Abstract
Infections caused by fungal biofilms with rapidly evolving resistance against the available antifungal agents are difficult to manage. These difficulties demand new strategies for effective eradication of biofilms from both biological and inert surfaces. In this study, polymeric micelles comprised of di-block polymer, poly-(ethylene glycol) methyl ether methacrylate and poly 2-(N,N-diethylamino) ethyl methacrylate polymer, P(PEGMA-b-DEAEMA), were observed to exhibit remarkable inhibitory effects on hyphal growth of Candida albicans (C. albicans) and C. tropicalis, thus preventing biofilm formation and removing existing biofilms. P(PEGMA-b-DEAEMA) micelles showed biofilm removal efficacy of > 40% and a 1.4-log reduction in cell viability of C. albicans in its single-species biofilms. In addition, micelles alone promoted high removal percentage in a mixed biofilm of C. albicans and C. tropicalis (~ 70%) and remarkably reduced cell viability of both strains. Co-delivery of fluconazole (Flu) and amphotericin B (AmB) with micelles showed synergistic effects on C. albicans biofilms (3-log reduction for AmB and 2.2-log reduction for Flu). Similar effects were noted on C. albicans planktonic cells when treated with the micellar system combined with AmB but not with Flu. Moreover, micelle-drug combinations showed an enhancement in the antibiofilm activity of Flu and AmB against dual-species biofilms. Furthermore, in vivo studies using Caenorhabditis elegans nematodes revealed no obvious toxicity of the micelles. Targeting morphologic transitions provides a new strategy for defeating fungal biofilms of polymorphic resistance strains and can be potentially used in counteracting Candida virulence.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Management of fungal infections has become particularly challenging within the clinical realm, due to the limited short list of available antifungal agents [1]. Invasive infections can be life-threatening with a reported mortality rate of up to 50%, causing about 1.5 million deaths per year among hospitalized patients with candidaemia [2, 3]. In immunocompromised individuals and individuals with implanted medical devices, fungal biofilm can cause fatal candidaemia and disseminated candidiasis [4, 5]. Candida albicans (C. albicans) is one of the major invasive pathogens involved in many fungal diseases, followed by Candida tropicalis (C. tropicalis) being the most important non-albicans Candida (NCA) species due to its virulence and resistance to clinical antifungals [6,7,8]. The Candida species polymorphic elements yeast, pseudohyphae and hyphae are often organized within a polysaccharide-rich matrix and form biofilm, the most virulent form of this opportunistic pathogenic microorganism [9,10,11]. Candida within biofilms are inherently unresponsive to currently available antifungals including azole and polyene drugs, and hence are responsible for therapy failures [2, 12]. Limited drug diffusion in the biofilm matrix, increased expression of genes involved in drug resistance, metabolic plasticity of biofilm-embedded cells and the presence of highly tolerant persister cells are the main factors contributing to biofilm resistance [13, 14]. In addition, polymicrobial biofilms of C. albicans and NCA species may promote synergistic relationships among the species, elevating the threat and becoming more difficult to eradicate [15, 16]. Each Candida species within a polymicrobial biofilm promotes different levels of resistance, increasing the challenge of treating fungal biofilm infections [13, 15]. Fluconazole (Flu) and amphotericin B (AmB) are commonly used antifungals in the clinic; however, the increased resistance of fungal biofilms to Flu (> 1000 times) compared with free floating (planktonic) cells, and systemic toxicity concerns for AmB have severely limited their therapeutic [12, 17].
During biofilm formation, the non-invasive budding form of C. albicans and C. tropicalis reversibly transitions into the invasive filamentous form, an important virulence factor in fungi pathogenesis and the eventual formation of a mature biofilm [2, 8, 18]. Therefore, targeting this virulent transition mode of Candida is a potential approach toward the development of new antifungal agents which have been designed to specifically target and act on biofilms [19]. Moreover, synergistic combinations of current drugs with anti-virulence molecules can address drawbacks associated with the toxicity and resistance development to drugs, and ultimately enhance their activity against fungal biofilms. This combination has been proven to be effective in increasing biofilm susceptibility and reducing the required effective antimicrobial dose of antifungals [20, 21]. In consideration of these points, the identification and development of novel molecules with antibiofilm activity is currently a research area of great clinical importance [22].
In previous work, we have provided evidence for the antibiofilm activity of the di-block copolymer micelles poly((ethylene glycol) monomethyl ether methacrylate)-block-(N,N-diethylaminoethyl methacrylate) P(PEGMA-b-DEAEMA), against pre-formed biofilms of C. albicans [23]. These micelles were pH-responsive, exhibited a high affinity towards C. albicans biofilms and enhanced the antifungal activity of encapsulated itraconazole against biofilms [23]. However, the mechanism involved in this activity, i.e. the role of the micelles themselves in preventing fungal biofilm formation, has not yet been investigated. This is the first report which shows the ability of P(PEGMA-b-DEAEMA) micelles alone in preventing C. albicans biofilm formation, when compared with other micelle-forming polymers. In addition, we investigate combinations of polymeric micelles with the antifungals Flu and AmB, for potential synergistic antimicrobial effects against single-species C. albicans and dual-species (C. albicans/C. tropicalis) biofilms.
Material
Fluconazole (Flu) and amphotericin B (AmB) were purchased from Sigma-Aldrich, Castle Hill, NSW, Australia. P(PEGMA-b-DEAEMA) polymer was synthesized as per published work [23]. Soluplus was kindly gifted by BASF (Ludwigshafen, Germany). Pluronic and Solutol HS 15 (polyethylene glycol 15-hydroxystearate) were purchased from Sigma-Aldrich, Munich, Germany. Polyethylene glycol-b-polyvinyl caprolactone (PEG-b-PCL) polymer was kindly provided by Dr. Anton Blencowe, University of South Australia. Phosphate-buffered saline (PBS), dimethyl sulfoxide (DMSO) and growth media (Sabouraud dextrose broth (SDB) as well as Sabouraud dextrose agar (SDA)) were purchased from BD Biosciences, North Ryde, NSW, Australia. The RPMI 1640 medium was procured from Thermo Fisher Scientific (Waltham, MA, USA). Organic solvents including acetone and methanol (all analytical grade) were purchased from Merck, Darmstadt, Germany Purified Milli-Q grade water (Millipore, MA, USA) was used throughout this study.
Methods
Preparation of polymeric micelles
Polymeric micelles based on graft copolymer polyvinyl caprolactam (PCL)-polyvinyl acetate (PVA)-polyethylene glycol (PEG) (Soluplus®, (Sol)), di-block copolymer polyethylene glycol-b-polyvinyl caprolactone (PEG-b-PCL), Solutol, Pluronic and pH-sensitive di-block copolymers poly-(ethylene glycol) methyl ether methacrylate-b-poly 2-(N,N-diethylamino) ethyl methacrylate P(PEGMA-b-DEAEMA) were prepared by an emulsion-solvent diffusion method [24, 25]. Briefly, each polymer was dissolved in 2 mL of acetone, followed by dropwise dispersion into Milli-Q-water under stirring for 24 h at room temperature to remove the organic solvent and form a micellar system. The resulting micelles with a final concentration (1 mg/mL) and (2 mg/mL) were subjected to centrifugation and filtration using a Haake Z36HK centrifuge (Wehingen, Germany) at 4499 × g for 15 min and a 0.45-μm filter membrane (Filtropur S 0.45, Sarstedt Technology Park, SA, Australia), respectively to sterilize and remove aggregated micelles. Subsequently, micelles were stored at 4°C until use for the following studies.
Fungal strains and culturing conditions
C. albicans strain ATCC 90028 and C. tropicalis ATCC 750 were employed in this study. Growing and maintaining of fungal cells were performed on the SDA agar medium by regular subculturing from a frozen glycerol stock of C. albicans and C. tropicalis. Fungal broth culture was prepared by transferring a loop of Candida colonies from freshly prepared SDA plates to inoculate SDB liquid medium at 37°C for 16–18 h with agitation. Then, Candida cells were harvested from the grown broth culture by centrifugation (4000 × g) for 5 min, followed by two washing steps with PBS (pH 7.2, 0.1 M) [26]. Washed cells were resuspended in RPMI 1640 medium buffered with MOPS (morpholino(propanesulfonic acid)) to a pH of 7 and adjusted to an optical density of 0.5 (~ 2 × 106 cells/mL) at 600 nm (OD600) using a UV/visible spectrophotometer (Jenway 6305, John Morris, Wayville, SA, Australia) [27, 28]. The obtained growth suspension was used as inoculum to form fungal planktonic cell culture and biofilms for subsequent antifungal studies.
Biofilm inhibition study
Crystal violet assay
Five different polymer-based micelles were investigated for their ability to inhibit C. albicans biofilms using a previously described method [29]. Briefly, 100 µL of prepared fungal suspension in RPMI medium (~ 2 × 106 cells/mL) was added to a 96-well polystyrene plate. Subsequently, 100 µL of each micellar system was added to the wells containing fungal cells, which were then incubated for 48 h at 37°C without agitation. Simultaneously, control samples were prepared by adding the RPMI medium without micelles into selected wells. At the end of incubation, biofilm formation was quantified by the crystal violet assay after aspirating the supernatants, washing the wells with PBS (pH 7.2, 0.1 M) to remove non-adherent cells and drying the plates for 1 h at 60°C. Plates were subjected to a staining step using crystal violet (0.1% CV solution) for 20 min, followed by washing to remove excess stain. Then, the stain was solubilized with 33% acetic acid and the absorbance was measured at 595 nm using a microtiter-plate reader (Bio-Rad, Hercules, CA, USA). The formed biofilms were quantified as the percentage of biomass formation calculated from the corresponding control (no treatment). Moreover, the time-dependent biofilm formation at different times of fungal cell attachment to the wells of 96-well plates was investigated by addition of micelles after allowing 0, 1, 2, 4, 6 and 24 h attachment of the fungal cells to the wells. The incubation period was continued for 24 h at 37°C after which the crystal violet assay was used to quantify C. albicans biofilm biomass. Under the same conditions, two independent biological replicates were performed for each assay.
Fluorescence imaging
To observe C. albicans biofilm formation in the presence of micelles, KOH-calcofluor white fluorescent stain (Sigma Aldrich, Castle Hill, NSW, Australia) was added to the wells after removing non-adherent cells following the directions of the manufacturer. After 1 min incubation at room temperature, wells were visualized using an inverted fluorescent microscopy (IX53 Olympus, Japan) with an ultraviolet filter cassette of 358/461 (excitation/emission) and imaged at ×40 magnification. The experiment was performed in duplicate.
Removal of preformed biofilm
To investigate the effect of micelles on the removal of biofilms, 48-h-old C. albicans biofilms were first grown in 96-well plates using 100 µL (~ 2 × 106 cells/mL) fungal inoculum and 37°C incubation temperature [29]. After 48 h incubation, 100 µL of micelles (1 mg/mL) was added to the PBS (pH 7.2, 0.1 M)-washed biofilms and plates were further incubated for 24 h at 37°C. Simultaneously, control samples were prepared by adding 100 µL RPMI medium without micelles into selected wells. Biofilm biomass was quantified by the previously described crystal violet assay and calculated as percentage of mass reduction in comparison with the corresponding control (biofilms with RPMI medium) [23]. Two experiments (six replicate for each) were performed at different time and under same conditions.
Effect of micelles on hyphae formation
Hyphae-inducing medium RPMI-1640 was used to study the effect of micelles on hyphae formation. The RPMI medium inoculated either with C. albicans or C. tropicalis (~ 2 × 106 cells/mL) was incubated with and without micelles (1 mg/mL) in 6-well tissue culture plates for 24 h with agitation at 37°C [30]. The morphological changes in the fungal cells were visualized under converted bright field microscopy using ×40 objective lens (IX53 Olympus, Japan). The experiment was performed in duplicate.
Antifungal combination therapy
Micelle antifungal combination against planktonic cells
Susceptibility of C. albicans planktonic cells to antifungal agents Flu and AmB in the present of micelles was determined using a cell viability assay. Briefly, drug solution in RPMI medium (1 µg/mL) and micelle solution (2 mg/mL) were prepared. A total of 100 µL (50 µL drug + 50 µL micelles) was transferred to microplates containing 100 µL of fungal suspension (~ 2 × 106 cells/mL). Wells containing only drug in DMSO (< 1%) or micelles were prepared to compare the antifungal activity of individual compounds with that of micelle-drug combinations. Wells containing only medium and fungi (i.e. without test compound) were included as negative and positive controls, respectively. After 48 h incubation at 37°C, aliquots of 100 µL of cell suspension were serially diluted in 0.9% sterile saline. Then, 10 µL of the diluted samples was spot-plated on SDA agar plates and incubated for 24 h at 37°C. After incubation, the total viable cells were determined and presented as colony forming units (CFU) [31]. Two independent experiments (4 replicate) were performed under equivalent conditions.
Micelle-antifungal combination against C. albicans biofilms
The effects of micelle-antifungal combinations were quantified in C. albicans biofilms using a previously described method [31]. Briefly, biofilms were grown on microplates for 48 h at 37°C using 100 µL of yeast suspension (~ 2 × 106 cells/mL). Subsequently, the biofilms were treated with Flu (500 µg/mL), AmB (2 µg/mL), micelles (2 mg/mL), Flu-micelles and AmB-micelles. Following 24 h incubation at 37°C, the exposed biofilms were detached from the wells and resuspended with sterile 0.9% saline. Following the serial dilution of suspended cells, the CFU grown on SDA agar plates were counted. The experiment was performed in duplicate.
Micelle-antifungal combination against dual-species biofilms
Dual-species biofilms of C. albicans and C. tropicalis were developed, following the previously mentioned protocol [15, 32]. Briefly, 14–16-h grown yeast culture of each strain was diluted in Spider medium (10 g nutrient broth, 10 g mannitol and 2 g K2HPO4 in 1 L Milli-Q water, pH 7.2) to 5 × 105 CFU/mL. This suspension was used as inoculum in a volume of 50 µL for each strain to inoculate 96-well plates, and dual-species biofilms were developed after 48 h incubation at 37°C. Following the incubation period, biofilms were washed with PBS and exposed to Flu, AmB, micelles, Flu-micelles and AmB-micelles. After 24 h incubation at 37°C, biofilms were washed with 0.9% saline and subjected to crystal violet assay as described previously to measure total biofilm biomass of dual-species biofilms. Another set of saline washed biofilms was subjected to a viability assay to quantify the biofilm-embedded cells. In this assay, biofilms were resuspended with 0.9% saline. Subsequently, the suspended cells were subjected to serial dilution and the CFU of each grown strain on selective chromogenic agar (CandiSelect) (Bio-Rad Laboratories Pty., Ltd., VIC, Australia). The experiment was performed in three replicates.
Toxicity study
Caenorhabditis elegans (C. elegans) AU37 (glp-4; sek-1) were provided from the Laboratory of Pharmaceutical Microbiology, Ghent University, Belgium, and used as an in vivo model to investigate the toxicity of the micelles. Maintenance and synchronization of the nematodes were performed following a previously described protocol [33, 34]. After synchronization, worms at L4 stages were dispersed into growth medium composed of 95% M9 buffer (3 g of KH2PO4, 6 g Na2HPO4, 5 g of NaCl and 1 M MgSO4 in 1 L of Milli-Q water), 5% brain heart infusion and 10 µg/mL cholesterol. Twenty to thirty worms were transferred into individual wells of 96-well plates and exposed to micelle solutions at concentrations of 0.5, 1 and 2 mg/mL. The total volume of each well was 100 µL. Plates were incubated for 72 h at 25°C. The viable worms were observed under an inverted microscope and counted in 24-h time intervals over 3 consecutive days. Wells containing nematodes without micelles and the toxic compound CuCl2 (63 µg/mL) were used as negative and positive controls, respectively. The live/dead worms of each treated nematode (six replicates, total: 120–180 nematodes/treatment) were monitored under bright-field inverted microscope at ×2 magnification and compared with the control samples to calculate relative nematode viability.
Statistical analysis
All data analyses were performed using GraphPad Prism 7.02 (La Jolla, CA, USA). P-values were obtained by one-way and two-way analysis of variance (ANOVA) with Tukey’s multiple-comparison test. Values were considered significant when the P-values < 0.05.
Results
Effect of copolymers on biofilm formation
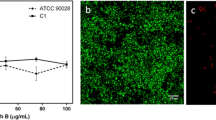
The formation of fungal biofilms in the presence of polymeric micelles quantified by crystal violet showed a maximum inhibition (> 90%) of biofilm by P(PEGMA-b-DEAEMA) micelles (Fig. 1a, b), followed by PEG-b-PCL micelles (approximately 30%) compared with the control. Fluorescence microscopy confirmed the inhibitory effects of P(PEGMA-b-DEAEMA) micelles on fungal biofilm development in comparison with biofilms not exposed to micelles (Fig. 2). While micrographs revealed the presence of budded yeast cells, true hyphae were absent. In contrast, none of the other polymeric micelles studied showed any inhibitory effects on hyphae formation and were comparable to the controls (Fig. 2). P(PEGMA-b-DEAEMA) micelles with the highest inhibitory effect on biofilms were further investigated for the biofilm inhibitory effects after 0, 1, 2, 3, 4 and 24 h of fungal cells attachment to the wells of 96-well plates before exposure to the micelles. The results indicated that inhibition of biofilm formation by micelles decreased as the post-inoculation time increased (Fig. 1c).
Inhibition of C. albicans biofilm formation. Effects of a series of polymeric micelles (1 mg/mL) after 48 h incubation at 37°C (a). Data represent the mean ± SD of two replicates, n = 12. Statistical comparison to control (untreated biofilms). ****P < 0.0001; ***P = 0.006; **P = 0.0002. Structure of P(PEGMA-b-DEAEMA). Adopted from The Royal Society of Chemistry, ref 23 (b). Effect of pH-sensitive micelles P(PEGMA-b-DEAEMA) as a function of time (c). Data represent the mean ± SD of two replicates, n = 6
Fluorescent staining of C. albicans biofilms with KOH-calcofluor white. Planktonic fungal cells were exposed to different polymeric micelles (1 mg/mL) at 37°C for 48 h. Biofilm formation inhibition was visualized by fluorescent microscopy after staining with calcofluor stain. The scale bar represents 100 µm
Effect on pre-grown biofilms
The efficacy of polymeric micelles against 48 h old C. albicans biofilms was investigated. As shown in Fig. 3, P(PEGMA-b-DEAEMA) micelles significantly reduced (P < 0.0001) the biomass of biofilms (~ 50%), followed by pluronic micelles to a lesser extent (P = 0.002, 20% biomass reduction). In contrast, none of the other tested polymeric micelles showed effects on fungal biofilms (P > 0.05).
Hyphae formation
Since hyphae are an essential structural element in the development of mature C. albicans as well as C. tropicalis biofilms, the effect of P(PEGMA-b-DEAEMA) micelles on hyphae formation was studied further. While yeast cells in the control samples underwent morphological transition from budding yeast cells to form a dense network of true hyphae as shown in Fig. 4a, yeast treated with P(PEGMA-b-DEAEMA) micelles failed to develop hyphae (Fig. 4b, d). Microscopic observations of C. albicans cells exposed to P(PEGMA-b-DEAEMA) micelles revealed that cells aggregated and had rough, proliferated surfaces in comparison with the cells of the control which were organized with smooth regular surfaces (Fig. 4b).
Effect of P(PEGMA-DEAEMA) micelles on fungal morphology. Untreated C. albicans cells (control) (a). C. albicans cells exposed to P(PEGMA-DEAEMA) micelles (1 mg/mL) for 48 h at 37°C (b). Untreated C. tropicalis. D) C. tropicalis exposed to micelles (1 mg/mL) for 48 h at 37°C (c). Inhibition of fungal hyphal growth was visualized by bright-field microscopy at 40 × magnification. The scale bar represents 20 µm
Combination of polymeric micelles and antifungal drugs
The combination of P(PEGMA-b-DEAEMA) blank micelles and the antifungal AmB was more effective as evidenced by a 1.8-log reduction of viable planktonic fungal cells compared with AmB alone (0.5 log reduction) (Fig. 5a). On calculation of the type of interaction (synergistic, indifferent, antagonistic) using the Bliss independence model, the micelle/AmB combination was found to be synergistic [35]. According to the model, values such as zero, above zero (positive) and below zero (negative) represent the following interactions respectively: indifferent, synergistic and antagonistic. In contrast the effectiveness of Flu alone and in combination with blank micelles against C. albicans cells were comparable (Fig. 5a). Interestingly, when treated by micelles alone, no notable antifungal activity against C. albicans planktonic cells was observed.
Effects of P(PEGMA-DEAEMA) micelle-antifungal combinations against C. albicans. Effect of combinations against C. albicans planktonic cells. CFU/mL of fungal cells after 24 h exposure to P(PEGMA-b-DEAEMA) blank micelles (1 mg/mL), AmB, AmB-micelles, Flu, Flu-micelles and medium as control (a). Effect of combinations against C. albicans biofilms. CFU/mL of biofilm-embedded cells after 24 h exposure to P(PEGMA-DEAEMA) blank micelles (2 mg/mL), AmB, AmB-micelles, Flu, Flu-micelles and medium as control (b). Data represent the mean ± SD of two replicates, n = 4. Statistical comparison to control (untreated sample). *P = 0.01; **P = 0.005; ***P = 0.0004
The combined effects of blank micelles and antifungals were also investigated against pre-grown C. albicans biofilms. The results of the viability assay indicated that when the antifungal agents were tested individually against 48-h-old C. albicans biofilms neither Flu nor AmB were effective (Fig. 5b). However, P(PEGMA-b-DEAEMA) micelles alone showed anti-biofilm activity against C. albicans biofilms (~ 1.5 log reduction) and was superior to the activity of both antifungals (< 0.5 log reduction). A physical mixture of Flu or AmB co-delivered with blank micelles (2 mg/mL) promoted synergistic effects against C. albicans biofilms and impressively reduced the population of biofilm-embedded fungal cells by 3 log for AmB and 2.2 log for Flu (Fig. 5b). These micelle-antifungal combinations were further tested against dual-species biofilms in which C. tropicalis were grown along with C. albicans. The biofilm biomass of dual-species biofilms was remarkably reduced after 24 h exposure to the blank micelles (up to 70% reduction) (Fig. 6a). Moreover, blank micelles co-delivered with physical mixtures of antifungals Flu and AmB promoted enhanced reduction in the biofilm biomass of these dual-species biofilms (up to 85% reduction) (Fig. 6a). When these polymicrobial biofilms were challenged with AmB and Flu alone, only 50% and less than 2% reduction in the biofilm biomass was observed, respectively. Importantly, blank micelles demonstrated comparable activity against biofilm-embedded fungal cells in C. albicans in both dual-species and single-species biofilms. In contrast, substantial anti-biofilm activity was observed by micelles alone against the NCA species, C. tropicalis, within dual-species biofilms (2.7-log reduction) (Fig. 6b). The reduction in the number of viable cells within dual-species biofilm was further enhanced after co-delivery of the micelles with antifungal agents. These micelle-AmB mixtures reduced C. albicans and C. tropicalis cell viability up to 2.7 log and 3.6 log respectively (Fig. 6b), whereas physical mixing with Flu reduced C. albicans and C. tropicalis cells viability up to 2.6 log and 3.7 log respectively.
Effects of P(PEGMA-DEAEMA) micelle-antifungal combinations against C. albicans and C. tropicalis in dual-species biofilms. Biofilm biomass reduction percentage of dual-species biofilms (a). Biofilm cells viability of dual-species biofilms. Biofilms were exposed to P(PEGMA-DEAEMA) blank micelles (2 mg/mL), AmB, AmB-micelles, Flu, Flu-micelles and medium as control for 24 h at 37°C (b). Data represent the mean ± SD of three replicates, n = 6. Statistical comparison to control (untreated biofilm). ****P < 0.0001; ***P ≤ 0.0006; **P = 0.005
Toxicity in C. elegans
The viability of nematodes in the presence of di-block P(PEGMA-b-DEAEMA) micelles at concentrations (0.5, 1 and 2) mg/mL was assessed visually by bright-field microscopy. Analysis revealed that more than 85% of the worms were alive (indicated by motility) after 72-h incubation. In control experiments, the mortality in nematodes treated with toxic positive control compound (CuCl2) was > 90%, while untreated negative control samples (worms in growth medium) demonstrated the lowest mortality rate (< 10%.).
Discussion
Candida biofilm elimination remains challenging due to the robust structures of biofilms that impede the efficacy of many antifungal agents via penetration and metabolic barriers. Thus, introduction of agents that attenuate the development of biofilms can be a novel and significant approach for the efficient elimination or prevention of fungal biofilms [1, 36]. Previously, we described the synthesis and physicochemical properties of pH-sensitive micelles based on P(PEGMA-b-DEAEMA)23. Notably, these micelles demonstrated a substantial activity against pre-formed single-species C. albicans biofilms. The present study investigated the underlying mechanisms of micelle-related antibiofilm activity and compared this activity with other polymeric micelles. In addition, we explored the potential of the co-delivery of antifungal agents with biofilm-busting micelles in defeating both the planktonic and biofilm mode of fungal growth.
A wide range of polymeric micelles have been successfully used as carrier systems for antibiofilm agents, but these micelles themselves did not exhibit anti-biofilm activity alone [23, 37, 38]. Recently, block copolymer nanoparticles (DA95B5) with a novel mechanism of action against bacterial biofilms have been reported [22]. The present study highlights that pH-responsive polymeric P(PEGMA-b-DEAEMA) micelles possess intrinsic antibiofilm activity. The efficacy of P(PEGMA-b-DEAEMA) micelles in blocking C. albicans biofilm formation as shown in Fig. 1a is likely attributed to a change in the net charge of the fungal cells or adherent surfaces resulting from an increase in the net positive charge of the micelles, induced by protonation of their polymer side chains within the acidic microenvironment of fungi (Fig. 1b) [23, 39]. P(PEGMA-b-DEAEMA) micelles with an increase in the zeta-potential (up to + 63 mV) has been recently reported to be produced in response to the low pH (i.e. pH < 6) [23]. These changes may keep fungal cells in suspension, preventing cell-to-cell interaction and adhesion, ultimately inhibiting biofilm formation (as schematically represented in Fig. 7a) [40]. For instance, the previously reported inhibition in Candida biofilms by chitosan was mainly attributed to the cationic charge of the polymer that interfered with the negative charge of the fungal cells, preventing them from adhesion and interaction to form mature biofilms [40, 41]. While the sizes of different micellar systems used in this study were comparable (< 100 nm) except Solutol (about 20 nm) (data not presented), the zeta potential of P(PEGMA-b-DEAEMA) micelles was significantly positive (> + 30 mV) at pH 7, and differed from other micelles which were nearly neutral (data not presented). The ionization and conformational changes in the structure of P(PEGMA-b-DEAEMA) micelles in response to different pH distinguished them from other polymeric micelles used in this study [23]. The micellar behaviour is due to the presence of (PDEAEMA) block copolymer which is stable at physiological pH (pKa of ~ 6.8) and susceptible to protonation under acidic conditions [42]. Considering the variation in the physicochemical property of each micellar system, the antibiofilm activity of micelles was investigated at different concentrations ((0.01, 0.05, 0.1, 0.5, 1, 2 and 3) mg/mL (data not presented). However, the antibiofilm activity of micelles at 1 and 2 mg/mL were significant compared with other concentrations at which the activity was negligible. Therefore, 1-mg/mL and 2-mg/mL concentrations of micelles were only considered in this study.
The results of biofilm inhibition by micelles at different post-inoculation times reveal that once the free cells get attached to the surface of the 96-well plate and start developing the biofilm, they become more tolerant to micelles (Fig. 1c). Indeed, compared with planktonic cells, mature biofilms of C. albicans have shown an up to 2000-fold increased tolerance towards antifungal agents [2, 18]. In addition, a variety of cellular and molecular changes occur during the development of Candida biofilms with distinctive differences compared with planktonic cells [36].
One important step in the biofilm life cycle of C. albicans is the ability of fungal yeast to transit to the hyphal form; this is considered a critical structural element of mature biofilms (as represented in Fig. 7b) [43]. In the present study, P(PEGMA-b-DEAEMA) micelles showed unique anti-filamentous activity in planktonic fungal cells, inhibiting hyphal formation and affecting yeast surfaces by modifying their thickness, smoothness and proliferation (Fig. 4a, b). This suppression phenomenon in the morphological switching suggests that biofilm inhibition and reduction by micelles are directly correlated with inhibition of hyphal growth (as schematically proposed in Fig. 7a, c). Since hyphae formation determines virulence of C. albicans and mediates invasion into the infected tissue, impediment of hyphal growth by polymeric micelles is a significant finding [4]. Recently, block copolymer nanoparticles (DA95B5) with a novel mechanism of action against bacterial biofilms have been reported [22]. These particles removed biofilms by promoting the dispersion of biofilm-associated bacteria without demonstrating bactericidal effects [22]. Herein, to the best of our knowledge we present the first extensive evidence of polymeric micelles with anti-fungal activity.
Synergistic and additive drug combinations are promising approaches for reducing the development of resistance often associated with monotherapy [44, 45]. In this study, the unique combination of antifungal AmB co-delivered with micelles displayed a synergistic effect against planktonic Candida cells, while the micellar system with Flu showed negligible improvement on the activity of pure drug (Fig. 5a). The synergic effects are possibly due to changes in the yeast cell wall established following the treatment of Candida with P(PEGMA-b-DEAEMA) micelles as shown in Fig. 4b. It is possible that these micelles enhance AmB access to the fungal cell membrane, where it binds to membrane ergosterol, establishing transmembrane pores and subsequent loss of cellular contents, leading to enhanced efficacy against free Candida cells [44, 46]. AmB is a poorly water-soluble agent (< 1 µg/mL and logP = 0.8), with amphiphilic and amphoteric behaviour [47, 48]. Therefore, AmB in water exists as a combination of water-soluble monomers and oligomers with non-water-soluble super-aggregates [47]. The state and extent of AmB aggregate can be reduced in the present of amphiphilic micelles; hence, a fraction of AmB is solubilized within the micelles [49]. In comparison with AmB, Flu has an increased water solubility (8 mg/mL), and a lower lipophilicity (logP = 0.5) [50]. The fungicidal or fungistatic action of Flu against C. albicans is based on the inhibition of a key role enzyme (14-alpha-demethylase) in biosynthesis of ergosterol of the fungal cytoplasmic membrane [51]. In addition, Flu inhibitory action is effective against fungi in their yeast-form of growth but inactive against fungal filamentous form [52].
Importantly, P(PEGMA-b-DEAEMA) micelles promoted a synergistic effect in combination with antifungal drugs AmB and Flu against C. albicans biofilms (Fig. 5b). The synergistic activity was likely due to the direct inhibitory effect of these micelles on the formation of fungal hyphae (Fig. 4b). Interestingly, the combination of such micelles with Flu was effective against pre-formed biofilms, reducing the viability of biofilm embedded cells, but did not alter the activity of Flu against free planktonic cells (Fig. 5a, b). It has been reported that the activity of combination therapy can vary between the planktonic and biofilm mode of growth of C. albicans [20, 53]. For example, synergy has been reported for the combination of AmB and shearinines, a hyphal growth inhibitor produced by Penicillium sp. against planktonic cells of C. albicans but showed marginal effects against C. albicans biofilms [54].
C. tropicalis shares several virulence factors associated normally with C. albicans, such as phenotypic switching, hyphae formation and biofilm production, which imposes a potent health threat by co-infection with C. albicans [15, 17]. Therefore, the present study also investigated the effects of micelles and antifungal combinations against closely related strains C. albicans and C. tropicalis in a dual-species biofilm. In accordance with the efficacy of P(PEGMA-b-DEAEMA) micelles against C. albicans in a single-species biofilm, the presence of the other species did not affect micellar activity against C. albicans in a dual-species biofilm. Moreover, the results indicated that even in dual-species biofilms, micelles alone and in combination with drugs promoted significant anti-biofilm effects by reducing the biofilm biomass and number of viable cells of each species (Fig. 6). In contrast, study by Carmello et al. on the effects of photodithiazine hydrogel against dual-species biofilms of C. albicans and C. tropicalis resulted in marginal reduction in the viability of both strains without any reduction in their biofilm biomass [16]. Present results highlighted the novelty of our micelles in defeating C. albicans and C. tropicalis in their dual-species biofilms by inhibiting the same process. It is known that C. tropicalis biofilms exhibit resistance to Flu. In line with this, we did not observe a reduction in the biomass and fungal cells of the dual-species biofilms by Flu (Fig. 6a) [55, 56].
Further, we challenged nematodes with the P(PEGMA-b-DEAEMA) micelles to investigate their toxicity. The high survival rate of nematodes indicated the compatible nature of micelles within the selected model. Generally, the micellar system containing the DEAEMA blocks were well-tolerated, and showed excellent compatibility against living cells as investigated in vitro [57, 58]. Consequently, the current study highlighted the critical role of P(PEGMA-b-DEAEMA) micelles in inhibiting fungal filament, a virulence factor involved in formation of C. albicans biofilms and could provide new, as well as safe, clinically relevant approach for defeating fungal biofilms.
Conclusion
The polymeric micelles P(PEGMA-b-DEAEMA) effectively inhibited C. albicans biofilm formation and removed pre-grown single- as well as dual-species fungal biofilm–associated C. albicans with C. tropicalis by preventing hyphal growth. Micelles alone showed antibiofilm activity against both single and dual-species biofilms, superior to the effects shown by antifungals Flu and AmB against the biofilms. Micelles interacted synergistically with antifungals Flu and AmB when co-delivered, enhancing their efficacy against pre-grown biofilms. Agents with anti-morphologic transition activity provides a new strategy for defeating biofilms of polymorphic resistance strains, and can be potentially used in counteracting Candida virulence.
Data availability
All data are available in the manuscript.
References
Prasad R, Shah AH, Dhamgaye S. Mechanisms of drug resistance in fungi and their significance in biofilms, in Antibiofilm Agents. Springer 2014. p. 45–65.
Tsui C, Kong EF, Jabra-Rizk MA. Pathogenesis of Candida albicans biofilm. FEMS Pathogens and Disease. 2016. 74(4): p. ftw018.
Voltan AR, et al. Fungal diseases: could nanostructured drug delivery systems be a novel paradigm for therapy? Int J Nanomed. 2016;11:3715.
Sudbery PE. Growth of Candida albicans hyphae. Nat Rev Microbiol. 2011;9(10):737–48.
Lohse MB, et al. Development and regulation of single-and multi-species Candida albicans biofilms. Nat Rev Microbiol. 2018;16(1):19.
Tan Y, et al. Efficacy of carboxymethyl chitosan against Candida tropicalis and Staphylococcus epidermidis monomicrobial and polymicrobial biofilms. Int J Biol Macromol. 2018;110:150–6.
Weerasekera MM, et al. Culture media profoundly affect Candida albicans and Candida tropicalis growth, adhesion and biofilm development. Memórias Do Instituto Oswaldo Cruz. 2016;111(11):697–702.
Jiang C, et al. Significance of hyphae formation in virulence of Candida tropicalis and transcriptomic analysis of hyphal cells. Microbiol Res. 2016;192:65–72.
Ramage G, Robertson SN, Williams C. Strength in numbers: antifungal strategies against fungal biofilms. Int J Antimicrob Agents. 2014;43(2):114–20.
Rodríguez-Cerdeira C, et al. Biofilms and vulvovaginal candidiasis. Colloids and Surfaces B: Biointerfaces; 2018.
Sawant B, Khan T. Recent advances in delivery of antifungal agents for therapeutic management of candidiasis. Biomed Pharmacother. 2017;96:1478–90.
Tobudic S, et al. Antifungal susceptibility of Candida albicans in biofilms. Mycoses. 2012;55(3):199–204.
Cavalheiro M, Teixeira MC. Candida biofilms: threats, challenges, and promising strategies. Front Med. 2018;5:28.
Fanning S, Mitchell AP. Fungal biofilms. PLoS Pathog. 2012. 8(4).
Pathirana RU, et al. Filamentous non-albicans Candida species adhere to Candida albicans and benefit from dual biofilm growth. Frontiers in microbiology. 2019;10:1188.
Carmello JC, et al. Photoinactivation of single and mixed biofilms of Candida albicans and non-albicans Candida species using Photodithazine®. Photodiagn Photodyn Ther. 2017;17:194–9.
Ramage G, et al. In vitro pharmacodynamic properties of three antifungal agents against preformed Candida albicans biofilms determined by time-kill studies. Antimicrob Agents Chemother. 2002;46(11):3634–6.
Mayer FL, Wilson D, Hube B. Candida albicans pathogenicity mechanisms. Virulence. 2013;4(2):119–28.
Gauwerky K, Borelli C, Korting HC. Targeting virulence: a new paradigm for antifungals. Drug Discovery Today. 2009;14(3–4):214–22.
Cui J, et al. Synergistic combinations of antifungals and anti-virulence agents to fight against Candida albicans. Virulence. 2015;6(4):362–71.
Nett JE. Future directions for anti-biofilm therapeutics targeting Candida. Expert review of anti-infective therapy. 2014;12(3):375–82.
Li J, et al. Block copolymer nanoparticles remove biofilms of drug-resistant Gram-positive bacteria by nanoscale bacterial debridement. Nano Lett. 2018;18(7):4180–7.
Albayaty YN, et al. pH-Responsive copolymer micelles to enhance itraconazole efficacy against Candida albicans biofilms. J Mater Chem B. 2020;8(8):1672–81.
Ahmed N, et al. Polymeric drug delivery systems for encapsulating hydrophobic drugs. Drug Delivery Strategies for Poorly Water-Soluble Drugs. 2013. p. 151.
Takahashi C, et al. Antibacterial activities of polymeric poly (dl-lactide-co-glycolide) nanoparticles and Soluplus® micelles against Staphylococcus epidermidis biofilm and their characterization. RSC Adv. 2015;5(88):71709–17.
Pierce CG, et al. A simple and reproducible 96-well plate-based method for the formation of fungal biofilms and its application to antifungal susceptibility testing. Nat Protoc. 2008;3(9):1494.
Lu M, et al. Gentamicin synergises with azoles against drug-resistant Candida albicans. Int J Antimicrob Agents. 2018;51(1):107–14.
Seneviratne C, et al. Cell density and cell aging as factors modulating antifungal resistance of Candida albicans biofilms. Antimicrob Agents Chemother. 2008;52(9):3259–66.
Haque F, et al. Inhibitory effect of sophorolipid on Candida albicans biofilm formation and hyphal growth. Sci Rep. 2016;6:23575.
Manoharan RK, et al. Alizarin and chrysazin inhibit biofilm and hyphal formation by Candida albicans. Front Cell Infect Microbiol. 2017;7:447.
Brilhante RSN, et al. Candida tropicalis from veterinary and human sources shows similar in vitro hemolytic activity, antifungal biofilm susceptibility and pathogenesis against Caenorhabditis elegans. Vet Microbiol. 2016;192:213–9.
Liu H, Kohler J, Fink GR. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science. 1994;266(5191):1723–6.
Richter K, et al. Taking the silver bullet colloidal silver particles for the topical treatment of biofilm-related infections. ACS Appl Mater Interfaces. 2017;9(26):21631–8.
Tampakakis E, Okoli I, Mylonakis EAC. elegans-based, whole animal, in vivo screen for the identification of antifungal compounds. Nat Protoc. 2008;3(12):1925.
Thangamani S, Younis W, Seleem MN. Repurposing celecoxib as a topical antimicrobial agent. Frontiers in microbiology. 2015;6:750.
Bandara H, Matsubara VH, Samaranayake LP. Future therapies targeted towards eliminating Candida biofilms and associated infections. Expert review of anti-infective therapy. 2017;15(3):299–318.
Albayaty YN, et al. Enzyme responsive copolymer micelles enhance the anti-biofilm efficacy of the antiseptic chlorhexidine. Int J Pharm. 2019.
Liu Y, et al. Surface-adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano. 2016;10(4):4779–89.
Miyake Y, et al. Antifungal drugs effect adherence of Candida albicans to acrylic surfaces by changing the zeta-potential of fungal cells. FEMS Microbiol Lett. 1990;69(3):211–4.
Martinez LR, et al. Demonstration of antibiofilm and antifungal efficacy of chitosan against candidal biofilms, using an in vivo central venous catheter model. J Infect Dis. 2010;201(9):1436–40.
Savard T, et al. Antimicrobial action of hydrolyzed chitosan against spoilage yeasts and lactic acid bacteria of fermented vegetables. J Food Prot. 2002;65(5):828–33.
Shahalom S, et al. Poly (DEAEMa-co-PEGMa): a new pH-responsive comb copolymer stabilizer for emulsions and dispersions. 2006;22(20):8311–8317.
Pierce CG, Saville SP, Lopez-Ribot JL. High-content phenotypic screenings to identify inhibitors of Candida albicans biofilm formation and filamentation. Pathogens and Disease. 2014;70(3):423–31.
Rodrigues ME, et al. Novel strategies to fight Candida species infection. Crit Rev Microbiol. 2016;42(4):594–606.
Tome M, et al. Synergistic and antagonistic effects of immunomodulatory drugs on the action of antifungals against Candida glabrata and Saccharomyces cerevisiae. PeerJ. 2018;6:e4999.
Rodrigues CF, Henriques M. Liposomal and deoxycholate amphotericin B formulations: effectiveness against biofilm infections of Candida spp. Pathogens. 2017;6(4):62.
Torrado J, et al. Amphotericin B formulations and drug targeting. 2008;97(7):2405–25.
Buehler DC, Marsden MD, Shen S. 8, Bioengineered vaults: self-assembling protein shell-lipophilic core nanoparticles for drug. 2014.
Vandermeulen G, et al. Encapsulation of amphotericin B in poly (ethylene glycol)-block-poly (ɛ-caprolactone-co-trimethylenecarbonate) polymeric micelles. 2006;309(1–2):234–240.
Muţ A, et al. Chitosan/HPMC-based hydrogels containing essential oils for topical delivery of fluconazole: Preliminary studies. 2018;66(2):248–56.
Corrêa JCR, Salgado HRN. Review of fluconazole properties and analytical methods for its determination. 2011;41(2):124–132.
Castelli MV, et al. Novel antifungal agents: a patent review (2011–present). 2014;24(3):323–338.
Bachmann SP, et al. Antifungal combinations against Candida albicans biofilms in vitro. Antimicrob Agents Chemother. 2003;47(11):3657–9.
You J, et al. Small-molecule suppressors of Candida albicans biofilm formation synergistically enhance the antifungal activity of amphotericin B against clinical Candida isolates. ACS Chem Biol. 2013;8(4):840–8.
Kawai A, Yamagishi Y, Mikamo H. In vitro efficacy of liposomal amphotericin B, micafungin and fluconazole against non-albicans Candida species biofilms. J Infect Chemother. 2015;21(9):647–53.
Ann Chai LY, Denning DW, Warn P. Candida tropicalis in human disease. Crit Rev Microbiol. 2010;36(4):282–98.
Yang YQ, et al. pH-sensitive micelles self-assembled from multi-arm star triblock co-polymers poly (ε-caprolactone)-b-poly (2-(diethylamino) ethyl methacrylate)-b-poly (poly (ethylene glycol) methyl ether methacrylate) for controlled anticancer drug delivery. Acta Biomater. 2013;9(8):7679–90.
Lin W, et al. pH-responsive micelles based on (PCL) 2 (PDEA-b-PPEGMA) 2 miktoarm polymer: controlled synthesis, characterization, and application as anticancer drug carrier. Nanoscale Res Lett. 2014;9(1):243.
Acknowledgements
The authors thank Dr. Anton Blencowe’s laboratory for providing the (PEG-b-PCL) polymer.
Funding
The Australian Research Council Centre for Excellent in Convergent Bio-Nano Science and Technology (CBNS) has supported this project.
Author information
Authors and Affiliations
Contributions
YNA designed the study, conducted the experiments, and collected and analysed the data. YNA and NT wrote the manuscript. CAP and NT designed the research and reviewed the manuscript. PDR, TPD, JFQ, and MRW synthesized the polymer P(PEGMA-b-DEAEMA) and reviewed the manuscript. All authors have approved the final manuscript.
Corresponding author
Ethics declarations
Consent for publication
All authors have consented to the publication.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Albayaty, Y.N., Thomas, N., Ramírez-García, P.D. et al. Polymeric micelles with anti-virulence activity against Candida albicans in a single- and dual-species biofilm. Drug Deliv. and Transl. Res. 11, 1586–1597 (2021). https://doi.org/10.1007/s13346-021-00943-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00943-4