption into tissues and tissue damage caused by fluoride ions are key in the treatment of HF burns.
Skin contact accounts for 70% of HF injuries. The severity of skin injury depends on HF contact time and concentration. Redness, swelling, heat, and pain appear after initial HF contact through the skin. Cloudy and creamy blisters may gradually occur, with ulcers and tissue necrosis developing in the final stage [2, 3]. HF burns are currently managed by removing HF, preventing systemic toxicity, applying supportive treatment, and managing local infection [4]. The common treatment methods include flushing with normal saline, the application of topical calcium ointments, the injection of calcium solutions, and even the surgical removal of affected parts.
Acinetobacter baumannii is an important opportunistic human pathogen, most strains of which have been isolated from hospitalized patients [5]. While A. baumannii does not easily cause disease under normal circumstances, it can cause skin and soft tissue infections, wound infections, meningitis, and bloodstream infections in hospitalized patients, especially those with low immunity after burns [6]. Additionally, clinical reports have described the increasing emergence of antibiotic-resistant strains of A. baumannii. Thus, the treatment of A. baumannii infections represents a challenge.
A variety of hydrogel dressings are available for wound care [7,8,9]. Hydrogels with different materials appropriate for medical use provide a moisturizing environment for wound repair; they can also reduce wound pain and bacterial infection. Hydrogels are easily applied, especially to burned and injured skin. In addition, hydrogels are compatible with several water-soluble drugs, allowing the use of antibiotics to treat infections. However, the limitations of hydrogels include cell cytotoxicity and biocompatibility in combination with certain drugs, as well as the biological activity of materials.
Herein, we used chitosan combined with different buffer solutions and calcium to prepare a hydrogel to provide efficient tissue repair as well as antibacterial effects in the treatment of injury from HF burn.
Materials and methods
Chemicals
HF (55%, pure, CAS number: 7664–39-3, ECHO CHEMICAL CO. LTD, Taipei, Taiwan), phosphate-buffered saline (PBS, Sigma-Aldrich Chemie GmbH, Steinheim, Germany), calcium gluconate (CG) gel (2.5%, Calgonate Corp., State of Florida, USA), calcium D-gluconate monohydrate (purity ≥ 98%, CAS number: 299–28-5, Echo Chemical Co. LTD, Taipei, Taiwan), boric acid (pure, CAS number: 10043–35-3, Echo Chemical Co. LTD, Taipei, Taiwan), Tris base (CAS number: 77–86-1, Sigma-Aldrich Chemie GmbH, Steinheim, Germany), hydrochloric acid (37%, CAS number: 7647–01-0, Sigma- Aldrich Chemie GmbH, Steinheim, Germany), and deacetylated chitosan (CAS number: 9012–76-4, Sigma-Aldrich Chemie GmbH, Steinheim, Germany) were purchased.
The 55% HF was diluted in deionized water to prepare 8% and 30% HF concentrations for the cell and animal experiments, respectively.
Fabrication of the buffer solutions and the chitosan-based hydrogels
Different buffer solutions have been combines with chitosan-based hydrogels, including phosphoric acid, citric acid, succinic acid, boric acid, and Tris. We used a chitosan-based hydrogel combined with either boric acid or Tris, plus CG gel as decontaminating agents for the absorption of fluoride ions after HF exposure, as these two types of buffer solutions yielded the best results in previous studies. First, 2% chitosan was dispersed in acetic acid (1% (v/v)) and gently stirred for 24 h at 40 °C to dissolve completely. The neutralized crystalline salt (boric acid or Tris base) was then dissolved in deionized water at a concentration of 0.1 M with CG (2.5% (w/v)) and added to the chitosan solution. Finally, the prepared solution was vortexed for 1 h. All hydrogels were washed with distilled water to remove residues that did not react. Finally, they were sterilized by ultraviolet light exposure for 1 h and stored at 4 °C.
The characterization of hydrogel
The formation of chitosan-based hydrogel was monitored by placing pre-gel solution containing chitosan, acetic acid, calcium D-gluconate monohydrate, 0.1 M Tris, or 0.1 M boric acid in the beaker, then observe the viscosity changes over time via the stopwatch.
In the swelling ratio (SR) test, the hydrogels were cut into samples of about 2 g then were soaked in water with a mild shaking motion. We measure the weight of hydrogels after 2, 4, 8, 16, 24, 48, and 72 h after removal of excess water carefully. The swelling ratios of hydrogels were calculated using the formula SR = (Wi − Wd)/Wd, where Wi and Wd are the weight of the swollen hydrogel and the weight of the dry hydrogel, respectively.
In vitro tests of calcium release in the chitosan-based hydrogel
A calcium colorimetric assay (Sigma-Aldrich Chemie GmbH, Steinheim, Germany) was used to test calcium release by the chitosan-based hydrogel. We added 500 μl of PBS to 200 μl of gel and incubated the combination at 37 °C to mimic the in vivo environment. The following procedure was duplicated every 6 h until after 48 h. First, ultrapure water was used to prepare the calcium standard solution. Then, a chromogenic reagent was added to each sample and standard. The solutions were mixed gently using a 200-μl tip and calcium assay buffer was added. The samples were then incubated at room temperature for 10 min. Absorbance was measured at 575 nm on a TECAN 200/200Pro multimode microplate reader (TECAN Trading AG, Männedorf, Switzerland).
Bacterial culture
Acinetobacter baumannii strain NTU58 (AB NTU58) was cultured at 37 °C in Mueller–Hinton broth (MHB, Lab M Limited., Heywood, UK) overnight with shaking at 250 rpm. Bacteria were then transferred to fresh MHB at a ratio of 1:100 and cultured for another 4 h until reaching log-phase growth. The cells were collected by centrifugation at 8000 × g for 10 min and washed with sterile PBS. We repeated the washing steps three times and then resuspended the cells in sterile PBS at a concentration corresponding to 1 × 108 colony-forming units (CFU)/mL.
In vitro antimicrobial study
The antimicrobial activities of the chitosan-based hydrogels were evaluated by Kirby–Bauer (KB) disc diffusion tests. AB NTU58 bacterial suspensions (1 × 108 CFU/mL) were prepared as described above. Then, 50 µl of bacterial suspension was transferred to Mueller–Hinton agar (MHA, Lab M Limited, Heywood, UK). The hydrogels (5-mm diameter) with 2% (w/v) of chitosan were loaded onto 10-mm sterile paper discs, which were then transferred to the dish. Sterile filter paper immersed in PBS was used as a control. The plates were incubated at 37 °C. The zones of bacterial inhibition were measured after 24 h of incubation [10].
Cell culture
Human dermal fibroblasts (HDFs) were purchased from the Bioresource Collection and Research Center (BCRE, Hsinchu City, Taiwan) and cultured in DMEM medium (Gibco; Thermo Fisher Scientific, Inc., MA, USA) containing 10% fetal bovine serum (Gibco; Thermo Fisher Scientific, Inc., MA, USA) and 1% penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific, Inc., MA, USA) at 37 °C in a 5% CO2 incubator. The morphologies of the HDFs were observed using an optical microscope (CiS, Nikon, Tokyo, Japan).
In vitro biocompatibility assay
The cytotoxicity of the chitosan-based hydrogel was investigated using the Cell Counting Kit-8 assay (CCK-8, Dojindo, Kumamoto, Japan). The HDFs were seeded onto a culture plate at a density of 6 × 103 cells per well and grown overnight. The hydrogel samples were exposed to UV light for 1 h for sterilization. When the cells reached 80–90% confluency, the hydrogels were gently placed on the well, allowing contact between the hydrogels and the cells. The HDFs were allowed to grow for 3 days with daily culture medium changes.
After 3 days, cells were washed twice with PBS, then 180 µL of culture medium was added, followed by 20 µL of the CCK-8 reagent. The cells were incubated at 37 °C for 2–4 h. The absorbance at 450 nm was then measured using a TECAN 200/200Pro multimode microplate reader (TECAN Trading AG, Männedorf, Switzerland). Background absorbance measured at 630 nm was subtracted from the final value. Each assay was performed in triplicate and the mean was calculated.
The effect of the chitosan-based hydrogel on the viability of HF-acid-injured HDFs
When cells reached 80–90% confluency, they were treated with 8% (v/v) HF for 5 min. We then divided the cells into four groups as follows: the control group cells were washed three times with PBS; the boric acid group cells were washed three times with boric acid buffer and then covered with the chitosan-based hydrogel containing boric acid and 2.5% CG; the Tris base buffer group was washed three times with Tris buffer and then covered with the chitosan-based hydrogel containing Tris and 2.5% CG; CG gel group. Following each treatment, the cells were seeded in 96-well plates at an initial density of 6 × 103 cells/well and incubated for 3 days. The effect of the hydrogel on the cell viability of HF-acid-injured HDFs was evaluated using a CCK-8 assay (Dojindo). Every 24 h, 20 µl of CCK-8 reagent was added and the plates were incubated for 1 h. The optical density was read at 450 nm. Cell proliferation was assessed using a TECAN 200/200Pro multimode microplate reader (TECAN Trading AG). Each assay was repeated in triplicate and the data were expressed as percentages of viable cells.
HF burn model
Eight-week-old male C57BL/6 mice were obtained from BioLASCO Taiwan Co., Ltd (Taipei City, Taiwan) and maintained at the National Defense Medical Center laboratory animal center (IACUC 20,229). The dorsal surfaces of the mice were shaved and depilated with depilatory cream before the experiment. The mice were divided into four groups, each containing five mice (n = 5). The mice were anesthetized with zoletil (5 mg/kg) and cutaneously exposed to 30% HF for 5 min.
After establishing the burn, elastic bandage tape was used to removing the upper epidermal layer. Then, 20 μl of an AB NTU58 bacterial suspension in PBS was smeared on the burn wounds [11]. One hour later, the control group mice were resuscitated with 1-ml sterile saline. The remaining three groups, A, B, and C, were treated as follows. The wounds in Group A were washed with 1-mL sterile saline, and a smear of 2.5% (w/v) CG gel was applied. The wounds in Group B were washed with 1-mL boric acid buffer solution; a smear of the chitosan-based hydrogel was then applied, which contained 0.1 M boric acid and 2.5% CG. The wounds in Group C were washed with Tris base buffer solution; a smear of the chitosan-based hydrogel was applied, which contained 0.1 M Tris base and 2.5% CG. The treatment period was 7 days, during which the dressing was changed daily. The tissue changes were recorded with a camera.
Histological analysis
The mice were divided into four groups (n = 5 for each group). Following the establishment of burns on their dorsal skin surface, the hydrogels were placed on the affected skin areas. The mice were sacrificed on days 3 and 7. Skin samples were fixed in 10% formalin before histological analysis.
To evaluate the impact of the chitosan-based hydrogel on mouse skin, dorsal skin samples were collected on days 3 and 7. All samples were processed and embedded in paraffin and sectioned (5 µm thick), followed by staining with hematoxylin and eosin (H&E) and Masson trichrome. To evaluate the effect of treatment, the sections were observed under a light microscope (CiS, Nikon, Tokyo, Japan).
Microbiological analysis
At 3 and 7 days after infecting the burn wounds, the mice were sacrificed. Skin samples from control and treated groups were excised (10 mm2). The tissue samples were then homogenized in 800 μl PBS, and ten-fold serially diluted bacterial colonies were enumerated by plating on MHA and incubated at 37 °C for 24 h. The results were normalized and expressed in log10 CFU bacterial load present in 1 g of tissue sample.
Statistical analysis
In this study, we conducted two-way ANOVA with Tukey’s multiple comparison test for days and groups. P < 0.05 were considered statistically significant. All experiments were conducted at least in triplicate for statistical analysis, with the results presented as means ± standard deviation.
Results
Preparation and evaluation of the hydrogel formula
This study prepared two chitosan-based hydrogel formulations containing either boric acid or Tris, as well as 2.5% (w/v) CG (CHS-BA-CG and CHS-Tris-CG, respectively). The physicochemical characteristics of these hydrogels were compared to those for a commercial CG gel.
Because of physical cross-linking, our pre-gel solution composed of chitosan, CG, Tris, or boric acid became viscous over time and finally transformed into polymer gels. As shown in Fig. 1a, all of the liquid levels of the initial pre-gel solution indicated high fluidity. While the gel was formatting, the viscosity of the system got increased and the fluidity was reduced as well until the gel finally formed. The CHI-Tris-CG gel was formed in 1 min, and the formation of CHI-BA-CG gel took 30 min (Table 1). There was no flowing phenomenon observed even the bottle was placed upside down. The formed polymer gel could be taken out of the bottle and had its shape kept well. As shown in Fig. 1b, the CHI-BA-CG gel had a perfect swelling in water and expanded to almost twice its initial size and still kept its shape, indicating the stable cross-linking of the gel network. On the other hand, the CHI-Tris-CG gel could also be expanded to 1.5 times its initial size and had its shape kept. A further quantitative study of the swelling behaviors was conducted by calculating the amount of absorbed water. In Fig. 1c, the swelling ratio of all the gels increased over time. We could see the rapid swelling process in the early stage from 1st to 10th hours. Afterward, the swelling was slow and finally approached an equilibrium state in the hour of 50.
In vitro test for chitosan-based hydrogel calcium release
As shown in Fig. 2, within the first hour, 7% of calcium was released from the CHS-Tris-CG gel, compared to 13% from the CHS-BA-CG gel. The amount of calcium released from two kinds of gels increased over time. After 5 h, 51% calcium was released from the CHS-BA-CG gel compared to 73% after 48 h. The calcium release from the CHS-Tris-CG gel was slower than that from the CHS-BA-CG gel; only 27% and 43% of the calcium was released after 5 h and 48 h, respectively.
In vitro antimicrobial activities of the chitosan-based hydrogels
The antibacterial activities of the chitosan-based hydrogels were tested against A. baumannii infection after HF burn using the disk diffusion test. As shown in Fig. 3, the two chitosan-based hydrogels produced larger zones of inhibition compared to that for the commercial CG gel and PBS groups. Compared to that of the CHS-Tris-CG group, the diameter of the inhibition zone was larger in the CHS-BA-CG group.
Cell morphology and viability after HF acid exposure
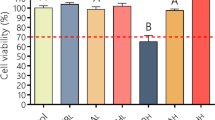
Optical microscopy was used to assess the change in cell morphology after HF acid exposure. Under the microscope, fibroblasts with a normal morphology had a star-like shape. As shown in Fig. 4a, at 24 h after HF burn, no significantly different changes in cell morphology were observed in any of the treated groups compared to the control group. However, at 72 h after HF burn, cells in the PBS and CG groups had a slender shape and showed unclear nucleoli. In comparison, CHS-Tris-CG- and CHS-BA-CG-treated cells showed noticeable nucleoli with a relatively plump cytoplasm.
After the fibroblasts were exposed to HF, we observed the cell viability for 3 consecutive days (Fig. 4b). Before HF exposure, the cell viability was maintained at 95–100%. All groups were then exposed to 8% HF for 5 min. Twenty-four hours after the HF burn, the cell viabilities in the PBS, CG, CHS-Tris-CG, and CHS-BA-CG groups were 70.66 ± 10.06%, 87.66 ± 2.52%, 71.00 ± 6.56%, and 76.00 ± 8.72%, respectively. At 48 h after HF burn, the viabilities were 62 ± 7.55%, 56.33 ± 6.03%, 78.00 ± 2.65%, and 78.33 ± 4.73%, respectively. At 48 h after HF burn, there was no significant difference in cell viability between the PBS and CG groups. However, the cell viabilities were significantly higher in the CHS-Tris-CG and CHS-BA-CG groups compared to that in the CG group.
Evaluation of the in vivo effects of hydrogels in a mouse model
After exposing the dorsum of mice to 30% HF, skin redness developed. As seen in Fig. 5, the group washed with PBS only (PBS group) and the CG group showed burn wounds on day 1. In contrast, the two chitosan-based hydrogel-treated groups showed no apparent dorsum wounds on day 1 after treatment. Moreover, the gels had formed a thin film on the skin surface that maintained skin moisture. On day 3, the no-treatment and CG groups showed reddish wounds on the dorsum; however, no visible wounds were observed in the two chitosan-based gel-treated groups. On day 7, all mice in PBS- and CG gel-treated groups were dead. In contrast, the dorsum wounds in the two chitosan-based hydrogel-treated groups were encrusted with scabs.
a The survival curve of each group (n = 5). b Wounds on the dorsum of mice after exposure to 30% HF. On day 1, a visible burn wound is visible in the PBS and CG groups (white arrow); on day 3, burn wounds are visible in the PBS and CG groups (white arrow), while there was no visible wound on the surface of the skin in the CHS-Tris-CG and CHS-BA-CG groups; on day 7, the wounds are encrusted with scabs on the dorsum of the CHS-Tris-CG and CHS-BA-CG-treated mice. HF hydrofluoric acid; PBS phosphate-buffered saline; CHS-Tris-CG chitosan, Tris, and calcium gluconate; CHS-BA-CG chitosan, boric acid, and calcium gluconate
Histopathological evaluation
H&E and Masson’s trichrome staining was used for histopathological evaluation. As shown in Fig. 6a, H&E staining revealed no significant damage in the epidermal and dermal layers in all groups on the first day after skin burn. On the third day, the keratin layer had disappeared in the PBS group, while the CG group showed keratin damage with a discontinuous epidermal layer and uneven thickness. The two chitosan-based hydrogel-treated groups showed slight keratin damage. On day 7, all mice in the PBS and CG groups were dead. Figure 6 shows the partial hyperplasia of the stratum corneum with a clear boundary between the epidermal and dermal layers in the CHS-Tris-CG group. The same tendency was observed in the CHS-BA-CG group. Furthermore, scabs were observed on the wounds.
Figure 6b shows the results of Masson’s trichrome staining in all of the groups after HF skin burn. On day 1 after skin burn, the cytoplasm obtained from the damaged cells from the dermal layers in the PBS group was reddish in color. The collagen density was also decreased. The CG group showed diffuse damage throughout the epidermal layer as well as decreased collagen density. No significant damage in the epidermal and dermal layers was observed in the CHS-Tris-CG group; however, slight keratin damage and decreased collagen density were observed in the CHS-BA-CG group. On day 3, the wounds in the CHS-Tris-CG and CHS-BA-CG groups started to scab and the collagen density increased. On day 7, the CHS-Tris-CG and CHS-BA-CG groups showed a clear dividing line between the epidermal and dermal layers, the density of collagen was remarkably increased, scabs had formed and a relatively normal keratin layer had been restored.
In vivo assessment of burn-induced microbial infection
The antimicrobial effects of the chitosan-based hydrogel against A. baumannii were evaluated in burned tissue obtained from sacrificed mice by homogenizing and quantifying the CFUs on day 3. The wounds treated with the two chitosan-based hydrogels showed significant decreases in bacterial counts compared to those in the PBS and CG groups. The same procedure executed on the day 7. The antimicrobial effects of CHI-BA-CG gel are still better than CHI-Tris-CG gel. However, there were no significant differences between two groups (Fig. 7).
Discussion
HF burns as an occupational injury are widely seen in both developing and industrialized countries. The major mechanisms underlying the toxic effects of HF are the high penetration rate in skin tissue and the release of large amounts of dissociated fluoride ions potentially leading to severe systemic toxicity [1]. The treatment principle includes the removal of any residual HF, neutralization of free fluoride ions, and prevention of HF-induced biological corrosion and progressive tissue destruction. In clinical practice, the first step in the treatment of HF burns is to wash the wound with abundant water. The second step is to neutralize dissociated fluoride ions to prevent deeper tissue damage [12]. The fluoride ions can bind to calcium and magnesium ions to form insoluble salts that are excreted from the human body. The fluoride neutralizers in clinical use may contain CG [13]. Thus, water flushes and the external application of fluoride neutralizers are among the standard treatments for HF [14]. The CG gel is suitable as a first-aid treatment for injuries due to its easy administration; however, both the gel and calcium penetrate poorly into tissue, which also limits their use for treating fluoride ions in deep tissues [15]. Although local tissue damage could be addressed, water flushes and neutralizers may not prevent systemic toxicity [4, 12].
Other adjuvant therapy for HF injury, such as topical CG injection to increase CG concentration to bind fluoride ions or the combination with infiltration aids such as dimethyl sulphoxide (DMSO), can help promote calcium penetrability [16]. However, excessive calcium in the tissue can lead to cellular toxicity and delay wound healing. In addition, the subcutaneous injection of high concentrations of calcium may cause vasculitis around the tissue, hypercalcemia, or even arrhythmia leading to death. Moreover, DMSO can cause protein degradation in the tissue and even tissue necrosis. Thus, the development of effective and safe therapy for the treatment of HF burns is needed to improve patient prognosis.
Several kinds of hydrogel are used as wound dressings to maintain wound moisture and remove necrotic tissue. Among hydrogel materials, chitosan is biodegradable, biocompatible, and bio-adhesive, making it suitable for preparations aimed at healing HF burn wounds [17, 18]. This study applied deacetylated chitosan-based hydrogels for the treatment HF. This composition has been reported to provide stability for the delivery of different therapeutic agents [19]. Additionally, we combined calcium and different buffer solutions to the chitosan-based gel for repair of the burn wound. Fluoride ions can form an insoluble complex with calcium and magnesium that is excreted from the body [20]. Calcium released from a hydrogel applied to the wound surface could prolong the tissue damage.
This study combined two buffer solutions, Tris and boric acid, with a hydrogel. Tris is an organic compound with high biocompatibility and low immunogenicity, which used in biochemistry as a buffer solution [21]. Boric acid is an antibacterial compound that has been used for the treatment of minor burns [22]. These buffer solutions maintain the pH on the skin surface after HF burns and prevent dissociated hydrogen ions from HF from penetrating deeper tissue and potentially causing severe damage. The present study performed in vitro and in vivo evaluations to compare the effects of these two buffer solutions combined with the hydrogel on tissue repair.
All formulations of the chitosan-based hydrogel were formed by simple mixing, to which 2.5% (w/v) CG was then added. As the result showed, in contrast to CHS-BA-CG, significant high gelation time and low swelling ratio were found on CHS-Tris-CG that calcium release ratio of CHS-BA-CG was higher than CHS-Tris-CG. As we know the hydrogen-bonding exhibit great effects on the viscosity properties of polymer gel. Tris has more hydrogen then Boric acid. Hydrogen bond interaction in polymer composites of CHI-Tris-CG more than CHI-BA-CG. We suggested that because of molecular interaction, Tris might be higher intermolecular force with CHS than CHS-BA-CG.
We used calcium colorimetric assay to measure the calcium release rates for the two buffer solution hydrogels. The release index did not differ significantly between the two hydrogels initially, after 1 h, and up to 3 h. After 4 h, the hydrogel with boric acid showed a calcium ion release rate of almost 40%, which increased to 76% at 48 h. Compared to boric acid, Tris has a higher viscosity, which may affect polymer swelling, which, in turn, affects calcium release from the hydrogel [23]. In contrast, the porous property of boric acid offers a larger surface area that allows the solvent cladding in hydrogel rapidly; moreover, the high dissolving rate in water allows easy calcium release [24]. One major mechanism of damage in HF burns is the easy skin penetration and dissociation of hydrogen ions. The high concentration of hydrogen ions may cause a pH decrease, leading to mucous membrane damage and even tissue necrosis [1, 25]. In this study, the chitosan-based hydrogel combined with a buffer solution slowed the hydrogen ion attack in tissues and maintained the pH at 6.5–7.5 to prevent further tissue damage.
We studied the cell viability and morphology change in three ways in a commercial CG gel, a chitosan-based hydrogel with Tris buffer solution, and a chitosan-based hydrogel with boric acid. After 48 h, the cells in the CG group appeared slender and with an unclear nucleolus and showed significantly decreased cell viability compared to those in the other two groups. In theory, the CG gel could address dissociated fluoride ions on the cell surface and prevent cell death [4]. However, some data have indicated that the CG gel does not adjust pH values; thus, it could not block hydrogen ion penetration into deep tissue. In contrast, the two chitosan-based hydrogels with buffer solutions could be used to flush damaged tissues, bind hydrogen ions to maintain the pH, preserve cell viability, and enhance cell proliferation. The 2.5% calcium added to the chitosan-based hydrogels allowed the release of calcium as the hydrogels degraded to minimize fluoride ion penetration into tissue and subsequent damage.
The in vivo model exhibited the same result; H&E and Masson’s trichrome staining showed signs of wound healing including connective tissue generation and skin lesion repair. The inflammatory cells infiltrated the epidermal and dermal layers, and epidermal damage with either detachment of the corneal layer or epidermal deroofing was observed in the PBS and CG groups. However, the two chitosan-based hydrogel-treated groups showed initiation of re-epithelialization through the damage, with gains of the corneal layer and epidermis observed on the seventh day. Moreover, less collagen damage was also observed in the two hydrogel-treated groups. Regrowth from the basal layer of the epidermis was observed on the third day in the two hydrogel-treated groups compared to the PBS and CG-treated groups. A remarkable rearrangement of collagen fiber order was noted on the seventh day in the two hydrogels treated groups, with the old debris and crusts pushed out with the recovery of the normal epidermal layer.
The results of the in vitro antibacterial study and in vivo evaluation of the chitosan-based hydrogels revealed two antimicrobial characteristics of chitosan and buffer solutions in this hydrogel. Chitosan is an antibacterial agent as it can bind with negatively charged bacteria and change bacterial membrane permeability, leading to bacterial death [26]. Another antibacterial mechanism of chitosan is the binding of metal ions to inhibit microbial proliferation [27]. The buffer solutions, Tris and boric acid, may cause bacteria dehydration to inhibit replication [28]. Moreover, the pH of the buffer solution can also prevent bacteria growth [29]. In this study, water-soluble chitosan combined with a buffer solution increased the surface area of the compounding interacting with bacteria, enhanced the positive charge of chitosan, and altered the membrane permeability, thus negatively affecting bacteria viability [30]. These data showed that the synergistic effect of chitosan with buffer solutions could enhance the antibacterial properties to prevent infection after HF burns.
In summary, the results of this study showed that chitosan-based hydrogels combined with a buffer solution could reduce discomfort from HF burns. The bridgeable hydrogel released calcium and buffer andante to prevent HF penetration into the skin and maintain the tissue pH. The results also showed the enhanced epithelization property of chitosan in wound healing as well as its antimicrobial effects. Treatment of HF burns with chitosan-based hydrogels combined with buffer solutions may be useful for skin care or tissue repair.
Availability of data and material
Data will be available on request to authors.
Change history
10 December 2021
A Correction to this paper has been published: https://doi.org/10.1007/s13346-021-01098-y
References
Bajraktarova-Valjakova E, et al. Hydrofluoric acid: burns and systemic toxicity, protective measures, immediate and hospital medical treatment. Open Access Maced J Med Sci. 2018;6:2257–69.
Sriamporn T, et al. Effect of different neutralizing agents on feldspathic porcelain etched by hydrofluoric acid. Eur J Dent. 2019;13:75–81.
Bossert D, et al. A hydrofluoric acid-free method to dissolve and quantify silica nanoparticles in aqueous and solid matrices. Sci Rep. 2019;9:7938.
McKee D, et al. A review of hydrofluoric acid burn management. Plast Surg (Oakv). 2014;22:95–8.
Babik J, Bodnarova L, Sopko K. Acinetobacter—serious danger for burn patients. Acta Chir Plast. 2008;50:27–32.
Eichenberger EM, Thaden JT. Epidemiology and mechanisms of resistance of extensively drug resistant gram-negative bacteria. Antibiotics (Basel). 2019; 8.
Dhaliwal K, Lopez N. Hydrogel dressings and their application in burn wound care. Br J Community Nurs. 2018;23(Sup9):S24-27.
Carvalho IC, Mansur HS. Engineered 3D-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration. Mater Sci Eng C Mater Biol Appl. 2017;78:690–705.
Francesko A, Petkova P, Tzanov T. Hydrogel dressings for advanced wound management. Curr Med Chem. 2018;25:5782–97.
Abidullah Khan, M.X., Tengjiao Wang, Chuangang You, Xingang Wang, Haitao Ren, Hongwei Zhou, Amin Khan, Chunmao Han, and Peng Li. Catechol crosslinked antimicrobial peptide hydrogels prevent multidrug-resistant Acinetobacter baumannii infection in burn wounds. Biosci Rep. 2019; 39.
Tatiya-Aphiradee N, Chatuphonprasert W, Jarukamjorn K. In vivo antibacterial activity of Garcinia mangostana pericarp extract against methicillin-resistant Staphylococcus aureus in a mouse superficial skin infection model. Pharm Biol. 2016;54:2606–15.
Wang X, et al. A review of treatment strategies for hydrofluoric acid burns: current status and future prospects. Burns. 2014;40:1447–57.
Roblin I, et al. Topical treatment of experimental hydrofluoric acid skin burns by 2.5% calcium gluconate. J Burn Care Res. 2006; 27: 889–94.
Mathieu L, et al. Water-based solutions are the best decontaminating fluids for dermal corrosive exposures: a mini review—letter to the editor. Clin Toxicol (Phila). 2014;52:149.
Lippert J, et al. Management of Hydrofluoric Acid Burns in the Emergency Department. Cureus. 2020;12:e7152.
Vijayan SM, et al. Calcium, magnesium and aluminium ions as decontaminating agents against dermal fluoride absorption following hydrofluoric acid exposure. Toxicol In Vitro. 2021;71:105055.
Yang YA, Campbell Ritchie, Everitt NM. Recombinant human collagen/chitosan-based soft hydrogels as biomaterials for soft tissue engineering. Mater Sci Eng C Mater Biol Appl. 2021; 121: 111846.
Dai T, et al. Chitosan preparations for wounds and burns: antimicrobial and wound-healing effects. Expert Rev Anti Infect Ther. 2011;9(7):857–79.
Ahmed EM. Hydrogel: Preparation, characterization, and applications: a review. J Adv Res. 2015;6:105–21.
Habuda-Stanic M, Ravancic ME, Flanagan A. A review on adsorption of fluoride from aqueous solution. Materials (Basel). 2014;7:6317–66.
Gomori G. Preparation of buffers for use in enzyme studies. Handbook of Biochemistry and Molecular Biology, 5th ed. CRC Press; 2018.
Iavazzo C, et al. Boric acid for recurrent vulvovaginal candidiasis: the clinical evidence. J Womens Health (Larchmt). 2011;20:1245–55.
El-Kased RF, et al. Honey-based hydrogel: in vitro and comparative In vivo evaluation for burn wound healing. Sci Rep. 2017;7:9692.
Abureesh MA, Oladipo AA, Gazi M. Facile synthesis of glucose-sensitive chitosan-poly(vinyl alcohol) hydrogel: drug release optimization and swelling properties. Int J Biol Macromol. 2016;90:75–80.
Odde S, et al. Hydration and dissociation of hydrogen fluoric acid (HF). J Phys Chem A. 2006;110:7918–24.
Nagy A, et al. Silver nanoparticles embedded in zeolite membranes: release of silver ions and mechanism of antibacterial action. Int J Nanomedicine. 2011;6:1833–52.
Shapi’i RA, et al. Antimicrobial properties of starch films incorporated with chitosan nanoparticles: In vitro and in vivo evaluation. Carbohydr Polym. 2020;230:115602.
Wooley RE, Jones MS. Action of EDTA-Tris and antimicrobial agent combinations on selected pathogenic bacteria. Vet Microbiol. 1983;8:271–80.
Wiegand C, et al. pH influence on antibacterial efficacy of common antiseptic substances. Skin Pharmacol Physiol. 2015;28:147–58.
Xing K, et al. Oleoyl-chitosan nanoparticles inhibits Escherichia coli and Staphylococcus aureus by damaging the cell membrane and putative binding to extracellular or intracellular targets. Int J Food Microbiol. 2009;132:127–33.
Funding
This work was supported by grants from Taipei Veterans General Hospital (V109C-012, V110C-009, VTA108-T-2–3, VTA109-T-3–2 andVTA110-T-5–2), Tri-Service General Hospital (TSGH-E-109237, TSGH-E-110205), the Ministry of National Defense-Medical Affairs Bureau (MAB-109–099, MAB-109–098), and the Ministry of Science and Technology (MOST 107–2314-B-075–066-MY3).
Author information
Authors and Affiliations
Contributions
Conception Fang-Ching Yeh, Ying-Fu Su, Chien-Yao Fu. Design of study and acquisition data Shu-Wei Huang, Shian-Chiuan Tzeng. Data analysis Shu-Wei Huang, Yi-Tzu Lee, Aristine Cheng, Wung-Chi Wang. Drafting of manuscript and critical revision Shu-Wei Huang, Ming-Hsien Chiang, Ying-Shih Su, Aristine Cheng.
Ethics declarations
Ethics approval
All the procedures performed in this study involving animals were in accordance with the ethical standards, and protocols were approved by the Institutional Animals Ethics Committee.
Consent to participate
All authors have read and approved the submitted manuscript.
Disclaimer
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Huang, SW., Yeh, FC., Ji, YR. et al. Chitosan-based hydrogels to treat hydrofluoric acid burns and prevent infection. Drug Deliv. and Transl. Res. 11, 1532–1544 (2021). https://doi.org/10.1007/s13346-021-01007-3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-01007-3