Abstract
Worldwide, colon cancer (CC) represents the fourth most common type of cancer and the fifth major cause of cancer-associated deaths. Surgical resection is considered the standard therapeutic choice for CC in early stages. However, in latter stages of the disease, adjuvant chemotherapy is essential for an appropriate management of this pathology. Metal-based complexes displaying cytotoxic properties towards tumor cells emerge as potential chemotherapeutic options. One metallodrug, oxaliplatin, was already approved for clinical use, playing an important role in the treatment of CC patients. Unfortunately, most of the newly designed metal-based complexes exhibit lack of selectivity against cancer cells, low solubility and permeability, high dose-limiting toxicity, and emergence of resistances. Nanodelivery systems enable the incorporation of metallodrugs at adequate payloads, solving the above-referred drawbacks. Moreover, drug delivery systems, depending on their physicochemical properties, are able to release the incorporated material preferentially at affected tissues/organs, enhancing the therapeutic activity in vivo, with concomitant fewer side effects. In this review, the general features and therapeutic management of CC will be addressed, with a special focus on preclinical or clinical studies using metal-based compounds. Furthermore, the use of different nanodelivery systems will also be described as tools to potentiate the therapeutic index of metallodrugs for the management of CC.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is a group of diseases in which abnormal cells divide without control, being able to invade nearby tissues, as well as to spread to distant parts of the body through circulatory and lymphatic systems [1]. This phenomenon can occur due to genetic predisposition, lifestyle habits or environmental factors, such as exposure to chemical carcinogens, ionizing radiation, or diseases and infections [1]. Indeed, cancer is one of the major causes of death worldwide and the increase of new cases year by year is alarming [2]. Colon cancer (CC), in particular, is one of the most dangerous malignant pathologies with both high incidence and mortality rates. Colon and rectal cancers are often grouped together because they share some similarities. Nevertheless, the colon and rectum are different in terms of location, blood supply, drainage, and innervation. Consequently, the invasive growth of primary tumor, as well as therapeutic options, are quite dissimilar [3]. In this review, although general considerations will be presented regarding cancer, the main focus will be on CC. Specifically, CC is developed due to changes in normal colonic epithelium, with formation of adenomatous polyps that might proliferate and increase in their dimension, leading to genetic and epigenetic mutations that accumulate over time [4–7]. Even though not all polyps evolve into invasive cancer, those with malignant features have the potential to metastasize. This uncontrolled growth is the reason why the polyps start invading nearby tissues, particularly the bowel wall, and eventually reaching distant sites through the lymphatic and circulatory systems [8]. In Fig. 1 are shown the progression of CC and the associated survival rates [9–11].
Risk factors for CC can be either modifiable or non-modifiable. Age and genetic predisposition are non-modifiable risk factors and, thus, the screening of people with age over 50 and/or with a family history of polyps and colorectal cancer (CRC) is highly recommended [8]. However, modifiable factors are also of major importance, since adequate lifestyle habits can prevent these malignant pathologies. Indeed, the consumption of red and processed meats, high-fat/low-fiber diets and alcohol intake have been associated to increased risks of CC or CRC [12, 13]. Other risk factors such as physical inactivity, overweight, or smoking may also contribute for cancer development [14, 15].
According to the International Agency for Research on Cancer, it was estimated for 2018 that 18.1 million new cancer cases were diagnosed and 9.6 million deaths were reported worldwide [16]. Regarding CC, 1.1 million patients were diagnosed (6.1% of the total number of new cancer cases) and more than 551,000 deaths were recorded (5.8% of total deaths from cancer). In Fig. 2a are shown the incidence, prevalence, and mortality associated to colon, rectum, and anus cancers for 2018. An alarming fact is represented in Fig. 2b, which shows the progression of the CC over the years (from 2018 to 2040). In 2040, it is expected that the incidence and mortality will almost duplicate in comparison to the predicted cases for 2018 [17]. Globally, CC seems to affect more males than females, and Asia counts with the highest incidence and mortality (49.2 and 49.3%, respectively), followed by Europe (28.5 and 28.5%, respectively) [5, 16]. Although the incidence and mortality in older adults have been decreasing, in adults younger than 50 years the increase of new cases constitutes an undeniable concern [18].
Clinical presentation and diagnosis
CC diagnosis is an important issue to be highlighted, consisting primarily of fecal immunochemical tests for hemoglobin, guaiac-based fecal occult blood tests, and multi-targeted stool DNA tests. Visual examination methodologies include computed tomographic colonography, sigmoidoscopy, and colonoscopy, being the latter considered the gold standard for CC detection [19]. The stage at which CC is diagnosed has a crucial influence on the type of treatment chosen. Based on this assumption, accurate and highly specific diagnostic methods are needed. An early diagnosis may improve therapeutic results and increase survival rates for CC patients [20, 21]. Nevertheless, at initial stages of the disease, patients may be asymptomatic or present non-specific symptoms, such as change in bowel habits, general abdominal discomfort, weight loss with no apparent cause, constant tiredness, nausea, or vomiting. In contrast, in advanced stages of the pathology, patients commonly suffer from abdominal pain, blood in stool, obstruction, or perforation. These symptoms, combined with all the diagnostic methodologies, are used to complement the medical information that is, subsequently, gathered during the process of cancer staging, allowing to plan the most adequate therapeutic strategies [21, 22].
Therapeutic management
As already described, most colon tumors develop when the normal colonic epithelium suffers histological, morphological, and genetic changes that accumulate over time [4]. The most common options for the treatment of CC include surgery, radiation therapy, chemotherapy, and targeted therapy [23] and, as aforementioned, the choice of the best treatment lies heavily on the stage of the disease (Table 1) [24, 25].
At present, the standard and the most effective therapeutic option for CC at early stages (0 and I) is surgery, aiming to completely remove the tumor, the major vascular pedicles and the lymphatic drainage basin of the affected colonic segment [21, 24, 25]. At these initial stages of the disease, it is uncommon the association of chemotherapy to surgical resection [23, 26].
At stage II, CC has proliferated through the muscle layer and serosa and spread to nearby organs, without reaching lymph nodes. In contrast, at stage III, cancer has already invaded surrounding lymph nodes. In both pathological conditions, radiation may be useful to reduce the size of unresectable tumors so they can later be surgically removed [26]. In addition, this therapeutic approach may also be used for patients who are not in physical conditions to undergo surgery [27]. Unfortunately, radiation is associated with severe side effects, such as nausea, stool leakage, skin irritation, fatigue, diarrhea or, in worse cases, small bowel disease that is, sometimes, as challenging as the treatment of cancer itself [28]. For CC management at stages II and III, a key therapeutic option is adjuvant chemotherapy aiming to eradicate micro-metastasis, prevent cancer recurrence and significantly improve overall survival rates [29, 30]. For these reasons, it is highly recommended to start adjuvant chemotherapy as soon as possible [31] in combination with radiation and surgery [11, 32].
At stage IV, CC has spread to other organs and tissues, highlighting chemotherapy and targeted therapies as crucial approaches to promote tumor shrinkage, relief of symptoms, and improved prognosis [23, 26, 33]. Regarding targeted therapies, they involve the use of monoclonal antibodies and small molecule inhibitors, being considered effective treatment options. Despite not being as efficacious as chemotherapy, a major advantage of targeted therapies is the reduced side effects [34].
As clinically proved, chemotherapy is, undoubtedly, one of the most important therapeutic options in the management of CC. However, its efficacy is often weakened by several drawbacks associated to its use, such as low specificity and inadequate pharmacokinetic and biodistribution profiles. Considering this, researchers have been exploring alternative and more effective and safer chemotherapeutic agents. One of this class of compounds are metallodrugs. Metal-based complexes display a wide spectrum of coordination numbers and geometries, varying oxidation states, as well as distinct thermodynamics and kinetics features [35, 36]. Importantly, metallodrugs can be structurally modified to improve selectivity and minimize possible undesirable effects [36]. In the next section, relevant information about metal-based complexes as potential therapeutic options against cancer in general and, particularly, in CC will be presented.
Metallodrugs used in colon cancer
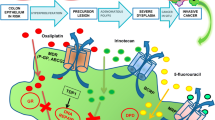
According to the National Cancer Institute, from the list of cancer drugs approved by the Food and Drug Administration (FDA), the only metal-based drug currently in use for CC is oxaliplatin (Fig. 3) [37, 38]. Oxaliplatin is commonly intravenously (i.v.) administered in combination with other anticancer drugs, such as 5-fluorouracil (5-FU) or capecitabine [39]. The chemotherapeutic regimen combining 5-FU, oxaliplatin, and leucovorin is designated as FOLFOX, whereas the double combination of capecitabine and oxaliplatin is named CAPOX. FOLFOX is one of the most used therapeutic regimens for the management of CC, especially at advanced stages of the disease (III and IV) [11, 39].
Chemical structure of oxaliplatin, the only metallodrug currently approved for CC treatment [40]
Additionally, randomized trials in patients’ stage IV, with resectable CRC liver metastases, were conducted, supporting the perioperative use of FOLFOX as a way to reduce tumor size and facilitate its surgical removal. However, contrary to the expected and compared to surgery alone, the perioperative FOLFOX chemotherapy has not demonstrated to enhance overall patient survival [21, 41, 42]. Therefore, in metastatic CRC, FOLFOX therapy is sometimes combined with other drugs or biologic targeted agents, such as irinotecan, bevacizumab, or cetuximab in patients with high tumor burden or rapid progressing disease. Nevertheless, these therapeutic regimens can also intensify side effects, such as nausea and vomiting, fatigue and cytopenia (reviewed in [39, 43])
Metal-based complexes as potential anticancer agents
The discovery of cisplatin [cis-diamminedichlorido platinum(II)] and its therapeutic properties revolutionized not only the treatment of cancer, but also encouraged the search for other metal complexes as promising tools for the treatment of various diseases, including CC (reviewed in [44, 45]). The potential of these metallodrugs can be related with a set of unique characteristics of metal elements, such as redox activity, charge variation, wide range of coordination geometries, and reactivity towards organic substrates. These properties are particularly useful in the design of a vast variety of molecules, allowing a wide array of interactions with several biomolecular targets [35, 46, 47].
Despite its success, cisplatin presents some limitations, such as low specificity, unsatisfactory biodistribution profile, toxic side effects, and emergence of resistances [48]. These limitations prompted the research for new platinum-based compounds, namely oxaliplatin and carboplatin, displaying better pharmacokinetic and pharmacodynamic properties than the original cisplatin [49, 50]. In addition, beyond the vast number of platinum drug analogues synthesized and studied, several other metal-based complexes have also demonstrated potential anticancer properties, as described in the next sections [51, 52].
Gold-based complexes
Gold has been used in medicine for thousands of years, but in the past century we have witnessed a substantial rise in gold-based complexes for the treatment of diseases, such as rheumatoid arthritis, infections, and cancer [53]. Gold(I) complexes, in particular, have been studied as anticancer agents for quite some time. For instance, gold(I) phosphine complexes, which are derived from the antirheumatic drug auranofin, have been considered as lead compounds to the development of gold metallodrugs. In this context, chloro gold(I) phosphane derivatives displayed IC50 values comparable to cisplatin (4.2–5.3 µM versus 7.0 µM, respectively) against the human CC cell line, HT-29. Moreover, cellular uptake studies showed that the most lipophilic compounds presented the highest uptake by CC cells, which can be correlated with their stronger antiproliferative activity [54]. In addition, gold-based complexes that contain the AuPEt3 moiety (Fig. 4a) have also demonstrated cytotoxic effects towards human colon cell lines, HCT-15 and LoVo, including the multidrug resistant (MDR) cell line (LoVo MDR) [54]. This last finding is of particular interest, since drug resistance is a serious problem that leads to the inefficacy of current standard chemotherapy. Additionally, gold(III) porphyrin complexes were found to exhibit in vitro antiproliferative properties towards the human CC cell line, HCT-116. Moreover, in a CC xenograft Balb/c murine model, a reduction on tumor progression was observed in comparison to untreated mice. Nevertheless, no positive control groups were included [55]. Water-soluble thiolate gold(I) complexes, of the type [Au(SR’)(PR3)] (Fig. 4b), displayed cytotoxic properties towards the human CC cell line WiDr, with ID50 values 2-fold lower than those obtained for cisplatin [56].
Copper-based complexes
In the search for new metallodrugs, copper has also attracted particular attention by virtue of being an endogenous metal and, therefore, better tolerated in vivo than other metals [58, 59]. Alongside its redox properties, copper is also known to form complexes with varied coordination numbers and geometries, which opened doors to a vast array of synthesizable compounds with different mechanisms of action [60, 61]. In this context, some studies have demonstrated that copper complexes (Fig. 5a) displayed inhibitory effects in nanomolar range towards CC cell lines, Colo 205 and Colo 320. Interestingly, the most active compound was 70 times more toxic towards cancerous cells than normal fibroblast MRC-5 cell line, showing the so desired selectivity [62]. Moreover, cytotoxic effects within the submicromolar range were also achieved using phosphine copper(I) complexes against a panel of CC cell lines (LoVo, DLD1, HCT-15 and SW480). Importantly, the antiproliferative activity of this complex in normal human fibroblasts was significantly lower, demonstrating its selectivity towards neoplastic cells. Also, this copper(I) complex was 5-fold more potent than oxaliplatin against LoVo MDR, suggesting its ability to overcome the oxaliplatin resistance. Of note, the results showed that, contrarily to oxaliplatin, the copper complex did not act via DNA fragmentation or activation of caspases, but by inhibition of caspase-3/7 activity [63]. Furthermore, Cuphen [Cu(phen)Cl2] (Fig. 5b) has demonstrated antiproliferative properties towards a diversity of cancer cell lines, including the murine CT-26 cells (IC50 = 1.8 µM). This compound did not elicit hemolytic activity up to 200 µM and displayed a good safety profile after i.v. administration in Balb/c mice at a dose of 1.5 mg/kg of body weight [64, 65].
Iron-based complexes
The first report on the potential anticancer properties of iron complexes was in 1984 by Köpf-Maier and collaborators, demonstrating that ferrocenium picrate and ferrocenium trichloroacetate salts induced oxidative DNA damage in Ehrlich ascites tumor in CF1 mice [66]. Like copper, iron-based complexes seem to induce lower toxicity since iron is naturally present in the human body. A study conducted by Estrada-Montaño and co-workers revealed that an iron(III) complex (Fig. 6) displayed cytotoxic properties towards the human CC cell line, HCT-15, in a higher extent than cisplatin, with IC50 values of 0.2 and 30 µM, respectively [67]. A series of iron(II)-based organometallic compounds has also been synthesized and their antitumor activity was evaluated in HCT-116 cells. The most promising candidate presented an IC50 value of 4.08 μM, similar to 5-FU (3.80 μM). However, these results were below expectations compared to oxaliplatin (0.45 μM) [68].
Example of a chemical structure of an iron-based complex [67]
Zinc-based complexes
Zinc-based complexes are another example of compounds that have demonstrated promising antitumor properties. Nevertheless, only few studies have described their activity on CC. An example is the work of Şen and collaborators, who evaluated the cytotoxic activity of thiosemicarbazone zinc complexes (Fig. 7) in human CC cell lines, DLD-1 and SW620. These compounds exhibited higher cytotoxicity than cisplatin, presenting a 30- and 4-fold reduction on the IC50 values for DLD-1 and SW620, respectively [69].
Example of a chemical structure of a zinc-based complex [69]
Vanadium-based complexes
The potential antitumor activity of vanadium complexes, including their mechanisms of action, have been extensively described [70–76]. In this context, among vanadium-based compounds, Metvan [bis(4,7-dimethyl-1,10-phenanthroline)sulfatooxidovanadium(IV)] was considered a landmark, since it was identified as one of the most promising multitargeted anticancer agent [77], showing high cytotoxic potential towards HT-29 cells, with an IC50 of 0.39 µM [78]. Reytman and collaborators (2018) showed that diaminotris(phenolato) vanadium(V) complexes (Fig. 8) arrested cell cycle in S phase, suggesting a possible interference with DNA synthesis, and induced apoptosis in human CC cells HT-29 [74]. Additionally, dioxovanadium complexes, with substituted salicylaldehyde derivatives, were also evaluated and demonstrated the ability to induce HT-29 cells death, possibly by activation of the Receptor Interacting Protein Kinase 3 [75]. Furthermore, a vanadium complex with N-(2-hydroxyacetophenone) glycinate induced apoptosis in the human CC cells HCT-116 via permeabilization of the mitochondrial outer membrane [76]. In vivo studies revealed its safety following intraperitoneal (i.p.) administration up to 50 mg/kg of body weight [79].
Example of chemical structures of vanadium-based complexes [74]
Ruthenium-based complexes
Among the diversity of metals studied, ruthenium compounds present a wide variety of coordination and organometallic chemistry [80]. Of note, ruthenium compounds present low toxicity and some are highly selective for cancer cells [81]. The anticancer activity of several ruthenium(II)-based organometallic compounds has been investigated. Towards HCT-116 cell line, one of these compounds (Fig. 9a) showed improved cytotoxic activity compared to oxaliplatin, promoting higher levels of caspase-3/7 activity and apoptosis in a dose-dependent manner [68]. Moreover, in in vitro studies with HCT-116 cells, ruthenium(II)-arene complexes (Fig. 9b) demonstrated to act as radiosensitizers when used simultaneously with clinically doses of radiotherapy, leading to cell cycle arrest in G2/M phase and apoptosis. These compounds were equally effective against both oxaliplatin-resistant and cisplatin-resistant cells and the results also indicate that this approach may be effective in p53-mutated tumors [82].
Iridium-based complexes
Iridium-based compounds have attracted interest due to their anticancer activity, versatile photophysical properties, diversity of molecular arrangements, as well as limited side effects. Interestingly, owing to their spectroscopic features, these complexes may also be used as imaging agents [83]. In a recent study, researchers prepared and tested a library of organometallic iridium(III) compounds (Fig. 10). Besides showing a preferential cytotoxic effect against p53-null HCT-116 cell line, a remarkable selectivity towards tumor versus normal cells was attained, opposed to cisplatin, which was also cytotoxic to healthy cells [84]. These data highlight the potential therapeutic advantages of these iridium(III) complexes against p53-mutated CC, as well as reduced unwanted side effects.
Examples of chemical structures of iridium-based complexes [84]
Palladium-based complexes
The coordination chemistry of palladium(II) and platinum(II) compounds has major similarities, prompting the investigation of palladium(II) complexes as antitumor agents. In order to ensure that the structural integrity of these complexes is maintained, an adequate choice of ligand is required to modify reactivity and lipophilicity, and to stabilize oxidation states [81]. In a recent study, a palladium(II) complex derived from 2-acetyl-5-chloro thiophene thiosemicarbazone (Fig. 11a) was more active towards the CRC cell lines SW620 and DLD-1, when compared to a normal colon cell line. Cisplatin, however, displayed higher cytotoxicity against the normal colon cells, leading the authors to suggest the possible utilization of this palladium(II) complex as a more effective and safer chemotherapeutic option for the treatment of aggressive and therapy-resistant cancers [69]. Another interesting work revealed that palladium(II) complexes with 1,7-bis(2-methoxyphenyl)hepta-1,6-diene-3,5-dione (Fig. 11b), containing a curcumin analogue, displayed a high antitumor effect against HT-29 and DLD-1 colon cancer cell lines, targeting stem-like tumor cell populations and reducing cell migration [85].
Drawbacks of metallodrugs
One of the most common arguments against the clinical use of metallodrugs is focused on the toxicity, such as nephrotoxicity, myelotoxicity, ototoxicity, neurotoxicity, nausea and vomiting (reviewed in [40, 86, 87]). Cisplatin, for example, is particularly known for its adverse effects, as aforementioned (reviewed in [48]). For that reason, many cisplatin analogues have been synthesized for the purpose of improving the therapeutic index of the original cisplatin. However, some of them have also demonstrated low selectivity towards tumor cells, severe side effects and development of drug resistances [87, 88]. In addition, most of metal-based complexes present low aqueous solubility, thus requiring the use of toxic solvents for parenteral administration. Furthermore, metallodrugs when orally administered may also present low bioavailability, primarily due to low solubility and low permeability, which are critical factors for crossing biological barriers and, consequently, reaching target tissues at therapeutic concentrations. In addition, the failure of therapeutic regimens including platinum drugs has been associated to the pharmacoresistance [34, 88, 89]. This phenomenon arises from various mechanisms, namely: 1) preventing drug influx into the cells; 2) promoting drug efflux via transport systems present in the cell membrane; 3) enzymatic inactivation of drugs by glutathione and metallothioneins; 4) countering drug activity by mutation or altered expression of the target; and 5) creation of more efficient repairing mechanisms [90, 91].
Therefore, in order to overcome the current limitations, new strategies have been adopted to facilitate the in vivo use of metallodrugs, many of which applying nanotechnological tools as safe solubilizers of selected compounds and specifically delivering them to tumor tissues or organs [86, 92].
Nanomedicines
Nanomedicine is a field that applies the knowledge of nanoscience in disease prevention, diagnosis, and treatment. Nanosized particles, according to their constituents, may display unique structural, chemical, magnetic, electrical, and biological properties that make them suitable tools for drug delivery. In fact, nanocarriers have allowed a preferential targeting of drugs towards the affected sites, reducing side effects by avoiding accumulation at healthy tissues or organs [45, 93–95]. When nanoformulated for oral administration, they have been reported to prevent gastrointestinal drug degradation and enhance bioavailability in comparison to the respective drugs in free form (reviewed in [96–98]). On the other hand, the administration of nanocarriers, with adequate physicochemical properties, has been able to present long blood circulation times, avoiding rapid clearance and enabling a sustained drug release of the incorporated compound [93, 96, 99].
Nanocarriers can deliver therapeutic agents to tumor sites by either passive or active targeting strategies. Passive drug targeting is mostly dependent on the nanocarrier characteristics, such as mean size, surface charge, blood circulation time, and tumor biology [93, 99, 100]. The leaky nature of tumor blood vessels and the poor lymphatic drainage is exploited by nanoparticles, enabling their extravasation and accumulation at tumor tissues, through the well-known enhanced permeation and retention (EPR) effect [101]. Active targeting, on the other hand, focuses on coating the nanocarrier surface with ligands to promote a specific recognition by receptors overexpressed at tumor cell surface [93, 99, 102]. Some examples include erlotinib, folic acid, and transferrin conjugation at nanoparticles surface, since epidermal growth factor receptor (EGFR), folate, and transferrin receptors are overexpressed in several human cancers, namely CC [103–107].
Drug delivery systems include nanoparticles of different natures, such as lipidic, polymeric, inorganic, or metallic-based systems, in which their efficacy is highly dependent on the size, shape, and other inherent biophysical/chemical characteristics [45, 96, 108]. Among lipid-based systems, liposomes represent the most well studied, safe, and accepted delivery system proved by the innumerous formulations already in clinical use [45, 89, 109, 110]. Another important class of delivery systems are the polymeric nanoparticles, in particular those based on poly(lactic-co-glycolic acid) (PLGA) [111–113]. In the next sections, examples of lipidic and polymeric-based nanocarriers will be addressed as tools to improve the therapeutic index of metal-based complexes (Fig. 12).
Lipid-based nanocarriers
Liposomes
As already mentioned, liposomes are, without any doubt, the most well-known and versatile nanodelivery system due to their unique properties. Liposomes are spherical vesicles constituted by one or more concentric lipid bilayers separated by aqueous compartments, with similar structures as biological membranes. The unique structure of liposomes allows the loading of hydrophilic and hydrophobic drugs in the aqueous compartment and lipid bilayer, respectively [109, 114]. Some examples include chemical drugs, proteins, enzymes, or genetic material that can be protected from premature degradation or chemical inactivation prior reaching the target tissues, while simultaneously minimizing the exposure to healthy tissues. Depending on the type of loaded material, a selection of most suitable phospholipids is required [115]. Moreover, the mean size of liposomes can range from few nanometers to several micrometers, and the ideal range for maximizing stability and extravasation to tumor sites is around 80 to 200 nm [114, 116, 117]. Overall, the design of liposomes with different phospholipids, mean size and surface charge will influence the pharmacokinetic and pharmacodynamic properties and, consequently, the therapeutic effect of the incorporated compounds. The longer the circulation time in bloodstream of liposomal formulations, the greater the possibility to extravasate to tumor sites. One strategy to prolong the blood circulation time of liposomes is achieved by including, in the lipid composition, the hydrophilic polymer polyethylene glycol (PEG) [93, 118–120].
Furthermore, in order to increase their target specificity, stimuli-responsive and targeted liposomes have been designed, contributing to improved therapeutic index of the incorporated compounds [115, 121, 122]. For instance, it is widely described that cancer tissues exhibit an acidic microenvironment (pH ≈ 6) compared to normal ones (pH ≈ 7.4) [123]. Therefore, a liposomal system that promotes a local pH-dependent drug release is advantageous. Typically, these liposomes contain specific lipids [e.g.: cholesteryl hemisuccinate (CHEMS) and dioleoyl phosphatidyl ethanolamine (DOPE)] that form stable bilayers at physiological pH; however, under acidic conditions, such as those observed in tumor microenvironment, the lipidic membrane destabilizes and its content is released [64, 123, 124].
Several studies have described promising outcomes of liposomal metal-based complexes (Table 2). Regarding targeted liposomes, an example was achieved by Kamps et al. [125], who developed long circulating oxaliplatin liposomes bearing at their surface a tumor cell-specific antibody, CC52. These immunoliposomes were evaluated in a rat model of colon adenocarcinoma induced with CC-531 cells. Biodistribution studies demonstrated that oxaliplatin immunoliposomes accumulated in liver metastatic tumor nodules in a significantly higher extent than non-targeted liposomes, emphasizing the advantages of using ligands to specifically target the site of action [125]. More recently, Zalba et al. [126] prepared long circulating oxaliplatin-loaded liposomes, with cetuximab as a targeting moiety for EGFR. In in vitro studies using human CC cell lines (HCT-116, SW480, HT-29, and SW620), these immunoliposomes proved to be advantageous in terms of cytotoxicity, when compared to the metallodrug in free form or incorporated in non-targeted liposomes. Interestingly, immunoliposomes were also effective against oxaliplatin-resistant cells (HT-29 and SW480). In a CC xenograft murine model induced with SW480 cells, EGFR-targeted oxaliplatin liposomes promoted a preferential accumulation at tumor sites as proved by the significant tumor growth reduction when compared to the metallodrug in free form or incorporated in non-targeted liposomes [126]. Zhang and collaborators demonstrated that a synergistic effect was achieved by co-loading oxaliplatin and irinotecan in liposomes both in vitro, towards murine (CT-26) and human (HCT-116) CC cells, and in vivo in a syngeneic CT-26 Balb/c mice model, exhibiting antitumor effect devoid of toxic side effects [127]. The research group of Bansal et al. [128] evaluated the anticancer potential of an oral nanoformulation of oxaliplatin incorporated in folic acid-conjugated liposomes. This nanoformulation was further entrapped in alginate beads coated with Eudragit-S-100 to protect it from hostile environment of gastrointestinal tract. In a xenograft CC SCID mice model (HT-29 cells) this oral formulation successfully accumulated at tumor sites revealing its potential for further clinical use, thus improving patient compliance [128].
The above described preclinical examples of liposomal metal-based complexes demonstrate their potential for further translation into real-time clinical applications. Indeed, several liposomal formulations with cytotoxic drugs have already been approved for clinical use. Doxil® was the first FDA approved liposomal formulation for cancer treatment, paving the way for many others to follow (reviewed in [116]). Nevertheless, some metal-based liposomal formulations for the treatment of cancer have already entered in clinical trials. In Table 3, some representative examples are shown.
SLNs
Solid lipid nanoparticles (SLNs) are colloidal particles of submicron size, ranging from 50 to 1000 nm, made of a lipid matrix solid at physiological temperature composed by well-tolerated lipids dispersed in an aqueous surfactant phase [139]. SLNs have been gaining popularity because they are able to carry both hydrophilic and hydrophobic drugs, preventing their degradation in vivo and providing sustained release. Various studies have pointed towards an increase in intracellular delivery of SLN encapsulated drugs, enhanced specificity, and decreased toxicity associated with antineoplastic agents [139, 140].
From a general point of view, SLNs are less toxic and more biocompatible compared to inorganic or polymeric nanoparticles [141, 142]. Taking into account their size and surface properties, SLNs can improve the uptake by cancer cells. They also offer the possibility of sterilization and economically feasible large-scale production, as well as the prospect of attaching a wide range of ligands at the surface. In addition, coating the particles with PEG leads to prolonged blood circulation time due to SLNs avoidance uptake by mononuclear phagocytic system (MPS) [142–144].
The use of SLNs in anticancer therapy can also allow their oral administration, leading to the employment of more convenient therapies over standard parental administration, which would undoubtedly improve patient compliance [89, 140]. Few studies have demonstrated that SLNs loaded with cytotoxic drugs are a capable nanotechnological strategy for the treatment of CC. Oxaliplatin loaded in SLNs with folic acid at their surface were developed to evaluate their cytotoxic properties towards human CC cells HT-29, which are known to overexpress folate receptors [104, 105]. Folic acid-coated oxaliplatin SLNs demonstrated higher cytotoxic activity in comparison to both non-functionalized SLNs and free metallodrug [86]. Another example of increased antitumor effect was demonstrated by Tummala et al. [145] who prepared oxaliplatin-loaded SLNs covalently conjugated with CD-253, a monoclonal antibody against tumor necrosis factor-related apoptosis-inducing ligand (TRAIL), for targeting CC HT-29 cells. These immune-nanoparticles displayed a 1.5-fold higher in vitro cytotoxicity activity than the free oxaliplatin [145].
Others
Considering the clinically available therapeutic regimen FOLFOX, Guo and collaborators developed aminoethyl anisamide (AEAA)-targeted PEGylated lipid nanoparticles loading the active form of oxaliplatin and folinic acid (named Nano-Folox). Interestingly, although 5-FU was not included in the nanoparticles, it was administered separately in free form. This combined therapy proved to be more potent than Nano-Folox alone or FOLFOX, showing higher tumor growth regression and increased mice survival, without systemic toxicity. Furthermore, the anti-PD-L1 monoclonal antibody in combination with Nano-Folox/5-FU displayed a synergistic effect, as demonstrated by the reduction of liver metastases in a preclinical mice model [146].
In another interesting work, Kotelevets and co-workers developed squalenoylated nanoparticles loaded with cisplatin (SQ-CDDP NP) for oral administration. Against a panel of human CC cell lines, SQ-CDDP NP demonstrated a higher cytotoxicity (10-fold), when compared to free cisplatin, inducing reactive oxygen species (ROS) generation, increasing the expression of stress-inducible genes, and promoting stress kinase cascades and apoptosis. In a mice model of CC and in comparison with free cisplatin, SQ-CDDP NP exhibited a greater antitumor activity, inhibiting liver and lymph nodes metastasis spread, with reduced toxicity associated [147].
Polymeric nanoparticles
The encapsulation of drugs in natural or synthetic biodegradable polymeric nanoparticles has also become an attractive approach in the field of nanomedicine for drug delivery [44, 148]. Polymers or copolymers may be used for polymeric nanoparticle preparation and their mean size may range from 10 to 1000 nm. Selected compounds may be loaded in the polymeric matrix, adsorbed or chemically linked to their surface. These systems might also provide good pharmacokinetic profiles, stability, safety, non-inflammatory, and non-immunogenic properties and avoid MPS capture, following an optimization of their characteristics [149].
It is essential that these polymeric nanoparticles present biodegradable properties to ensure their safety for drug delivery and appropriate drug release rates. Depending on the target organ, certain polymers may possess a specific set of properties suitable for that specific target or for different routes of drug administration, such as i.v. and oral [148]. These biodegradable polymers can be natural, such as alginate, hyaluronic acid, dextran, chitosan (polysaccharides), collagen, albumin, elastin, and gelatin (proteins) or they can be synthetic materials obtained from various methods, such as PLGA, poly(lactic acid), PEG, poly-l-glutamic acid or poly(acrylic acid), among others [149]. The usefulness of polymeric nanoparticles as delivery systems for metal-based complexes is stated by relevant examples that have entered in clinical trials for cancer treatment, other than colon. ProLindac, which is a (diaminocyclohexane)platinum(II) (DACHPt) polymer prodrug, is in phase II evaluation for the treatment of ovarian cancer in combination with paclitaxel [150]. Cisplatin loaded in polymeric micelles, combined with gemcitabine, have also entered in a phase I/II clinical trial for the treatment of pancreatic cancer [151].
PLGA, an FDA approved biodegradable copolymer, has attracted attention in the development of drug delivery systems and its advantages in cancer are supported either by in vitro or in vivo studies [44, 152–154]. Additionally, because of its slow-release profile (depending on the properties of the copolymers), PLGA nanoparticles may allow a controlled and sustained drug release from days to weeks at the target sites [155, 156]. The polymeric systems may also be coated with PEG, improving their stability and enhancing blood circulation time [157].
Mattheolabakis et al. [158] evaluated the antitumor activity of cisplatin loaded in PLGA-monomethoxyPEG (mPEG) nanoparticles in an HT-29 SCID mice model. The in vivo results showed that the administration of cisplatin loaded in PLGA-mPEG nanoparticles displayed high therapeutic efficacy, delaying tumor growth and improving survival rates compared to mice treated with cisplatin in the free form [158]. In another study, cholesterol-coated PLGA nanoparticles loaded with oxaliplatin and retinoic acid exhibited in vivo downregulation of cell factors, responsible for inducing metastasis and drug resistance to oxaliplatin [159].
Another work focused on a cisplatin analogue, kiteplatin, a metallodrug active against oxaliplatin-resistant LoVo/LoVo-OXP CC cells [160]. In this study, kiteplatin was loaded in PLGA-PEG nanoparticles and tested in vivo in a C57/BL mice Lewis lung carcinoma model. Results showed that the kiteplatin loaded in polymeric nanoparticles exhibited greater anticancer activity and lower toxicity, compared to mice receiving the free metallodrug [161]. Additionally, the group of de Moraes Profirio described the variables taken into consideration when designing PLGA nanosystems loaded with carboplatin, resulting in an enhanced cellular uptake in comparison to the compound in the free form [162].
In addition to PLGA nanoparticles, some metal-based complexes loaded in other polymers have been developed and their cytotoxic properties evaluated both in vitro and in vivo. For instance, Hackl and co-workers designed a poly(organo)-phosphazene biodegradable carrier loading organoruthenium and rhodium complexes to improve their pharmacodynamic and toxicity profiles. In a CT-26 CC murine model, mice treated with these complexes, loaded in the polymeric system, displayed significantly fewer side effects than free compounds, with similar therapeutic effect [163]. Another research work focused on the development of an oral delivery system to specifically target drugs to the colon. β-Lactoglobulin nanoparticles coated with pectin were loaded with a platinum complex, the bipyridine ethyl dithiocarbamate Pt(II) nitrate. In simulated gastrointestinal conditions, the β-lactoglobulin-pectin nanosystem was stable under acidic conditions, while at pH 7, the complex was released, demonstrating its suitability for colon delivery. Lastly, the in vitro cytotoxic effect of these nanoparticles was shown to be higher towards HCT-116 cells compared to the free complex [164]. Jain et al. [165] developed chitosan nanoparticles loading oxaliplatin, with or without hyaluronic acid at their surface, for oral administration. To protect them from gastrointestinal degradation, all nanoparticles were encapsulated in Eudragit S100–coated pellets. In a HT-29 C57/BL murine model, the targeted nanoformulation displayed higher tumor inhibitory effect in comparison to oxaliplatin in free form or incorporated in non-targeted nanoparticles [165]. Saber and coworkers have described the development of nano-cubosomes co-loading cisplatin and metformin. In vitro, a synergistic effect was obtained, confirmed by the enhanced cytotoxicity towards HCT-116 CC cells. The increased levels of ROS and caspase-3 activity, as well as the inhibition of mammalian target of rapamycin (mTOR), caused both by AMPK activation and p-Akt suppression, were attributed by the authors for the enhanced cytotoxic effect observed for this combined therapeutic strategy [166].
Polymeric micelles loading platinum-based complexes have also been explored. In this context, cisplatin was successfully incorporated in poly(ethylene glycol)-poly(glutamic acid) copolymers and tested both in vitro and in vivo. Curiously, against CT-26 cells, free drug presented a lower IC50 than nanoformulated form (0.56 and 7.5 µg/mL, respectively). Nevertheless, in a CT-26- bearing Balb/c mice model, although both nanoformulated and free forms of cisplatin displayed significant antitumor activity, the complete tumor regression was only achieved for mice treated with cisplatin-loaded polymeric micelles [167]. In another study, a platinum-based complex, DACHPt, was also incorporated into the same polymeric system. This nanoformulation demonstrated to be effective towards orthotopic and metastatic tumor models. Importantly, this polymeric micelle nanoformulation displayed higher antitumor activity and lower toxicity than the clinically approved oxaliplatin [168].
Conclusions and future perspectives
Some metals, such as copper, iron, and zinc, are naturally present in the human body and are involved in multiple biological processes, namely redox chemistry, enzyme function, and oxygen transport. Due to their unique features, the use of metals and metal-based complexes for medical applications has been an ancient practice for thousands of years. The understanding of metals properties and their interaction with biological systems has greatly improved in the last century, providing countless opportunities to develop novel therapies. In this sense, the discovery and clinical approval of cisplatin represented an unparalleled landmark in cancer treatment. Indeed, this metal-based complex remains an important part of standard chemotherapeutic regimens towards different types of cancer. The clinical success of this metallodrug was followed by endeavors to develop novel platinum analogues with less side effects, resulting in the approval of carboplatin and oxaliplatin. Oxaliplatin, specifically, is widely used for CC management, a severe and prevalent malignant disease with alarming mortality rates.
In addition to the aforementioned examples, some metal-based structures display high versatility for other biomedical applications. For example, supramolecular coordination complexes (SCCs) are being investigated not only as cytotoxic anticancer agents [169], but also as drug delivery systems. Interestingly, 3D-assembled SCCs, including metallacages, demonstrated high potential for the delivery of cisplatin [170, 171]. Another area of research deals with metallo-prodrugs that, when activated, may favor their selectivity towards CC cells [172]. For instance, a recent work explored an organoruthenium prodrug for targeting plectin, a cytoskeleton protein [172]. These examples and the ones described in previous sections show that metal complexation provides a myriad of biomedical applications. There is a growing awareness for understanding the interaction of metal-based complexes within biological systems, vital for a successful clinical translation [173, 174].
Despite the evidenced therapeutic potential of metal-based complexes, constant efforts to further improve their therapeutic index are being carried out. In this context, new delivery strategies using nanoscale particles have emerged as promising tools to enhance the pharmacodynamic, pharmacokinetic, and safety profiles of these new compounds. For instance, nanocarriers can modify their biodistribution profile and ensure that metal-based complexes are protected from the surrounding medium, avoiding speciation and off-target effects due to uncontrolled reactivity [175]. Particularly, lipidic and polymeric nanoformulations have led to significant breakthroughs in targeting chemotherapeutic agents to cancer tissues, with recent preclinical and clinical studies indicating that nanomedicine plays a crucial role in the future treatment of CC. Lipid- and polymer-based nanosystems possess distinct properties, turning them more suitable, for i.v. and oral administration, respectively. Overall, both nanotechnological approaches aim to improve the therapeutic index of associated metal-based complexes.
It is worthy to note that malignant pathologies like CC are multifactorial diseases, which often require combinatorial approaches to achieve satisfactory therapeutic outcomes. In this sense, the co-administration of drugs loaded into a single nanosystem constitutes an advantageous strategy to improve the efficacy of the active principles, minimize toxicity, and overcome drug resistance emergence. In this review, some examples of synergism between a metal-based complex and other non-metallic compounds were addressed, emphasizing the potential of this approach for cancer treatment. It is evident that the vast majority of examples depicted in this review are intended for i.v. administration. Nevertheless, studies demonstrating the effectiveness of oral delivery for CC were also highlighted, as this administration route would positively impact patient compliance.
The development and optimization of nanomedicines continues to be a hot topic, attracting attention from both academia and industry. The high number of marketed nanoformulated drugs, with focus on liposomes, proves the success of nanocarriers implementation for improved clinical outcomes [176]. Despite representing promising therapeutic options, the translation of nanomedicines from the bench to the market is hampered by few aspects. In terms of regulatory agencies, for example, a case-by-case approach is often used for evaluating novel nanoproducts. The complexity of nanotechnology field requires specific regulatory frames that must be carefully revised and updated. Also, as newly nanoproducts are developed, different risks to environment and adverse events to humans may be found, raising novel questions regarding adequacy of existing safety and efficacy requirements. Accordingly, the nanosystems addressed in this review, like other nanoparticles, must be subjected to a rigorous understanding and standardization of the manufacture process, including validated quality control procedures.
Overall, the development of new nanoformulations of metal-based complexes is promising for CC treatment and could be expanded to other pathologies. Combining the versatility and intrinsic antitumor activity of these compounds with nanotechnological tools provides, undoubtedly, an advantageous opportunity for advancing diseases management. An exhaustive therapeutic validation of these potential nanomedicines is, of course, mandatory for successfully entering clinical trials and, further reaching the market.
Data availability
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Nabi K, Le A. The intratumoral heterogeneity of cancer metabolism. Adv Exp Med Biol. 2018;1063:131–45.
Mattiuzzi C, Lippi G. Current Cancer Epidemiology glossary. J Epidemiol Glob Health. 2019;9:217–22.
Li F, Lai M. Colorectal cancer, one entity or three. J Zhejiang Univ Sci B. 2009;10:219–29.
Cappell MS. Pathophysiology, clinical presentation, and management of colon cancer. Gastroenterol Clin North Am. 2008;37:1–24.
Mattiuzzi C, Sanchis-Gomar F, Lippi G. Concise update on colorectal cancer epidemiology. Ann Transl Med. 2019;7:609.
Ahmed M. Colon cancer: a clinician’s perspective in 2019. Gastroenterol Res. 2020;13:1–10.
Ilyas M, Straub J, Tomlinson IPM, Bodmer WF. Genetic pathways in colorectal and other cancers. Eur J Cancer. 1999;35:1986–2002.
Balchen V, Simon K. Colorectal cancer development and advances in screening. Clin Interv Aging. 2016;11:967–76.
Virk GS, Jafri M, Mehdi S, Ashley C. Staging and survival of colorectal cancer (CRC) in octogenarians: Nationwide Study of US Veterans. J Gastrointest Oncol. 2018;10:12–8.
Johns Hopkins Colon Cancer Center. Sporadic (Nonhereditary) Colorectal Cancer: Introduction [Internet]. 2013 [cited 2020 Aug 4]. Available from: https://www.hopkinsmedicine.org/gastroenterology_hepatology/_pdfs/small_large_intestine/sporadic_nonhereditary_colorectal_cancer.pdf.
American Cancer Society. Survival Rates for Colorectal Cancer [Internet]. 2020 [cited 2020 Aug 26]. Available from: https://www.cancer.org/cancer/colon-rectal-cancer/detection-diagnosis-staging/survival-rates.html.
Pan P, Yu J, Wang LS. Colon cancer: what we eat. Surg Oncol Clin N Am. 2018;27:243–67.
Testino G, Leone S, Sumberaz A, Borro P. Alcohol and cancer. Alcohol Clin Exp Res. 2015;39:2261–2261.
Parajuli R, Bjerkaas E, Tverdal A, Selmer R, Le ML, Weiderpass E, et al. The increased risk of colon cancer due to cigarette smoking may be greater in women than men. Cancer Epidemiol Biomarkers Prev. 2013;22:862–71.
Baena R, Salinas P. Diet and colorectal cancer. Maturitas. 2015;80:258–64.
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424.
GLOBOCAN [Internet]. 2020 [cited 2020 Aug 31]. Available from: https://gco.iarc.fr/.
Stoffel EM, Murphy CC. Epidemiology and mechanisms of the increasing incidence of colon and rectal cancers in young adults. Gastroenterology. 2020;158:341–53.
Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJY, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut. 2015;64:1637–49.
Burt RW. Colon cancer screening continues as pivotal to cancer prevention. J Natl Compr Cancer Netw. 2013;11:1457–8.
Vogel JD, Eskicioglu C, Weiser MR, Feingold DL, Steele SR. The American Society of Colon and Rectal Surgeons clinical practice guidelines for the treatment of colon cancer. Dis Colon Rectum. 2017;60:999–1017.
Labianca R, Beretta GD, Kildani B, Milesi L, Merlin F, Mosconi S, et al. Colon cancer. Crit Rev Oncol Hematol. 2010;74:106–33.
Hagan S, Orr MCM, Doyle B. Targeted therapies in colorectal cancer—an integrative view by PPPM. EPMA J. 2013;4:3.
Gulbake A, Jain A, Jain A, Jain A, Jain SK. Insight to drug delivery aspects for colorectal cancer. World J Gastroenterol. 2016;22:582–99.
National Cancer Institute. Colon Cancer Treatment (PDQ®): Health Professional Version. [Internet]. Bethesda (MD); 2020 [cited 2020 Aug 10]. Available from: https://www.cancer.gov/types/colorectal/hp/colon-treatment-pdq.
Hodgkinson N, Kruger CA, Abrahamse H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells. Tumor Biol. 2017;39:1010428317734691.
Willett CG, Duda DG, Czito BG, Bendell JC, Clark JW, Jain RK. Targeted therapy in rectal cancer. Oncology. 2007;21:1055–65.
Ashburn JH, Kalady MF. Radiation-induced problems in colorectal surgery. Clin Colon Rectal Surg. 2016;29:85–91.
Bender U, Rho YS, Barrera I, Aghajanyan S, Acoba J, Kavan P. Adjuvant therapy for stages II and III colon cancer: risk stratification, treatment duration, and future directions. Curr Oncol. 2019;26:S43-52.
Gelibter AJ, Caponnetto S, Urbano F, Emiliani A, Scagnoli S, Sirgiovanni G, et al. Adjuvant chemotherapy in resected colon cancer: When, how and how long? Surg Oncol Elsevier. 2019;30:100–7.
Chan GHJ, Chee CE. Making sense of adjuvant chemotherapy in colorectal cancer. J Gastrointest Oncol. 2019;10:1183–92.
McKeown E, Nelson DW, Johnson EK, Maykel JA, Stojadinovic A, Nissan A, et al. Current approaches and challenges for monitoring treatment response in colon and rectal cancer. J Cancer. 2014;5:31–43.
Kotelevets L, Chastre E, Desmaële D, Couvreur P. Nanotechnologies for the treatment of colon cancer: from old drugs to new hope. Int J Pharm. 2016;514:24–40.
Van Der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24:3834–48.
Ott I, Gust R. Non platinum metal complexes as anti-cancer drugs. Arch Pharm (Weinheim). 2007;340:117–26.
Martín J, Alés MR, Asuero GA. An overview on ligands of therapeutically interest. Pharm Pharmacol Int J. 2018;6:198–214.
Brenner H, Kloor M, Pox CP. Colorectal cancer Lancet England. 2014;383:1490–502.
National Cancer Institute. Drugs approved for colon and rectal cancer [Internet]. 2019 [cited 2020 Aug 2]. Available from: https://www.cancer.gov/about-cancer/treatment/drugs/colorectal.
Wu C. Systemic Therapy for colon cancer. Surg Oncol Clin N Am. Elsevier Inc; 2018;27:235–42.
Culy CR, Clemett D, Wiseman LR. Oxaliplatin drugs. 2000;60:895–924.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol. 2013;14:1208–15.
Nordlinger B, Sorbye H, Glimelius B, Poston GJ, Schlag PM, Rougier P, et al. Perioperative chemotherapy with FOLFOX4 and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC Intergroup trial 40983): a randomised controlled trial. Lancet. 2008;371:1007–16.
Cremolini C, Loupakis F, Antoniotti C, Lupi C, Sensi E, Lonardi S, et al. FOLFOXIRI plus bevacizumab versus FOLFIRI plus bevacizumab as first-line treatment of patients with metastatic colorectal cancer: updated overall survival and molecular subgroup analyses of the open-label, phase 3 TRIBE study. Lancet Oncol England. 2015;16:1306–15.
Mir M, Ahmed N, Rehman AU. Recent applications of PLGA based nanostructures in drug delivery. Colloids Surf B Biointerfaces. 2017;159:217–31.
Pinho JO, Matias M, Gaspar MM. Emergent nanotechnological strategies for systemic chemotherapy against melanoma. Nanomaterials. 2019;9:1455.
de Almeida A, Oliveira BL, Correia JDG, Soveral G, Casini A. Emerging protein targets for metal-based pharmaceutical agents: an update. Coord Chem Rev. 2013;257:2689–704.
Sullivan MP, Holtkamp HU, Hartinger CG. Antitumor Metallodrugs that Target Proteins. In: Sigel A, Sigel H, Freisinger E, Sigel RKO, editors. Met Dev Action Anticancer Agents. Berlin: De Gruyter; 2018. p. 351–86.
Ghosh S. Cisplatin: the first metal based anticancer drug. Bioorg Chem. 2019;88:102925.
Wong E, Giandornenico CM. Current status of platinum-based antitumor drugs. Chem Rev. 1999;99:2451–66.
Ho YP, Au-Yeung SCF, To KKW. Platinum-based anticancer agents: Innovative design strategies and biological perspectives. Med Res Rev. 2003;23:633–55.
Frezza M, Hindo S, Chen D, Davenport A, Schmitt S, Tomco D, et al. Novel metals and metal complexes as platforms for cancer therapy. Curr Pharm Des. 2010;16:1813–25.
Ndagi U, Mhlongo N, Soliman M. Metal complexes in cancer therapy - an update from drug design perspective. Drug Des Devel Ther. 2017;11:599–616.
Casini A, Sun RWY, Ott I. Medicinal chemistry of gold anticancer metallodrugs. In: Sigel A, Sigel H, Freisinger E, Sigel R, editors. Met Dev Action Anticancer Agents. Berlin, Boston: De Gruyter; 2018. p. 199–217.
Scheffler H, You Y, Ott I. Comparative studies on the cytotoxicity, cellular and nuclear uptake of a series of chloro gold(I) phosphine complexes. Polyhedron. 2010;29:66–9.
Tong KC, Lok CN, Wan PK, Hu D, Fung YME, Chang XY, et al. An anticancer gold(III)-activated porphyrin scaffold that covalently modifies protein cysteine thiols. Proc Natl Acad Sci. 2020;117:1321–9.
Miranda S, Vergara E, Mohr F, de Vos D, Cerrada E, Mendía A, et al. Synthesis, characterization, and in vitro cytotoxicity of some gold(I) and trans platinum(II) thionate complexes containing water-soluble PTA and DAPTA ligands. X-ray crystal structures of [Au(SC4H3N2)(PTA)], trans-[Pt(SC4H3N2)2(PTA)2], trans-[Pt(SC5H4N)2. Inorg Chem Am Chem Soc. 2008;47:5641–8.
Gandin V, Fernandes AP, Rigobello MP, Dani B, Sorrentino F, Tisato F, et al. Cancer cell death induced by phosphine gold(I) compounds targeting thioredoxin reductase. Biochem Pharmacol. 2010;79:90–101.
Santini C, Pellei M, Gandin V, Porchia M, Tisato F, Marzano C. Advances in copper complexes as anticancer agents. Chem Rev. 2014;114:815–62.
Montagner D, Fresch B, Browne K, Gandin V, Erxleben A. A Cu(ii) complex targeting the translocator protein: in vitro and in vivo antitumor potential and mechanistic insights. Chem Commun. 2017;53:134–7.
Leite SMG, Lima LMP, Gama S, Mendes F, Orio M, Bento I, et al. Copper(II) complexes of phenanthroline and histidine containing ligands: synthesis, characterization and evaluation of their DNA cleavage and cytotoxic activity. Inorg Chem. 2016;55:11801–14.
Nunes CJ, Otake AH, Bustos SO, Fazzi RB, Chammas R, Da Costa Ferreira AM. Unlike reactivity of mono- and binuclear imine-copper(II) complexes toward melanoma cells via a tyrosinase-dependent mechanism. Chem Biol Interact. 2019;311:108789.
Bacher F, Wittmann C, Nové M, Spengler G, Marć MA, Enyedy EA, et al. Novel latonduine derived proligands and their copper(II) complexes show cytotoxicity in the nanomolar range in human colon adenocarcinoma cells and: in vitro cancer selectivity. Dalt Trans. 2019;48:10464–78.
Gandin V, Pellei M, Tisato F, Porchia M, Santini C, Marzano C. A novel copper complex induces paraptosis in colon cancer cellsviathe activation of ER stress signalling. J Cell Mol Med. 2012;16:142–51.
Pinho JO, Amaral JD, Castro RE, Rodrigues CMP, Casini A, Soveral G, et al. Copper complex nanoformulations featuring highly promising therapeutic potential in murine melanoma models. Nanomedicine. 2019;14:835–50.
Nave M, Castro RE, Rodrigues CMP, Casini A, Soveral G, Gaspar MM. Nanoformulations of a potent copper-based aquaporin inhibitor with cytotoxic effect against cancer cells. Nanomedicine. 2016;11:1817–30.
Köpf-Maier P, Köpf H, Neuse EW. Ferricenium complexes: a new type of water-soluble antitumor agent. J Cancer Res Clin Oncol. 1984;108:336–40.
Estrada-Montaño AS, Ryabov AD, Gries A, Gaiddon C, Le Lagadec R. Iron(III) pincer complexes as a strategy for anticancer studies. Eur J Inorg Chem. 2017;2017:1673–8.
Florindo PR, Pereira DM, Borralho PM, Rodrigues CMP, Piedade MFM, Fernandes AC. Cyclopentadienyl–ruthenium(II) and iron(II) organometallic compounds with carbohydrate derivative ligands as good colorectal anticancer agents. J Med Chem. 2015;58:4339–47.
Şen B, Kalhan HK, Demir V, Güler EE, Kayalı HA, Subaşı E. Crystal structures, spectroscopic properties of new cobalt(II), nickel(II), zinc(II) and palladium(II) complexes derived from 2-acetyl-5-chloro thiophene thiosemicarbazone: anticancer evaluation. Mater Sci Eng C. 2019;98:550–9.
Pessoa JC, Etcheverry S, Gambino D. Vanadium compounds in medicine. Coord Chem Rev. 2015;301–302:24–48.
Kioseoglou E, Petanidis S, Gabriel C, Salifoglou A. The chemistry and biology of vanadium compounds in cancer therapeutics. Coord Chem Rev. 2015;301–302:87–105.
León IE, Cadavid-Vargas JF, Tiscornia I, Porro V, Castelli S, Katkar P, et al. Oxidovanadium(IV) complexes with chrysin and silibinin: anticancer activity and mechanisms of action in a human colon adenocarcinoma model. J Biol Inorg Chem. 2015;20:1175–91.
Kowalski S, Wyrzykowski D, Inkielewicz-Stępniak I. Molecular and cellular mechanisms of cytotoxic activity of vanadium compounds against cancer cells. Molecules. 2020;25:1757.
Reytman L, Hochman J, Tshuva EY. Anticancer diaminotris(phenolato) vanadium(V) complexes: ligand-metal interplay. J Coord Chem. 2018;71:2003–11.
Rana SR, McCaffrey V, Rabquer BJ. Vanadium complex induced cancer cell death via RIPK3 activated necroptosis. FASEB J. 2018;31:876.6.
Sinha A, Banerjee K, Banerjee A, Sarkar A, Ahir M, Adhikary A, et al. Induction of apoptosis in human colorectal cancer cell line, HCT-116 by a vanadium- Schiff base complex. Biomed Pharmacother. 2017;92:509–18.
Sanna D, Ugone V, Micera G, Buglyó P, Bíró L, Garribba E. Speciation in human blood of Metvan, a vanadium based potential anti-tumor drug. Dalt Trans. 2017;46:8950–67.
León IE, Ruiz MC, Franca CA, Parajón-Costa BS, Baran EJ. Metvan, bis(4,7-Dimethyl-1,10-phenanthroline)sulfatooxidovanadium(IV): DFT and spectroscopic study—antitumor action on human bone and colorectal cancer cell lines. Biol Trace Elem Res. 2019;191:81–7.
Sinha A, Banerjee K, Banerjee A, Das S, Choudhuri SK. Synthesis, characterization and biological evaluation of a novel vanadium complex as a possible anticancer agent. J Organomet Chem. 2014;772–773:34–41.
Bratsos I, Gianferrara T, Alessio E, Hartinger CG, Jakupec MA, Keppler BK. Ruthenium and other non-platinum anticancer compounds. In: Alessio E, editor. Bioinorg Med Chem. Weinheim, Germany: Wiley-VCH Verlag GmbH & Co. KGaA; 2011. p. 151–74.
Lazarević T, Rilak A, Bugarčić ŽD. Platinum, palladium, gold and ruthenium complexes as anticancer agents: current clinical uses, cytotoxicity studies and future perspectives. Eur J Med Chem. 2017;142:8–31.
Carter R, Westhorpe A, Romero M, Habtemariam A, Gallevo C, Bark Y, et al. Radiosensitisation of human colorectal cancer cells by ruthenium(II) arene anticancer complexes. Sci Rep. 2016;6:20596.
Ma DL, Wu C, Wu KJ, Leung CH. Iridium(III) complexes targeting apoptotic cell death in cancer cells. Molecules. 2019;24:2739.
Lord RM, Zegke M, Henderson IR, Pask CM, Shepherd HJ, McGowan PC. β-Ketoiminato iridium(III) organometallic complexes: selective cytotoxicity towards colorectal cancer cells HCT116 p53 -/-. Chem - A Eur J. 2019;25:495–500.
Fischer-Fodor E, Mikláš R, Rišiaňová L, Cenariu M, Grosu IG, Virag P, et al. Novel palladium(II) complexes that influence prominin-1/CD133 expression and stem cell factor release in tumor cells. Molecules. 2017;22:561.
Rajpoot K, Jain SK. Colorectal cancer-targeted delivery of oxaliplatin via folic acid-grafted solid lipid nanoparticles: preparation, optimization, and in vitro evaluation. Artif Cells, Nanomedicine, Biotechnol. 2018;46:1236–47.
Mjos KD, Orvig C. Metallodrugs in medicinal inorganic chemistry. Chem Rev. 2014;114:4540–63.
Stewart DJ. Mechanisms of resistance to cisplatin and carboplatin. Crit Rev Oncol Hematol. 2007;63:12–31.
Bayón-Cordero L, Alkorta I, Arana L. Application of solid lipid nanoparticles to improve the efficiency of anticancer drugs. Nanomaterials. 2019;9:474.
Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol. 2019;53:148–58.
Komarova NL, Wodarz D. Drug resistance in cancer: principles of emergence and prevention. Proc Natl Acad Sci. 2005;102:9714–9.
Sguizzato M, Cortesi R, Gallerani E, Drechsler M, Marvelli L, Mariani P, et al. Solid lipid nanoparticles for the delivery of 1,3,5-triaza-7-phosphaadamantane (PTA) platinum (II) carboxylates. Mater Sci Eng C. 2017;74:357–64.
Belfiore L, Saunders DN, Ranson M, Thurecht KJ, Storm G, Vine KL. Towards clinical translation of ligand-functionalized liposomes in targeted cancer therapy: challenges and opportunities. J Control Release. 2018;277:1–13.
Sanna V, Pala N, Sechi M. Targeted therapy using nanotechnology: focus on cancer. Int J Nanomedicine. 2014;9:467–83.
Silva CO, Pinho JO, Lopes JM, Almeida AJ, Gaspar MM, Reis C. Current trends in cancer nanotheranostics: metallic, polymeric, and lipid-based systems. Pharmaceutics. 2019;11:22.
Patra JK, Das G, Fraceto LF, Campos EVR, Rodriguez-Torres MDP, Acosta-Torres LS, et al. Nano based drug delivery systems: recent developments and future prospects. J Nanobiotechnology. 2018;16:71.
Griffin BT, Guo J, Presas E, Donovan MD, Alonso MJ, O’Driscoll CM. Pharmacokinetic, pharmacodynamic and biodistribution following oral administration of nanocarriers containing peptide and protein drugs. Adv Drug Deliv Rev. 2016;106:367–80.
Durán-Lobato M, Niu Z, Alonso MJ. Oral delivery of biologics for precision medicine. Adv Mater. 2020;32:1901935.
Attia MF, Anton N, Wallyn J, Omran Z, Vandamme TF. An overview of active and passive targeting strategies to improve the nanocarriers efficiency to tumour sites. J Pharm Pharmacol. 2019;71:1185–98.
Mishra B, Chaurasia S. Design of novel chemotherapeutic delivery systems for colon cancer therapy based on oral polymeric nanoparticles. Ther Deliv. 2017;8:29–47.
Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65:271–84.
Banerjee A, Pathak S, Subramanium VD, Dharanivasan G, Murugesan R, Verma RS. Strategies for targeted drug delivery in treatment of colon cancer: current trends and future perspectives. Drug Discov Today. 2017;22:1224–32.
Gaspar MM, Radomska A, Gobbo OL, Bakowsky U, Radomski MW, Ehrhardt C. Targeted delivery of transferrin-conjugated liposomes to an orthotopic model of lung cancer in nude rats. J Aerosol Med Pulm Drug Deliv. 2012;25:310–8.
Song H, Su C, Cui W, Zhu B, Liu L, Chen Z, et al. Folic acid-chitosan conjugated nanoparticles for improving tumor-targeted drug delivery. Biomed Res Int. 2013;2013:1–6.
Sharma M, Malik R, Verma A, Dwivedi P, Banoth GS, Pandey N, et al. Folic acid conjugated guar gum nanoparticles for targeting methotrexate to colon cancer. J Biomed Nanotechnol. 2013;9:96–106.
Santos-Rebelo A, Kumar P, Pillay V, Choonara YE, Eleutério C, Figueira M, et al. Development and mechanistic insight into the enhanced cytotoxic potential of parvifloron D albumin nanoparticles in EGFR-overexpressing pancreatic cancer cells. Cancers (Basel). 2019;11:1733.
Elechalawar CK, Sridharan K, Pal A, Ahmed MT, Yousuf M, Adhikari SS, et al. Cationic folate-mediated liposomal delivery of bis-arylidene oxindole induces efficient melanoma tumor regression. Biomater Sci. 2017;5:1898–909.
Fernandez-Fernandez A, Manchanda R, McGoron AJ. Theranostic applications of nanomaterials in cancer: drug delivery, image-guided therapy, and multifunctional platforms. Appl Biochem Biotechnol. 2011;165:1628–51.
Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9:12.
Lamichhane N, Udayakumar T, D’Souza W, Simone C II, Raghavan S, Polf J, et al. Liposomes: clinical applications and potential for image-guided drug delivery. Molecules. 2018;23:288.
Kennedy PJ, Sousa F, Ferreira D, Pereira C, Nestor M, Oliveira C, et al. Fab-conjugated PLGA nanoparticles effectively target cancer cells expressing human CD44v6. Acta Biomater. 2018;81:208–18.
Csaba N, Caamaño P, Sánchez A, Domínguez F, Alonso MJ. PLGA: poloxamer and PLGA: poloxamine blend nanoparticles: new carriers for gene delivery. Biomacromol. 2005;6:271–8.
Cadete A, Alonso MJ. Targeting cancer with hyaluronic acid-based nanocarriers: recent advances and translational perspectives. Nanomedicine. 2016;11:2341–57.
Cruz MEM, Simões SI, Corvo ML, Martins MBF, Gaspar MM. Formulation of NPDDS for macromolecules. In: Pathak Y, Thassu D, editors. Drug Deliv Nanoparticles Formul Charact. New York, USA: Informa Healthcare; 2009. p. 35–49.
Yang C, Merlin D. Lipid-based drug delivery nanoplatforms for colorectal cancer therapy. Nanomaterials. 2020;10:1424.
Bozzuto G, Molinari A. Liposomes as nanomedical devices. Int J Nanomedicine. 2015;10:975–99.
Perrie Y, Ramsay E. Nanomedicines: exploring the past, present and future. Drug Discov World. 2017;18:17–22.
Allen TM, Cullis PR. Drug delivery systems: entering the mainstream. Science. 2004;303:1818–22.
Gabizon AA. Stealth liposomes and tumor targeting: one step further in the quest for the magic bullet. Clin Cancer Res. 2001;7:223–5.
Allen TM, Cullis PR. Liposomal drug delivery systems: from concept to clinical applications. Adv Drug Deliv Rev. 2013;65:36–48.
Deshpande S, Sharma S, Koul V, Singh N. Core-shell nanoparticles as an efficient, sustained, and triggered drug-delivery system. ACS Omega. 2017;2:6455–63.
Lila ASA, Ishida T. Liposomal delivery systems: design optimization and current applications. Biol Pharm Bull. 2017;40:1–10.
Simões S, Nuno Moreira J, Fonseca C, Düzgüneş Pedroso N, De Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56:947–65.
Fan Y, Chen C, Huang Y, Zhang F, Lin G. Study of the pH-sensitive mechanism of tumor-targeting liposomes. Colloids Surfaces B Biointerfaces. 2017;151:19–25.
Kamps J, Koning G, Velinova M, Morselt H, Wilkens M, Gorter A, et al. Uptake of long-circulating immunoliposomes, directed against colon adenocarcinoma cells, by liver metastases of colon cancer. J Drug Target. 2000;8:235–45.
Zalba S, Contreras AM, Haeri A, ten Hagen TLM, Navarro I, Koning G, et al. Cetuximab-oxaliplatin-liposomes for epidermal growth factor receptor targeted chemotherapy of colorectal cancer. J Control Release. 2015;210:26–38.
Zhang B, Wang T, Yang S, Xiao Y, Song Y, Zhang N, et al. Development and evaluation of oxaliplatin and irinotecan co-loaded liposomes for enhanced colorectal cancer therapy. J Control Release. 2016;238:10–21.
Bansal D, Gulbake A, Tiwari J, Jain SK. Development of liposomes entrapped in alginate beads for the treatment of colorectal cancer. Int J Biol Macromol. 2016;82:687–95.
Stathopoulos GP, Boulikas T. Lipoplatin formulation review article. J Drug Deliv. 2012;2012:1–10.
Jehn CF, Boulikas T, Kourvetaris A, Possinger K, Lüftner D. Pharmacokinetics of liposomal cisplatin (lipoplatin) in combination with 5-FU in patients with advanced head and neck cancer: first results of a phase III study. Anticancer Res. 2007;27:471–5.
Kosmas C, Angel J, Athanasiou A, Rapti A, Karanikas C, Lambaki S, et al. 9088 Phase III study of lipoplatin plus gemcitabine versus cisplatin plus gemcitabine in advanced NSCLC; interim analysis. Eur J Cancer Suppl. 2009;7:531.
Dragovich T, Mendelson D, Kurtin S, Richardson K, Von Hoff D, Hoos A. A phase 2 trial of the liposomal DACH platinum L-NDDP in patients with therapy-refractory advanced colorectal cancer. Cancer Chemother Pharmacol. 2006;58:759–64.
Hang Z, Cooper MA, Ziora ZM. Platinum-based anticancer drugs encapsulated liposome and polymeric micelle formulation in clinical trials. Biochem Compd. 2016;4:1.
Seetharamu N, Kim E, Hochster H, Martin F, Muggia F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010;30:541–6.
Kim ES, Lu C, Khuri FR, Tonda M, Glisson BS, Liu D, et al. A phase II study of STEALTH cisplatin (SPI-77) in patients with advanced non-small cell lung cancer. Lung Cancer. 2001;34:427–32.
de Jonge MJA, Slingerland M, Loos WJ, Wiemer EAC, Burger H, Mathijssen RHJ, et al. Early cessation of the clinical development of LiPlaCis, a liposomal cisplatin formulation. Eur J Cancer. 2010;46:3016–21.
U.S. National Health Institute. Phase I/II Study to evaluate the safety and tolerability of LiPlaCis in patients with advanced or refractory tumours (LiPlaCis) [Internet]. 2019 [cited 2020 Sep 10]. Available from: https://clinicaltrials.gov/ct2/show/NCT01861496.
Stathopoulos GP, Boulikas T, Kourvetaris A, Stathopoulos J. Liposomal oxaliplatin in the treatment of advanced cancer: a phase I study. Anticancer Res. 2006;26:1489–93.
Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: composition, properties and therapeutic strategies. Mater Sci Eng C. 2016;68:982–94.
Mei L, Zhang Z, Zhao L, Huang L, Yang XL, Tang J, et al. Pharmaceutical nanotechnology for oral delivery of anticancer drugs. Adv Drug Deliv Rev. 2013;65:880–90.
Muller RH, Shegokar R, Keck CM. 20 years of lipid nanoparticles (SLN & NLC): present state of development & industrial applications. Curr Drug Discov Technol. 2011;8:207–27.
Gaspar DP, Gaspar MM, Eleutério CV, Grenha A, Blanco M, Gonçalves LMD, et al. Microencapsulated solid lipid nanoparticles as a hybrid platform for pulmonary antibiotic delivery. Mol Pharm. 2017;14:2977–90.
Madan J, Pandey RS, Jain V, Katare OP, Chandra R, Katyal A. Poly (ethylene)-glycol conjugated solid lipid nanoparticles of noscapine improve biological half-life, brain delivery and efficacy in glioblastoma cells. Nanomed Nanotechnol, Biol Med. 2013;9:492–503.
Wissing SA, Kayser O, Müller RH. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–72.
Tummala S, Gowthamarajan K, Satish Kumar M, Praveen T, Yamjala K, Tripuraneni NS, et al. Formulation and optimization of oxaliplatin immuno-nanoparticles using Box-Behnken design and cytotoxicity assessment for synergistic and receptor-mediated targeting in the treatment of colorectal cancer. Artif Cells, Nanomedicine, Biotechnol. 2016;44:1835–50.
Guo J, Yu Z, Das M, Huang L. Nano codelivery of oxaliplatin and folinic acid achieves synergistic chemo-immunotherapy with 5-fluorouracil for colorectal cancer and liver metastasis. ACS Nano. 2020;14:5075–89.
Kotelevets L, Chastre E, Caron J, Mougin J, Bastian G, Pineau A, et al. A squalene-based nanomedicine for oral treatment of colon cancer. Cancer Res. 2017;77:2964–75.
George A, Shah PA, Shrivastav PS. Natural biodegradable polymers based nano-formulations for drug delivery: a review. Int J Pharm. 2019;561:244–64.
Ahlawat J, Henriquez G, Narayan M. Enhancing the delivery of chemotherapeutics: role of biodegradable polymeric nanoparticles. Molecules. 2018;23:1–20.
Nowotnik DP. AP5346 (ProLindacTM), A DACH platinum polymer conjugate in phase II trials against ovarian cancer. Curr Bioact Compd. 2011;7:21–6.
Mochida Y, Cabral H, Kataoka K. Polymeric micelles for targeted tumor therapy of platinum anticancer drugs. Expert Opin Drug Deliv. 2017;14:1423–38.
Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: an overview of biomedical applications. J Control Release. 2012;161:505–22.
Acharya S, Sahoo SK. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv Drug Deliv Rev. 2011;63:170–83.
Mirakabad FST, Nejati-Koshki K, Akbarzadeh A, Yamchi MR, Milani M, Zarghami N, et al. PLGA-based nanoparticles as cancer drug delivery systems. Asian Pacific J Cancer Prev. 2014;15:517–35.
Matoba T, Egashira K. Nanoparticle-mediated drug delivery system for cardiovascular disease. Int Heart J. 2014;55:281–6.
Chaudhary Z, Ahmed N, Rehman AU, Khan GM. Lipid polymer hybrid carrier systems for cancer targeting: A review. Int J Polym Mater Polym Biomater. 2018;67:86–100.
Sousa AR, Oliveira MJ, Sarmento B. Impact of CEA-targeting nanoparticles for drug delivery in colorectal cancer. J Pharmacol Exp Ther. 2019;370:657–70.
Mattheolabakis G, Taoufik E, Haralambous S, Roberts ML, Avgoustakis K. In vivo investigation of tolerance and antitumor activity of cisplatin-loaded PLGA-mPEG nanoparticles. Eur J Pharm Biopharm. 2009;71:190–5.
de S. L. Oliveira ALC, de Araújo Júnior RF, de Carvalho TG, Chan AB, Schomann T, Tamburini F, et al. Effect of oxaliplatin-loaded poly (d,l-lactide-co-glycolic acid) (PLGA) nanoparticles combined with retinoic acid and cholesterol on apoptosis, drug resistance, and metastasis factors of colorectal cancer. Pharmaceutics. 2020;12:193.
Margiotta N, Marzano C, Gandin V, Osella D, Ravera M, Gabano E, et al. Revisiting [PtCl2(cis -1,4-DACH)]: an underestimated antitumor drug with potential application to the treatment of oxaliplatin-refractory colorectal cancer. J Med Chem. 2012;55:7182–92.
Margiotta N, Savino S, Denora N, Marzano C, Laquintana V, Cutrignelli A, et al. Encapsulation of lipophilic kiteplatin Pt(IV) prodrugs in PLGA-PEG micelles. Dalt Trans. 2016;45:13070–81.
de Moraes PD, Pessine FBT. Formulation of functionalized PLGA nanoparticles with folic acid-conjugated chitosan for carboplatin encapsulation. Eur Polym J. 2018;108:311–21.
Hackl CM, Schoenhacker-Alte B, Klose MHM, Henke H, Legina MS, Jakupec MA, et al. Synthesis and in vivo anticancer evaluation of poly(organo)phosphazene-based metallodrug conjugates. Dalt Trans. 2017;46:12114–24.
Izadi Z, Divsalar A, Saboury AA, Sawyer L. β-Lactoglobulin-pectin nanoparticle-based oral drug delivery system for potential treatment of colon cancer. Chem Biol Drug Des. 2016;88:209–16.
Jain A, Jain SK, Ganesh N, Barve J, Beg AM. Design and development of ligand-appended polysaccharidic nanoparticles for the delivery of oxaliplatin in colorectal cancer. Nanomed Nanotechnol, Biol Med. 2010;6:179–90.
Saber MM, Al-mahallawi AM, Nassar NN, Stork B, Shouman SA. Targeting colorectal cancer cell metabolism through development of cisplatin and metformin nano-cubosomes. BMC Cancer. 2018;18:822.
Nishiyama N, Okazaki S, Cabral H, Miyamoto M, Kato Y, Sugiyama Y, et al. Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice. Cancer Res. 2003;63:8977–83.
Wu H, Cabral H, Toh K, Mi P, Chen Y-C, Matsumoto Y, et al. Polymeric micelles loaded with platinum anticancer drugs target preangiogenic micrometastatic niches associated with inflammation. J Control Release. 2014;189:1–10.
Pöthig A, Casini A. Recent developments of supramolecular metal-based structures for applications in cancer therapy and imaging. Theranostics. 2019;9:3150–69.
Woods B, Wenzel MN, Williams T, Thomas SR, Jenkins RL, Casini A. Exo-functionalized metallacages as host-guest systems for the anticancer drug cisplatin. Front Chem. 2019;7:68.
Han J, Räder AFB, Reichart F, Aikman B, Wenzel MN, Woods B, et al. Bioconjugation of supramolecular metallacages to integrin ligands for targeted delivery of cisplatin. Bioconjug Chem. 2018;29:3856–65.
Meier-Menches SM, Zappe K, Bileck A, Kreutz D, Tahir A, Cichna-Markl M, et al. Time-dependent shotgun proteomics revealed distinct effects of an organoruthenium prodrug and its activation product on colon carcinoma cells. Metallomics. 2019;11:118–27.
Steel TR, Hartinger CG. Metalloproteomics for molecular target identification of protein-binding anticancer metallodrugs. Metallomics. 2020;12:1627–36.
Anthony EJ, Bolitho EM, Bridgewater HE, Carter OWL, Donnelly JM, Imberti C, et al. Metallodrugs are unique: opportunities and challenges of discovery and development. Chem Sci. 2020;11:12888–917.
Wenzel M, Casini A. Mass spectrometry as a powerful tool to study therapeutic metallodrugs speciation mechanisms: current frontiers and perspectives. Coord Chem Rev. 2017;352:432–60.
Crommelin DJA, van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256–63.
Funding
Fundação para a Ciência e a Tecnologia (FCT) for financial support through Projects UIDB/04138/2020, UIDP/04138/2020 and PTDC/MED-QUI/31721/2017, as well as for the PhD fellowship SFRH/BD/117586/2016.
Author information
Authors and Affiliations
Contributions
All authors whose names appear on the submission made substantial contributions to the article. Pedro Farinha—writing and original draft preparation; Pedro Farinha, Jacinta O. Pinho, Mariana Matias and Maria Manuela Gaspar—writing, editing, and critical revision of intellectual content; Pedro Farinha, Jacinta O. Pinho, Mariana Matias, and Maria Manuela Gaspar—conceptualization and supervision. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval and consent to participate
No human subjects or animal studies were involved in the manuscript.
Consent for publication
All authors have given their consent for publication.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Farinha, P., Pinho, J.O., Matias, M. et al. Nanomedicines in the treatment of colon cancer: a focus on metallodrugs. Drug Deliv. and Transl. Res. 12, 49–66 (2022). https://doi.org/10.1007/s13346-021-00916-7
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-021-00916-7