Abstract
The objective of the present work was to develop and optimize multiparticulate pH-dependent bioadhesive pellets of curcumin and cyclosporine for the management of intestinal bowel disease (IBD). The bioadhesive sustained release pellets were intended for targeting the affected site for an improved therapeutic effect. Bioadhesive pellet cores of curcumin and cyclosporine were formulated using Carbopol 940 (CP940) and hydroxypropyl cellulose (HPC-H) by the extrusion/spheronization method, and drug delivery to the colon was controlled by the pH-sensitive polymer Eudragit® S100. Microcrystalline cellulose (Avicel PH101) was found to be the best forming agent for pellet core. The ratio of CP940 to HPC-H was kept at 1:1 to achieve 100% bioadhesion. The in vitro dissolution profiles of coated pellets depicted that 12.327 ± 0.342% of curcumin and 14.751 ± 0.112% of cyclosporine were released at the end of 6 h (at pH 6.8), whereas 71.278 ± 0.100% of curcumin and 76.76 ± 0.195% of cyclosporine were released at the end of 24 h (at pH 7.4). The drug release profile was found to follow zero-order kinetics for both drugs. The selected formulation was evaluated on an acetic acid-induced ulcerative colitis in the rat model to evaluate the efficiency of drug-loaded pellets coated with Eudragit®S100. The pharmacodynamic study revealed the therapeutic efficacy of Eudragit®S100-coated pellets of curcumin and cyclosporine in alleviating the conditions of the acetic acid-induced colitis model as reflected by weight gain as well as improvement of clinical, macroscopic and microscopic parameters of induced colitis, as compared with free curcumin and cyclosporine. The combination of curcumin and cyclosporine has been proven to have a synergistic effect for the successful management of IBD when used in a low dose as compared with individual drugs with high doses. Hence, curcumin- and cyclosporine-loaded bioadhesive pellets may act as a promising targeted drug delivery system in the management of IBD.

Graphical abstract
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Oral colon targeted drug delivery is broadly utilized in the treatment of intestinal bowel disease (IBD) viz. Crohn’s disease and ulcerative colitis (UC) [1, 2]. The treatment strategies include the utilization of aminosalicylates, corticosteroids, immunosuppressants, antibiotics and biologics [3, 4]. The conventional drug delivery system of these therapeutic agents causes unfavourable adverse drug reactions and side effects to neighbour organs and furthermore limits the therapeutic efficacy of the medications [3,4,5]. For this purpose, a colon targeted drug delivery system (CDDS) has been developed for the management of IBD with a goal to increase the drug localization and drug accessibility at the target site, dose reduction and the reduction of adverse effects fundamentally encountered when the drugs are released in the gastrointestinal tract (GIT) [3,4,5,6]. Moreover, a colon targeted drug delivery system guarantees maximum drug release in the colonic region with less vacillations in drug concentration at the site, presenting a better therapeutic management of the disease [1, 2, 4, 7]. IBD is characterized by the aggravation of the intestinal mucous layer, bringing about decimation of intestinal motility, thereby causing bloody and mucous diarrhoea, which leads to the variation of the intestinal pH, intestinal volume, intestinal microbial flora and mucosal integrity [3,4,5]. For such conditions, the multiparticulate targeted drug delivery system with better mucoadhesion, and a higher residence time of the formulation and localized delivery of the drug to the inflamed tissue is highly preferred. Various multiparticulates like pellets, granules, microspheres and nanoparticles have been broadly studied for their therapeutic efficacy in the management of IBD [8,9,10,11]. As a result of their smaller particle size, these systems are capable of passing through the GIT easily when contrasted with single-unit conventional dose structures. As the units of multiparticulate frameworks are distributed unreservedly all through the GIT, their transport is affected to a lesser degree than single-unit formulations by the transit time of food [7]. For intestinal delivery, multiparticulates are favourable as compared with single-unit dosage forms because of better distribution along the intestinal wall, and the increased total surface area available for adhesive bonds [12]. Besides, the gastrointestinal transit of multiparticulate formulations is less variable than single-units, and transit through the colon is slower because of a sieving effect, with monolithic dosage forms moving quicker [13, 14]. In this way, a tremendous amount of work has been done on the development of mucoadhesive nanoparticles [15, 16], microparticles [17, 18] and pellets in the millimetre range [14, 19] for the treatment of ulcerative colitis. There is a clear trend in colon drug delivery to develop multiparticulate formulations instead of single-unit systems. The advantages are similar as for mucoadhesive multiparticulates i.e. prolonged transit time, closer contact to the diseased tissue and more reproducible drug release [20, 21]. The development of colon-targeted pellet formulations has already led to the launch of several products in the market (Apriso and Salofalk Granu-Stix) [20, 21], while the colonic-targeted formulations based on multiparticulates or nanoparticles require further advancement, particularly in regard to their systemic toxicity profile and manufacturability on an industrial scale. Multiparticulates for colon delivery are an intriguing option to nanoparticles as they are not internalized by epithelial cells, and consequently, there is a much lower risk of systemic drug toxicity [22]. There have been numerous reports on multiparticulate colon drug delivery systems in the micro-size range; the vast majority of them are dependent on bacteria-degradable or pH-sensitive release mechanisms [11]. Mucoadhesive functionalization of multiparticulate colon drug delivery systems can increase the colonic transit time and thus they can improve their therapeutic efficiency. In general, processing of multiparticulates is simpler when compared with nanocarriers because of the improved flowability of larger particles and the possibility of utilizing easier standardized processes appropriate for scale-up. Pellets in the millimetre range have better flowability and show better mucoadhesive performance because of the increased surface area accessible for mucoadhesive bonds as contrasted to large tablets [23].
The pH-dependent drug delivery systems developed using Eudragit® S100 polymer have unique properties, for example, they improve therapeutic efficiency by confining drugs to the target site, increase bioavailability, facilitate intimate contact of formulation to the affected site and prolong residence time in the target region [12]. It was accounted that the pH-dependent system was utilized to deliver mesalazine at the colonic site using Eudragit® S100 and Eudragit® L100 and methacrylic acid copolymers [24, 25]. Therefore, pH-dependent polymer-coated bioadhesive pellets have a potential benefit for the treatment of IBD [6, 20, 26]. Furthermore, it is also reported that the utilization of bioadhesive polymers in pharmaceutical formulations could advantageously improve the therapeutic efficiency by localizing the drug to the target tissue [27]. It has also been indicated that the utilization of mucoadhesive agents such as Carbopol 940 (CP940) and hydroxypropyl cellulose (HPC-H) brought about a strong retention of particles at the mucosal surface which preferentially extends the retention time of the formulation in the target region [14, 21, 26]. The colonic pH is fundamentally low in certain IBD conditions like ulcerative colitis. Under this acidic condition, the bioadhesive property of Carbomer® is progressively enhanced in the colon as compared with the small intestine [14, 28]. The combination of the bioadhesion concept alongside a colon-targeted drug delivery system will contribute to a more efficient targeting to the colon and avoid the drug release at the upper GI tract [14].
Statistically, it was observed that very nearly 25% of the patients with IBD are analysed in the initial 2 decades of their life, not many of them being diagnosed in the early period of youth (i.e. 13–18 years of age). Furthermore, it is additionally reported from various countries that the pervasiveness of IBD is expanding broadly in adolescence [12]. Upon current examination, the most elevated yearly occurrence of IBD in Europe was found to be 24.3 per 100,000 person-years for UC, and 12.7 per 100,000 person-years for CD, that in North America was 19.2 per 100,000 person-years for UC and 20.2 per 100,000 person-years for CD and that in Asia and Middle East was 6.3 per 100,000 person-years for UC and 5.0 per 100,000 person-years for CD [29, 30].
As the reason for IBD is as yet obscure, the present treatment regimen involves the utilization of anti-inflammatory agents, immunomodulators, corticosteroids and antibiotics. From the class of anti-inflammatory agent’s curcumin, the principal active component is widely utilized as the treatment regimen for IBD because of its high inhibitory action on interleukin and cytokine development factors. Curcumin was found to be isolated from the rhizomes of the plant Curcuma longa and has different organic, biological and pharmacological activities, such as antioxidant, anti-carcinogenic, anti-inflammatory, anti-virus, anti-proliferative and chemo-preventive activities [31]. The primary mechanism by which curcumin intercedes these effects is associated with the activity of suppression of the nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). Additionally, curcumin suppresses interleukin-1 (IL-1), interleukin-2 (IL-2), B lymphocytes and tumour necrosis factor-alpha (TNF-α), the principle inflammatory mediators in the regulation of inflammatory responses. For these crucial activities, curcumin is considered a potential and effective drug in the management of IBD [32].
Curcumin in combination with cyclosporine is found to be effective in subsequent studies for the treatment of IBD because of its complementary mechanism of action to decrease the aggravation and henceforth exhibit an appealing alternative for adjunct therapy in managing IBD [7, 29]. Thus, cyclosporine was chosen as the second medication of decision to demonstrate the synergistic effect. Cyclosporine is an immunomodulator drug and is thought to act by decreasing pro-inflammatory lymphokines by inhibiting their antigen-induced secretion through the binding of calcium-calmodulin-dependent protein phosphatase calcineurin. The significant property of cyclosporine is its ability to hinder the production of cytokines, which are engaged in the regulation of T cell activation. Particularly, it inhibits the synthesis of interleukin 2 (IL-2) and interleukin 1 (IL-1) at the level of gene transcription. This action is accomplished by complex formation with cyclophilin, which leads to reduced function of effector T cells. Various studies have reported that curcumin can modulate the proliferation and activation of the T cell. It can also induce apoptosis to reduce the proliferation of B cell lymphoma. It can likewise inhibit the proliferation induced by concanavalin A, phytohemagglutinin (PHA) and phorbol-12-myristate-13-acetate (PMA) of lymphocytes. Likewise, curcumin additionally suppresses IL-2 synthesis and IL-2-induced proliferation of lymphocytes, suggesting that it exhibits immunosuppressive properties [29]. It was also reported that curcumin inhibits the proliferation induced by PMA and anti-CD28 antibody as well as the proliferation induced by PHA of T lymphocytes isolated from healthy donors. In correlation, cyclosporine has demonstrated to have a guarantee in the induction of remission of refractory ulcerative colitis and suppresses PHA-induced T cell proliferation but not the ones induced by PMA and anti-CD28 antibodies [33]. Thus, curcumin can overcome the resistance of PMA and CD28 pathway to cyclosporine, featuring its immunomodulating properties. Hence, curcumin, when added to both immunomodulator and non-immunomodulator medication, shows a synergistic effect [29].
The present research work is an innovative concept for exploring the pH-dependent solubility of Eudragit®S100, bioadhesion and swelling properties of HPC-H and CP940, anti-inflammatory effects of curcumin and the synergistic effect when utilized in combination with cyclosporine. In this research study, we prepared curcumin and cyclosporine bioadhesive pellets containing CP940 and HPC-H for colonic delivery for the potential benefit for the treatment of IBD. Pellets were prepared by the extrusion/spheronization method with CP940 and HPC-H as bioadhesive core material. Prepared pellets were coated with Eudragit® S100, a pH-sensitive polymer to deliver both the drugs at the target site so as to minimize the drug release at the upper GIT and prevent adverse drug reactions to the neighbouring organs. The acetic acid-induced ulcerative colitis model was chosen to assess the in vivo efficacy of curcumin and cyclosporine pellets [26].
Materials and methods
Materials
Curcumin (99.9% pure) was procured from Otto Chemie pharmaceuticals (Mumbai, India). Cyclosporine was received as a gift sample from Concord Biotech Limited (Ahmedabad, India). Eudragit® S100 was purchased from Evonik India Pvt. Ltd. (Mumbai, India). Microcrystalline cellulose PH 101 (Avicel PH101), Carbopol 940 (CP940) and hydroxypropyl cellulose (HPC-H) were received from Signet Chemical Corporation (Mumbai, India). Acetic acid was purchased from S. D. Fine Pvt., Ltd. (Mumbai, India).
Bioadhesive pellet preparation
Pellets of different compositions were prepared by an extrusion/spheronization method as shown in Table 1. All the excipients were sifted through 40# mesh separately and mixed in geometric proportion and blended for about 10 min. The dry powder blend was homogenized in a mortar and then wetted using purified water as a granulating liquid until a wet mass was obtained. The plastic mass was fed into the one-screw axial extruder (Umang Pharm Tech., Mumbai, India) operated at a speed of 100 rpm with the perforations of 1.2 mm in diameter to form the extrudate. The extrudates were then placed into the spheronizer (Umang Pharm Tech., Mumbai, India) operated at a speed of 1500 rpm with the perforations of 3.2 mm in diameter for 3 min to form the pellets. The prepared pellets were dried in a tray dryer (Dolphin, Mumbai, India) at 40 °C for 0.5 h and then dried in a fluidized bed dryer (Glatt Integrated Process, Mumbai, India) at an inlet temperature of 40 °C and airflow rate of 80 m3/h until the product temperature reached 40 °C for 0.5 h. The pellets were then sifted through a 40# mesh sieve for separation of fines and the pellets that were retained on the sieve were selected.
Enteric coating of pellets
Enteric coating of the bioadhesive pellets with the material such as Eudragit® S100 (pH-sensitive polymer), talc (anti-tacking agent) and triethyl citrate (plasticizer) as an outer layer can improve the concentration of curcumin and cyclosporine at the affected site in the colon. For coating of the drug-loaded pellet core, the polymer percentage attempted were 1%w/v, 2%w/v and 3%w/v using isopropyl alcohol (IPA): Acetone as a solvent in the ratio of 1:1. 2% w/v of Eudragit® S100 in IPA: Acetone in the ratio of 1:1 was selected for coating of pellets using fluidized bed coater (Glatt Integrated Process, Mumbai, India). Eudragit® S100 (2%w/v), talc (0.5%w/v) and triethyl citrate (0.2%w/v) in the concentration of and lake sunset yellow colour were dispersed into the above solution under magnetic stirring (Labmann Scientific Instruments Pvt. Ltd. Chennai) at the rate of 500 rpm, and stirring was continued for 15 min. to obtain a final dispersion. The coating level was set to increase to a 10% weight of the total pellets. Sixty grams of pellets, with a diameter of 0.8–1.00 mm, was transferred into a chamber equipped with a bottom spray nozzle of 0.5 mm diameter. Coating dispersions were sprayed at the rate of 1.5 ml/min under an atomizing pressure of 0.1 MPa, the fluid airflow rate of 18 m3/h; the inlet air temperature was maintained at 50 °C, the outlet air temperature was maintained at 40 °C and the voltage was 50 Hz. Coated pellets were further fluidized for 15 min. at 50 °C for 0.5 h and kept in an oven at 40 °C for 2 h to complete the coating film formation.
Pellet characterization and evaluation
The size of pellets and size distribution were determined prior to the coating process by performing a sieve analysis. Hardness, friability, pellet sphericity [34, 35], bulk density, tap density, % Carr’s compressibility index, Hausner’s ratio, % yield, angle of repose and flow rate were evaluated according to USP criteria in an uncoated pellet sample fraction from 0.9–1.00 mm diameter. Pellet dimensions (pellet size) were derived using a digital vernier calliper and the developed Eudragit® S100-coated bioadhesive pellets were characterized for their surface morphology using a scanning electron microscopy (SEM) under × 200 magnification power. The friability was tested in a friabilator (Erweka, Mumbai, India) equipped with a plastic drum on both sides.
Bioadhesiveness test/mucoadhesion test
The mucoadhesive performance of uncoated pellets was assessed ex vivo on colon mucosa of the rat and goat. The colon was cut longitudinally with a variable length and cleaned by a phosphate buffer (pH 7.4), placed on polyethene support, spread and held in position with the help of pins. Uncoated pellet cores were placed uniformly on the mucosa of the colon. The tissue was then placed in a container maintained at 92.5% relative humidity and room temperature for 20 min to allow the polymer to hydrate and to interact with the glycoprotein and also to prevent the drying of the mucus. After 20 min, the mucosa was washed for 5 min with a phosphate buffer (pH 7.4) at the rate of 1 ml/min using a syringe. Finally, the number of pellets on the mucosa was calculated. A larger number of the pellets in the mucosa would indicate a better adhesion of the pellets. This study was performed in triplicate.
Swelling index
The swelling index of uncoated and Eudragit® S100-coated pellets was carried out to check the swelling behaviour of CP940 and HPC-H. Swelling of uncoated and Eudragit® S100-coated pellets was carried out in 0.1 N hydrochloric acid (HCl) (pH 1.2) and phosphate buffer (pH 7.4).
Bioadhesion testing
Bioadhesion testing was determined using an apparatus which was adapted in-house. It consists of a wooden block, which was provided by a pulley at one end. A ground glass slide was fixed on this block. Uncoated pellets were placed uniformly on the colon mucosa of goat and were placed on this ground slide. The colon mucosa was then sandwiched between this slide and another glass slide having the dimension of the fixed ground slide and provided with a hook. The top plate was then subjected to a pull of 5 g weight increment with the help of a string attached to the hook, and the time (sec.) required by the top slide to cover a distance was noted. The total weight (g) required to detach the pellets with the help of the upper glass slide from the mucous membrane was taken as a measure of mucoadhesive strength. From this mucoadhesive strength, the force of adhesion was calculated using the following formula [36]:
Drug content
The bioadhesive pellets were triturated using a mortar and pestle, and the powder blend equivalent to 30 mg of the drug (curcumin 20 mg, cyclosporine 10 mg) was dispersed in 10 ml of distilled water in 10 ml amber-coloured volumetric flask. The resultant dispersion was subjected to sonication for 10 min. and then filtered through a nylon filter (0.45 μm). The filtrate was then appropriately diluted and the percent drug content was estimated by calculating the peak area and calculating the quantity of curcumin and cyclosporine in the formulation at the wavelength of 214 nm using a high-performance liquid chromatography (HPLC) (make- Agilent Technologies, Mumbai, India; model- Agilent 1260 Infinity Quaternary LC; column- Eclipse XDB- C18 (4.6 × 150 mm, 5 μ particle size); mobile phase- acetonitrile: water: methanol (50:10:40 v/v/v); flowrate- 0.5 ml/min.; temperature- 70 °C ± 0.02 °C; injection volume- 20 μl detector- photo diode array detector) [20].
The in vitro dissolution profile of bioadhesive pellets coated with a pH-sensitive polymer
The in vitro release of the drug from the bioadhesive pellets was investigated using a USP type 1 dissolution rate test apparatus (Basket type, ElectroLab Pvt. Ltd., ET-11L, Mumbai, India) at a rotational speed of 100 rpm. Five hundred milligrams of pellets in size 0 hard gelatin capsule shell equivalent to 30 mg of the drug (20 mg of curcumin and 10 mg of cyclosporine) was placed in the basket. The apparatus was maintained at 37 °C ± 0.5 °C. The dissolution was carried out in 250 ml of 0.1 N HCL with 2% SLS (sodium lauryl sulphate) for 2 h followed by 250 ml of a phosphate buffer pH 6.8 with 2% SLS for additional 4 h (total 6 h) followed by a phosphate buffer 7.4 with 2% SLS for additional 18 h. Five milliliters of aliquot was withdrawn from the dissolution apparatus at specific time intervals of 1, 2, 3, 4, 5, 6, 7, 8, 9, 10 and 24 h and was filtered through a 0.45-μm syringe filter and analysed using HPLC. Sink conditions were maintained by replenishing the same amount of medium. This study was performed in triplicate [37].
In vivo studies to evaluate the efficacy of the developed formulation
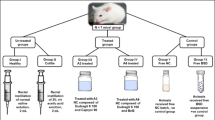
“All institutional and national guidelines for the care and use of laboratory animals were followed.” Male Wistar rats of weight 170–200 g approx. were procured from the National Institute of Bioscience (Pune, India). Rats were randomly divided into six groups each containing five animals. They were housed under standard conditions of temperature and relative humidity with 12 h light/dark cycle. The animals were fed on a standard commercial pelleted diet and water ad libitum. The Institutional Animal Ethical Committee approved the experimental protocols as per CPCSEA guidelines through the research project no. CPCSEA/IAEC/BNCP/P-79/2017. Twenty-four hours prior to the induction of colitis, the rats were deprived of food but not of water. Colonic inflammatory lesions (ulcerative colitis) were induced in rats by instilling 1 ml acetic acid (4% v/v) into the colon anus (intra-rectal route) using a soft 6-Fr paediatric catheter under mild anaesthesia (sodium thiopental) [38]. The catheter was inserted into the anus up to a length of 6 cm, and then acetic acid was administered. Before removing the catheter, 2 ml of air was injected to spread the acetic acid completely in the colon. Rats were observed for rectal bleeding and watery stools which confirmed the induction of ulcerative colitis. For 2 days, the rats were housed without any treatment to maintain the development of a full IBD model. A healthy group received saline solution instead of an acetic acid solution. Two days after the induction, the groups received drug-containing pellets. The rats were treated once in a day for the next eight continuous days. The rats were randomly divided into six groups: normal control/healthy group (1); negative control/untreated group (2); marketed formulation/standard control (mesacol tablet 500 mg; dose 20 mg/kg; orally: intragastric route) group (3); cyclosporine pellets (equivalent to 20 mg/kg; orally: intragastric route) group (4); curcumin pellets (equivalent to 40 mg/kg; orally: intragastric route) group (5); and optimized test formulation bioadhesive pellets of curcumin and cyclosporine coated with Eudragit® S100 (equivalent to 30 mg/kg curcumin 20 mg/kg, cyclosporine 10 mg/kg; orally: intragastric route) group (6). Animals were weighed just before treatment and just before autopsy to determine whether colitis had an effect on body weight. After 24 h of the last drug administration, the animals were sacrificed. The colon was excised and its length was measured. The colon was opened longitudinally, rinsed in ice-cold normal saline, cleaned of fat and mesentery, blotted on filter paper and weighed. The ratio of colon weight in milligrams to total body weight in grams was taken as the colon mass index and was used as a measure of the degree of colonic oedema and severity of inflammation [31]. Ulcer projections were assessed visually and the specimen was fixed in 10% v/v formalin solution and preserved for evaluation/examination of histopathological studies [26, 39].
Efficacy study parameters of ulcerative colitis model
Colon/body weight ratio
Samples of the resected colon tissue were opened longitudinally, rinsed with ice-cold physiological saline to remove luminal content and weighed. The colon/body weight ratio was calculated as a quotient of the colon wet’ weight compared with the total body weight of each rat [20, 26].
Histological assessment of rat colon
Histological assessment was used to measure colonic injury. Colonic tissue samples taken from the distal colon were immersed in 10% phosphate-buffered formalin and subsequently embedded in paraffin. Sections of 3 mm thickness were stained with haematoxylin and eosin. The slides were then evaluated by a pathologist for epithelial damage, architectural changes, polymorphonuclear leukocyte (PMN) infiltration, mononuclear cell infiltration and ulceration.
Results
Bioadhesive pellet core preparation
The core of pellets was prepared by an extrusion/spheronization method. Pellets from 0.9 to 1.0 mm interval were selected by sieve analysis and used for fluidized bed coating. The results of pellet sphericity and percentage yield are shown in Table 2. Formulation batch B1 and B2 had a higher concentration of CP940 and HPC-H due to which the extrudates formed were fluffy and after spheronization, excessive fines were produced. However, the formulation batch B3, B4 and B5 had the lowest polymer concentration which produced pellets with good sphericity and a maximum yield as compared with B1 and B2. Therefore, B3, B4 and B5 were taken up for further evaluation parameters.
Aqueous vehicle (water)
Avicel PH101 pellets were successfully formed using water as a granulating liquid. Hence, water is considered the choice of granulating fluid over isopropyl alcohol in preparation of Avicel PH101 pellets [40]. The formulation batch B3 exhibited the best sphericity and highest yield compared with B1, B2, B4 and B5. Therefore, pellets of formulation batch B3 were selected and used for the subsequent fluidized bed coating and for further evaluation.
Bioadhesiveness test/mucoadhesion test
The quantitative adhesion of pellets on excised intestinal tissue is shown in Fig. 1. A larger number of the pellets on the mucosa indicate a better adhesion of the pellet. The results showed that CP940, as a high molecular polymer and stable in a highly viscous medium, when used with HPC-H, is a commonly used bioadhesive material; a synergistic effect was obtained. Polymer ratios 2:1, 1:1 and 1:2 (CP940:HPC-H) were evaluated. The mucoadhesion effect was varied with the variation of their ratio. The ratio of (1:1 CP940: HPC-H i.e. B3; Fig.1a) had the best bioadhesion ability, and almost 100% of pellets adhered to the mucosa as shown in Fig. 2. Therefore, this ratio was selected for the preparation of pellets.
Determination of bioadhesive properties of different proportions of bioadhesive polymer in pellets. a Rat colon indicating 100% bioadhesion of pellets with 1:1 (CP940: HPC-H) polymer ratio (B3). b Rat colon indicating 80% bioadhesion of pellets with 2:1 (CP940: HPC-H) polymer ratio (B4). c Rat colon indicating 40% bioadhesion of pellets with 1:2 (CP940: HPC-H) polymer ratio (B5)
Determination of bioadhesive properties of different proportions of bioadhesive polymer in pellets. Graphical data representing (a) 100% adhered pellet particles on rat colon with 1:1 (CP940: HPC-H) polymer ratio (B3); (b) 80% adhered pellet particles on rat colon with 2:1 (CP940: HPC-H) polymer ratio (B4); and (c) 40% adhered pellet particles on rat colon with 1:2 (CP940: HPC-H) polymer ratio (B5). *Data is expressed as mean ± SD, n = 3
Swelling index
Bioadhesive polymers possess numerous hydrophilic functional groups, such as hydroxyl and carboxyl groups. These groups allow hydrogen bonding with the substrate and swelling in the aqueous media, thereby allowing maximal exposure of potential anchor sites. In addition, swollen polymers have the maximum distance between their chains leading to increased chain flexibility and efficient penetration of the substrate [41].
The swelling index of uncoated pellets and Eudragit® S100-coated pellets was carried out on batches B3, B4 and B5. Table 3 compares the % swelling of uncoated and coated pellets. It was observed that the formulation batch B3 showed no swelling in 0.1 N HCl (pH 1.2) and 70% swelling at pH 7.4 (colonic pH) as compared with formulation batches B4 and B5. Therefore, batch B3 was taken up for further studies.
Bioadhesion testing
Bioadhesion testing was carried out on goat colonic mucosa. Formulation batch B3 showed the maximum force of adhesion of pellets on colonic mucosa. The force required to detach the pellets from the mucosa of batch B3 was greater as compared with batches B4 and B5 as shown in Table 4. This study was performed in triplicate.
Pellet characterization and evaluation
The particle size of the pellet was determined using a digital vernier calliper. Batch B3 had maximum yield, sphericity and bioadhesion as compared with other batches; hence, formulation batch B3 was evaluated for flow properties. Thirty pellets of formulation batch B3 were taken for size analysis. The %RSD (relative standard deviation) of uncoated and coated pellets was found to be 1.12 and 1.24 respectively as shown in Table 5.
Drug content
Drug content of the formulation was found to be 97.34 ± 0.5% for curcumin and 95.68 ± 0.63% for cyclosporine. Hence, it complies with the USP pharmacopoeial limit of 90–110%.
Characterization of bioadhesive pellets containing curcumin and cyclosporine coated with the pH-sensitive polymer by scanning electron microscopy analysis
The developed bioadhesive pellets coated with Eudragit® S100 exhibited good spherical geometry as evidenced by the SEM photograph in Fig. 3 and Fig. 4 respectively. Also, the surface of the pellet appears smooth and intact.
The in vitro dissolution profile of bioadhesive pellets coated with Eudragit® S100
In vitro dissolution studies were performed on 5%, 10% and 20% weight gain of pellets of formulation batch B3 after coating with Eudragit® S100. In the formulation with 5% Eudragit® S 100 coating on formulation batch B3, more than 10% of curcumin and cyclosporine was released at pH 1.2 (stomach pH), whereas more than 70% of cyclosporine was released at pH 6.8 (intestinal pH) and at pH 7.4 (colonic pH), almost 90% of cyclosporine was released which was undesirable. Whereas in the formulation with 10% Eudragit® S 100 coating on formulation batch B3, more than 10% of cyclosporine was released at pH 1.2 (stomach pH), more than 70% of cyclosporine was released at pH 6.8 (intestinal pH) and almost 80% of cyclosporine was released at pH 7.4 (colonic pH) which was not desirable. In contrast, the formulation with 20% Eudragit® S 100 coating on formulation batch B3, both the drugs, curcumin and cyclosporine, showed no drug release at pH 1.2 (stomach pH) and less than 15% release at pH 6.8 (intestinal pH) and almost 80% release at pH 7.4 (colonic pH) at the end of 24 h which was desirable.
The in vitro release results of 20% Eudragit® S100 coating on the pellets of formulation batch B3 proved that curcumin and cyclosporine showed a gradient of pH-sensitive release characteristics as shown in Fig. 5. The drug release profile was fit into various kinetic models to check out for the kinetics by which the drug was released from the polymer matrix [42]. We can summarize this as, as the pH increased, Eudragit® S100 gradually dissolved, water infiltrated through the membrane gradually into the pellet core, leading to volume expansion and successive swelling of CP940 and (HPC-H), which ensured not only a quick release of drug in the colon but also a strong adhesion of pellet cores to the colonic mucosa. Therefore, the drug release profile indicated that Eudragit® S100 outer coating effectively inhibited the drug release in the upper digestive tract and increased the localization of drug-loaded pellets in the colon affected regions.
In vitro dissolution profile of Eudragit® S100-coated curcumin and cyclosporine bioadhesive pellets for the first 2 h at pH 1.2, followed for 4 h at pH 6.8 and finished at pH 7.4 for 24 h. (♦) release of cyclosporine from 5% wt. gain of pellets (i.e. 5% Eudragit® S 100 coating on B3), (■) release of curcumin from 5% wt. gain of pellets (i.e. 5% Eudragit® S 100 coating on B3), (▲) release of cyclosporine from 10% wt. gain of pellets (i.e. 10% Eudragit® S 100 coating on B3), (×) release of curcumin from 10% wt. gain of pellets (i.e. 10% Eudragit® S 100 coating on B3), (>|<) release of cyclosporine from 20% wt. gain of pellets (i.e. 20% Eudragit® S 100 coating on B3) and (●) release of curcumin from 20% wt. gain of pellets (i.e. 20% Eudragit® S 100 coating on B3). *Data are shown as mean ± SD (n = 3)
Animal studies to evaluate the efficacy of the developed formulation
Effect on body weight
One of the major symptoms of ulcerative colitis is a reduction in body weight. Reduction in body weight is considered a sign of the severity of the disease [30]. Acetic acid-induced colitis in the negative group led to inhibition in the weight gain of rats by P < 0.001 while in the animals treated with the test formulation i.e. formulation batch B3 of bioadhesive pellets containing curcumin and cyclosporine coated with 20% Eudragit® S100 as a pH-sensitive polymer tend to restore the weight at a normal level. These results shown in Fig. 6 emphasized that the bioadhesive pellets of formulation batch B3 coated with 20% Eudragit® S100 (test formulation) improved the therapeutic efficacy of curcumin and cyclosporine.
Determination of increase in body weight. Effect of test formulation batch B3 with 20% Eudragit® S100 coating (bioadhesive pellets of curcumin and cyclosporine), marketed formulation (standard control), cyclosporine pellets with 20% Eudragit® S100 coating, curcumin pellets with 20% Eudragit® S100 coating on the bodyweight of animals with acetic acid-induced colitis measured from the time of induction of colitis until sacrifice. ***P < 0.001 vs. normal control group; ###P < 0.001 vs. negative control group; @P < 0.05 vs. standard control group. Data are expressed as mean ± SD (n = 5)
Colon mass index
Colon mass index (CMI) is a measure of the severity of the disease. Induction of colitis with acetic acid was associated with an increase in colon mass index [31]. The colon mass index of animals in the negative group was found to be significantly higher than the normal control group indicating the induction of ulcerative colitis. Treatment with 20% Eudragit® S100-coated bioadhesive pellets of curcumin and cyclosporine (i.e. formulation batch B3) tends to prevent the increase in colon mass index compared with the standard control group (@@@P < 0.001), group dosed with 20% Eudragit® S100-coated curcumin pellets ($$$P < 0.001) and group dosed with 20% Eudragit® S100-coated cyclosporine pellets (!!P < 0.01) as shown in Fig. 7. The results indicate that the bioadhesive pellets of curcumin and cyclosporine coated with 20% Eudragit® S100 on formulation batch B3 (test formulation) improved the therapeutic efficacy of both the drugs.
Determination of CMI. Effect of test formulation batch B3 with 20% Eudragit® S100 coating (bioadhesive pellets of curcumin and cyclosporine), marketed formulation (standard control), cyclosporine pellets with 20% Eudragit® S100 coating, curcumin pellets with 20% Eudragit® S100 coating on the colon mass index of animals with acetic acid-induced colitis. ***P < 0.001 vs. normal control group; ###P < 0.001 vs. negative control group; @@@P < 0.001 vs. standard control group; $$$P < 0.001 vs. curcumin pellets group; @@P < 0.01 vs. standard control group;!!P < 0.01 vs. cyclosporine pellets group. Data are expressed as mean ± SD (n = 5)
As shown in Fig. 8, the thickness of the negative control group (Fig. 8b) colon is significantly higher than the normal control group (Fig. 8a) which indicates that the disease was induced in the rats, whereas the thickness of the colon of the test formulation group (Fig. 8f) is quite similar to that of the normal control group (Fig.8a) indicating that the disease has been treated with the test formulation (i.e. bioadhesive pellets of curcumin and cyclosporine of formulation batch B3 coated with 20% Eudragit® S100).
Photographs of representative rat colon indicating tissue sections from acetic acid installation site (a- Normal control group; b- Negative control group; c- Standard control group; d- Cyclosporine pellets with 20% Eudragit® S100 coating group; e- Curcumin pellets with 20% Eudragit® S100 coating group; f- Cyclosporine and curcumin pellets group (test formulation batch B3 with 20% Eudragit® S100 coating), equivalent magnification for all images, original size)
Histological (microscopical) examination of rat colon
In order to process for microscopic studies, approx. 5-μm-thick paraffin sections were stained in haematoxylin and eosin (H & E). The stained sections were examined for any inflammatory changes like infiltration of cells, necrotic foci, damage to tissue structures, damage to the nucleus, congestion, aggregation of cells, presence of inflammatory cells, and morphology of villi. As shown in Fig. 9c and d, the negative control group showed focal and diffuse lymphoid aggregates, mononuclear cell infiltration (+++), multifocal necrosis (+), denudation/sloughing of lining epithelium, diffuse mononuclear cell infiltration (++), test formulation (pellets made up of curcumin and cyclosporine with 20% Eudragit® S100 coating) group (Fig. 9k and l) showed a normal histological structure of colonic mucosa, whereas the standard control group (Fig. 9e and f), curcumin pellets with 20% Eudragit® S100 coating group (Fig.9i and j) and cyclosporine pellets with 20% Eudragit® S100 coating group (Fig. 9g and h) showed slight focal lymphoid aggregates and denudation of epithelial lining. These results proved that the prepared pellets of curcumin and cyclosporine pellets in combination improved the therapeutic effect of both the drugs for the treatment of colitis. Results of in vivo studies on male Wistar rats proved that the low doses of developed bioadhesive pellets of curcumin and cyclosporine coated with 20% Eudragit® S100 in combination showed improved weight gain (P < 0.001), colon mass index (P < 0.001) and showed comparative reduction in infiltration of immune cells, reduction in necrosis as indicated by histopathological examination compared with the group treated with pure curcumin and cyclosporine pellets with high doses of both the drugs given individually in two different groups as shown in Fig. 9. In vivo clinical activity on the rats showed that curcumin and cyclosporine, when loaded in colon adhesive pellets coated with the pH-sensitive carrier, exerted a higher efficacy for the management of IBD.
Histological specimens of rats. a Normal control group × 10. b Normal control group × 45. c Negative control group × 10. d Negative control group × 45. e Standard control group × 10. f Standard control group × 45. g Cyclosporine pellets with 20% Eudragit® S100 coating group × 10. h Cyclosporine pellets with 20% Eudragit® S100 coating group × 45. i Curcumin pellets with 20% Eudragit® S100 coating group × 10. j Curcumin pellets with 20% Eudragit® S100 coating group × 45. k Test formulation batch B3 with 20% Eudragit® S100 coating group × 10. l Test formulation batch B3 with 20% Eudragit® S100 coating group × 45 (n = 5); *× 10 and × 45, magnification power of microscope
Discussion
Bioadhesive pellets of curcumin and cyclosporine were successfully prepared by the extrusion-spheronization technique. It was observed that Avicel PH101, when used as a diluent, imparted maximum sphericity to the pellet core. Better sustained release, good sphericity and less percent of fines were observed in formulation batch B3 as compared with formulation batch B1 and B2. This could be attributed to a higher concentration of MCC and 11.5% of overall polymer concentration of CP940 and HPC-H in the ratio of 1:1. Animal studies revealed that pellets with CP940 and HPC-H in the ratio of 1:1 gave excellent bioadhesion, thus increasing the residence time in the rat colon. Furthermore, enteric coating of formulation batch B3 with 20% Eudragit® S100 showed pH-dependent site specific release at pH 7.4 of almost 80% of curcumin and cyclosporine. The in vitro results also indicated that Eudragit® S100 coating leads to the minimal absorption of curcumin and cyclosporine in the jejunum and upper ileum and increased the localization in the colon affected areas, which proved that Eudragit® S100 coating was able to prevent the drug release in the upper GIT. In vivo histological assessment proved that the group administered with low doses of curcumin and cyclosporine pellets in combination showed the normal pathological structure of the mucosa as compared with the groups receiving pure curcumin and cyclosporine pellets individually in a high dose [31, 43,44,45,46,47,48,49]. The results proved that low doses of curcumin and cyclosporine pellets in combination act synergistically and therefore can be used in the management of IBD.
Conclusion
A promising targeted drug delivery system of curcumin and cyclosporine was developed for the treatment of IBD. Curcumin- and cyclosporine-loaded colon adhesive pellets were prepared by coating the core of the bioadhesive pellet with Eudragit® S100 as an outer layer for pH control. CP940 and HPC-H being a bioadhesive polymer served to impart good swelling ability, strong adhesion to the mucosal surface, better permeability and provided a sustained release pattern for the drug release at the affected site. Coating of pellets with Eudragit® S100 (pH-sensitive polymer) helped to achieve targeting of the drug to the intestinal region and reduced adverse reactions to non-target sites. The results of the in vitro release of curcumin and cyclosporine from the coated pellets proved that the release was pH-sensitive. On the other hand, the results of the adhesion indicated that a strong adhesion was obtained. In vivo clinical activity on the rats showed that curcumin and cyclosporine together in a lower dose, in bioadhesive pellets coated with the pH-sensitive carrier, exerted a higher efficacy for the management of the disease as compared with individual drugs with higher doses. Thus, it can be concluded that a combination of curcumin and cyclosporine can have a synergistic effect for successful management of IBD when used in a low dosage as compared with individual drugs with high doses [31, 43,44,45,46,47,48,49]. Hence, bioadhesive pellets of curcumin and cyclosporine coated with Eudragit® S100 can be a promising approach for targeting both the drugs to the intestinal region for efficient management of IBD.
References
Malayandi R, Kondamudi PK, Ruby PK, Aggarwal D. Biopharmaceutical considerations and characterizations in development of colon targeted dosage forms for inflammatory bowel disease. Drug Deliv Transl Res. 2014;4:187–202.
Vemula SK, Veerareddy PR, Devadasu VR. Pharmacokinetics of ketorolac tromethamine compression-coated tablets for colon delivery. Drug Deliv Transl Res. 2014;4:310–9.
Sohail M, Mudassir, Minhas MU, Khan S, Hussain Z, de Matas M, et al. Natural and synthetic polymer-based smart biomaterials for management of ulcerative colitis: a review of recent developments and future prospects. Drug Deliv Transl Res. 2019;9:595–614.
Jain SK, Jain A. Target-specific drug release to the colon. Expert Opin Drug Deliv. 2008;5:483–98.
Guo Y, Zong S, Pu Y, Xu B, Zhang T, Wang B. Advances in pharmaceutical strategies enhancing the efficiencies of oral colon-targeted delivery systems in inflammatory bowel disease. Molecules. 2018;23.
Varshosaz J, Emami J, Tavakoli N, Minaiyan M, Rahmani N, Dorkoosh F, et al. Colon specific delivery of budesonide based on triple coated pellets: in vitro/in vivo evaluation. Acta Pharma. 2012;62:341–56.
Sharma S, Sinha VR. Current pharmaceutical strategies for efficient site specific delivery in inflamed distal intestinal mucosa. J Control Release. 2018;272:97–106.
Collnot E-M, Ali H, Lehr C-M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J Control Release. 2012;161:235–46.
Das S, Deshmukh R, Jha A. Role of natural polymers in the development of multiparticulate systems for colon drug targeting. Syst Rev Pharm. 2010;1:79.
Jose S, Dhanya K, Cinu TA, Aleykutty NA. Multiparticulate system for colon targeted delivery of ondansetron. Indian J Pharm Sci. 2010;72:58–64.
Asghar LFA, Chandran S. Design and evaluation of matrix base with sigmoidal release profile for colon-specific delivery using a combination of Eudragit and non-ionic cellulose ether polymers. Drug Deliv Transl Res. 2011;1:132–46.
Patel MM. Formulation and development of di-dependent microparticulate system for colon-specific drug delivery. Drug Deliv Transl Res. 2017;7:312–24.
Abrahamsson B, Alpsten M, Jonsson UE, Lundberg PJ, Sandberg A, Sundgren M, et al. Gastro-intestinal transit of a multiple-unit formulation (metoprolol CR/ZOK) and a non-disintegrating tablet with the emphasis on colon. Int J Pharm. 1996;140:229–35.
Varum FJO, Veiga F, Sousa JS, Basit AW. Mucoadhesive platforms for targeted delivery to the colon. Int J Pharm. 2011;420:11–9.
Takeuchi H, Yamamoto H, Kawashima Y. Mucoadhesive nanoparticulate systems for peptide drug delivery. Adv Drug Deliv Rev. 2001;47:39–54.
Sosnik A, Das Neves J, Sarmento B. Mucoadhesive polymers in the design of nano-drug delivery systems for administration by non-parenteral routes: a review. Prog Polym Sci. 2014;39:2030–75.
De Ascentiis A, deGrazia JL, Bowman CN, Colombo P, Peppas NA. Mucoadhesion of poly(2-hydroxyethyl methacrylate) is improved when linear poly(ethylene oxide) chains are added to the polymer network. J Control Release. 1995;33:197–201.
Pengpong T, Sangvanich P, Sirilertmukul K, Muangsin N. Design, synthesis and in vitro evaluation of mucoadhesive p-coumarate-thiolated-chitosan as a hydrophobic drug carriers. Eur J Pharm Biopharm. 2014;86:487–97.
Cao QR, Liu Y, Xu WJ, Lee BJ, Yang M, Cui JH. Enhanced oral bioavailability of novel mucoadhesive pellets containing valsartan prepared by a dry powder-coating technique. Int J Pharm. 2012;434:325–33.
Bautzová T, Rabišková M, Béduneau A, Pellequer Y, Lamprecht A. Bioadhesive pellets increase local 5-aminosalicylic acid concentration in experimental colitis. Eur J Pharm Biopharm. 2012;81:379–85.
Li C, Bhatt PP, Johnston TP. Evaluation of a mucoadhesive buccal patch for delivery of peptides: in vitro screening of bioadhesion. Drug Dev Ind Pharm. 1998;24:919–26.
Nidhi, Rashid M, Kaur V, Hallan SS, Sharma S, Mishra N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: a brief review. Saudi Pharm J. 2016;24:458–72.
He P, Davis SS, Illum L. In vitro evaluation of the mucoadhesive properties of chitosan microspheres. Int J Pharm. 1998;166:75–88.
Senturk DS. Size dependent properties of hollow gold nanoparticles: a theoretical investigation. Acta Phys Pol A. 2016;129:531–4.
Khan MZI, Štedul HP, Kurjaković N. A pH-dependent colon-targeted oral drug delivery system using methacrylic acid copolymers. II. Manipulation of drug release using Eudragit® L100 and Eudragit S100 combinations. Drug Dev Ind Pharm. 2000;26:549–54.
Xu M, Sun M, Qiao H, Ping Q, Elamin ES. Preparation and evaluation of colon adhesive pellets of 5-aminosalicylic acid. Int J Pharm. 2014;468:165–71.
Akiyama Y, Nagahara N, Kashihara T, Hirai S, Toguchi H. In vitro and in vivo evaluation of mucoadhesive microspheres prepared for the gastrointestinal tract using polyglycerol esters of fatty acids and a poly(acrylic acid) derivative. Pharm Res. 1995;12:397–405.
Dar MJ, Ali H, Khan A, Khan GM. Polymer-based drug delivery: the quest for local targeting of inflamed intestinal mucosa. J Drug Target. 2017:582–96.
Ali T, Shakir F, Morton J. Curcumin and inflammatory bowel disease: biological mechanisms and clinical implication. Digestion. 2012;85:249–55.
Fakhoury M, Negrulj R, Mooranian A, Al-Salami H. Inflammatory bowel disease: clinical aspects and treatments. J Inflamm Res. 2014:113–20.
Jurenka JS. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern Med Rev. 2009;14:141–53.
Sesarman A, Tefas L, Sylvester B, Licarete E, Rauca V, Luput L, et al. Co-delivery of curcumin and doxorubicin in PEGylated liposomes favored the antineoplastic C26 murine colon carcinoma microenvironment. Drug Deliv Transl Res. 2019;9:260–72.
Tirkey N, Kaur G, Vij G, Chopra K. Curcumin, a diferuloylmethane, attenuates cyclosporine-induced renal dysfunction and oxidative stress in rat kidneys. BMC Pharmacol. 2005;5:1–10.
Rezaei H, Lim CJ, Lau A, Sokhansanj S. Size, shape and flow characterization of ground wood chip and ground wood pellet particles. Powder Technol. 2016;301:737–46.
Heng PWS, Wong TW, Chan LW. Influence of production variables on the sphericity of melt pellets. Chem Pharm Bull. 2000;48:420–4.
Carvalho FC, Bruschi ML, Evangelista RC, Gremião MPD. Mucoadhesive drug delivery systems. Braz J Pharm Sci. 2010;46:1–17.
Nugent SG, Kumar D, Rampton DS, Evans DF. Intestinal luminal pH in inflammatory bowel disease: possible determinants and implications for therapy with aminosalicylates and other drugs. Gut. 2001:571–7.
Sawarkar SP, Deshpande SG, Bajaj AN, Nikam VS. I n vivo evaluation of 5-ASA colon-specific tablets using experimental-induced colitis rat animal model. AAPS PharmSciTech. 2015;16:1445–54.
Kedia S, Bhatia V, Thareja S, Garg S, Mouli VP, Bopanna S, et al. Low dose oral curcumin is not effective in induction of remission in mild to moderate ulcerative colitis: results from a randomized double blind placebo controlled trial. World J Gastrointest Pharmacol Ther. 2017;8:147.
Tiwari R, Agarwal SK, Tiwari S. Formulation and multivariate optimization of microcrystalline cellulose pellets of highly water soluble drug. Int J Drug Deliv. 2013;5:206–13.
Inoue T, Osaki K. Role of polymer chain flexibility on the viscoelasticity of amorphous polymers around the glass transition zone. Macromolecules. 1996;29:1595–9.
Baishya H. Application of mathematical models in drug release kinetics of carbidopa and levodopa ER tablets. J Dev Drugs. 2017;06:1–8.
Ekström GM. Oxazolone-induced colitis in rats: effects of budesonide, cyclosporin A, and 5-aminosalicylic acid. Scand J Gastroenterol. 1998;33:174–9.
Brumatti LV, Marcuzzi A, Tricarico PM, Zanin V, Girardelli M, Bianco AM. Curcumin and inflammatory bowel disease: potential andlimits of innovative treatments. Molecules. 2014;19:21127–53.
Feldman M, Cryer B. The New England Journal of Medicine Downloaded from nejm.org at CASE WESTERN RESERVE UNIVERSITY on November 14, 2013. For personal use only. No other uses without permission. Copyright © 1994 Massachusetts Medical Society. All rights reserved. Am J Cardiol. 2013;84:404–9.
Sandborn WJ. A critical review of cyclosporine therapy in inflammatory bowel disease. Inflamm Bowel Dis. 1995;1:48–63.
Sandborn W, Tremaine W. Cyclosporine treatment of inflammatory bowel disease. Mayo Clin Proc. 1992;67:981–90.
Malmary MF, Houti I, Labat C, Batalla A, Moussamih S, Bouguettaya D, et al. Chronopharmacokinetics of cyclosporine A following a single i.v. dose in the Wistar rat. Eur J Pharm Sci. 1995;3:49–56.
Bachrach W. Medical treatment of ulcerative colitis. Clin Symp. 1955;7:103–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics statement
All institutional and national guidelines for the care and use of laboratory animals were followed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Desai, N., Momin, M. Colon targeted bioadhesive pellets of curcumin and cyclosporine for improved management of inflammatory bowel disease. Drug Deliv. and Transl. Res. 10, 1288–1301 (2020). https://doi.org/10.1007/s13346-020-00756-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-020-00756-x