Abstract
To test whether sulpiride (SP), an anti-psychotic and prokinetic drug, shows beneficial effects on experimental murine colitis, a colon-targeted prodrug of SP, 5-(aminoethanoylsulfamoyl)-N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxybenzamide (glycylsulpiride (GSP)), was synthesized and its colonic delivery and therapeutic activity against 2,4-dinitrobenzenesulfonic acid (DNBS)–induced rat colitis were assessed. Synthesis of GSP was verified by infrared and proton nuclear magnetic resonance spectroscopy. GSP was converted to SP when incubated with the cecal contents but not when incubated with the small intestinal contents. The percent conversion was about 50.5% at 6 h and 67.7% at 10 h. Colonic delivery of GSP was examined by comparison with sulfasalazine (SSZ), a colon-specific prodrug of 5-aminosalicylic acid currently used for the treatment of inflammatory bowel disease. The two prodrugs accumulated similar concentrations of the corresponding parent drugs in the cecum at 2, 4, and 6 h after oral gavage. Although oral gavage of GSP released millimolar level of SP in the large intestine, SP was hardly detected in the blood. GSP improved colonic damage score and reduced myeloperoxidase activity up to 80.5% in the inflamed colon in a dose-dependent manner. Moreover, GSP was able to reduce the levels of inflammatory mediators in the inflamed colon. Overall, the anti-colitic effectiveness of GSP and SSZ was similar. In conclusion, colonic delivery of SP ameliorates DNBS-induced colitis in rats with no significant systemic absorption of SP. Thus, colon-targeted SP may be therapeutically switched to an anti-colitic drug.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Inflammatory bowel disease (IBD), comprising ulcerative colitis (UC) and Crohn’s disease (CD), is an idiopathic and chronic inflammatory disorder in the gastrointestinal tract, mainly the large intestine [1, 2]. Although the exact molecular etiology remains to be elucidated, IBD is caused by dysregulated immune responses to host intestinal microflora, leading to destructive inflammatory reactions [3, 4]. For the treatment of IBD, various therapeutic agents including steroids, aminosalicylates, immunosuppressants, and biologics have been developed [4, 5].
Colon-specific drug delivery is a pharmaceutical strategy for the efficient treatment of colonic diseases such as UC and CD [6, 7]. In general, colonic delivery of a drug, achieved by pharmaceutical formulation and prodrug approach, increases colonic concentration and decreases systemic absorption of the drug, thus increasing therapeutic benefits and decreasing adverse effects of the drug [7,8,9]. For this reason, the delivery technique is adapted for the development of anti-colitic therapeutic agents [7]. Sulfasalazine (SSZ) is a representative colon-specific prodrug of 5-aminosalicylic acid (5-ASA), an anti-inflammatory agent applicable to the treatment of IBD. Although sulfasalazine is widely used in clinics, its side effects caused by sulfapyridine, the colon-specific carrier of the prodrug, limits its clinical use. In addition, SSZ is not effective for severe IBD [10, 11]. Therefore, development of alternative anti-colitic agents with improved efficacy and safety is warranted.
Recently, it is suggested that colon-specific drug delivery is a promising strategy for therapeutic switching, also known as drug repurposing, repositioning, and reprofiling, of a drug to anti-colitic therapeutics [12]. Major reasons limiting therapeutic switching of a drug include the following: drug dose for switched therapeutic effects can be higher, likely causing side effects that are not elicited otherwise, and a pharmacological effect of the drug becomes a side effect upon therapeutic switching. Colon-specific drug delivery is thought to efficiently tackle these obstacles, considering therapeutic and toxicological benefits afforded by the delivery technique [7].
Sulpiride (SP) is a selective dopamine D2 and D3 antagonist therapeutically used for the treatment of schizophrenia, neurosis, and depression [13]. The drug with anti-psychotic and anti-depressant activity is also classified as a prokinetic drug promoting gastrointestinal mobility [14]. SP alone or in combination with gastric acid regulators elicits anti-gastric and anti-duodenal ulcer effects probably by improving blood flow to the gastroduodenal mucosa and enhancing mucus secretion, which may be relevant to anti-colitic effects of dopamine antagonists [15, 16]. A recent report indicating that SP is effective in resolving chronic tongue ulcers further supports the feasibility of using the anti-psychotic drug to the treatment of various diseases such as colitis where mucosal ulcer formation is one of the pathological features [17, 18].
Amino acid conjugation with a drug is a typical strategy to design a colon-specific prodrug of the drug [7]. Amino acids such as glycine and aspartic acid are safe and increase the hydrophilicity of drugs conjugated with them. In addition, amide and carbonylaminosulfone bonds, formed by amino acid conjugation with drugs with a carboxylic acid and a sulfonamide group, are susceptible to colonic microbial enzymes while stable to host digestive enzymes [19]. These properties of amino acids are necessary and sufficient to make them suitable colon-specific carriers [7]. In this study, it was investigated whether colonic delivery of SP is a feasible way to therapeutically switch SP to an anti-colitic drug. 5-(Aminoethanoylsulfamoyl)-N-[(1-ethylpyrrolidin-2-yl)methyl]-2-methoxybenzamide, glycine-conjugated sulpiride (glycylsulpiride (GSP)), was synthesized as a prodrug to deliver SP to the large intestine. Colon specificity of GSP along with restricted systemic absorption of SP was tested in vitro/in vivo and its therapeutic activity against colitis was evaluated in a 2,4-dinitrobenzenesulfonic acid (DNBS)–induced rat colitis model.
Materials and methods
Materials
SP and N-(tert-butoxycarbonyl)glycine (N-(t-Boc)glycine), 1,1′-carbonyldiimidazole (CDI) were purchased from Tokyo Kasei Kogyo Co. (Tokyo, Japan). Trifluoroacetic acid, SSZ, and DNBS were purchased from Sigma Chemical Co. Inc. (St. Louis, MO, USA). Reaction solvents were obtained from Junsei Chemical Co. (Tokyo, Japan). ELISA kits were obtained from R & D Systems (Minneapolis, MN, USA). All other chemicals were of reagent grade, commercially available products.
Synthesis of GSP
N-(t-Boc)Glycine (2 mmol) was dissolved in 15 mL of acetonitrile followed by the addition of CDI (2.4 mmol) and triethylamine (TEA, 1 mmol) and stirred for 1 h at room temperature. SP (1 mmol) was added to the reaction mixture and then stirred at room temperature for 48 h. After the removal of acetonitrile by evaporation, the residue was dissolved in dichloromethane (30 mL), washed with 5% NaHCO3, and dried over Na2SO4, which was subjected to flash evaporation to obtain N-(t-Boc)glycylsulpiride. N-(t-Boc)Glycylsulpiride was treated with trifluoroacetic acid/dichloromethane (1:1) at room temperature for 1.5 h. After evaporation, the residue was dissolved in 5% NaHCO3 (15 mL) followed by the addition of dichloromethane (30 mL), and the organic layer was isolated and dried with anhydrous Na2SO4 and evaporated. The residue was dried in an oven for 24 h to obtain the final product GSP. IR spectra were recorded on a Varian FT-IR spectrophotometer (Varian, Palo Alto, CA, USA). 1H-NMR spectra were obtained by using a Varian AS 500 spectrometer and the chemical shifts are reported in parts per million downfield from tetramethylsilane. Results are as follows: yield 50%; mp 200–203 °C; IR (nujol mull), νmax (cm−1) 1633 (C=O, CONH), 1732 (C=O, SO2CONH); 1H-NMR (DMSO-d6) δ = 1.19 (t, 3H, J = 8 Hz), 1.57–1.90 (m, 4H), 1.77–2.06 (m, 2H), 2.84 (m, 2H), 3.18–3.52 (m, 2H), 3.34 (m, 1H), 3.41–3.61 (m, 2H), 3.91 (s, 3H), 7.33(d, 1H, J = 9 Hz), 8.01 (dd, 1H, J = 9 Hz, J = 2.5), 8.27 (d, 1H, J = 2.5), 8.71 (s, 1H), 10.02 (s, 1H).
Apparent partition coefficient and chemical stability
To a solution of GSP (10 mL, 25 μM) in pH 7.4 isotonic phosphate buffer pre-saturated with 1-octanol, 1-octanol which was pre-saturated with pH 7.4 isotonic phosphate buffer (10 mL) was added. The mixture was shaken for 12 h and then left to stand for 3 h for phase separation at room temperature. The concentration of GSP in the aqueous phase was determined by using a UV spectrophotometer. The apparent partition coefficients were calculated by employing the equation (Co − Cw) ∕ Cw, where Co and Cw represent the initial and equilibrium concentrations of the drug in the aqueous phase, respectively (Nakamura et al. 1992). The same experiment was performed with SP.
GSP was placed in pH 1.2 hydrochloric acid buffer or in pH 6.8 isotonic phosphate buffer (500 μM, USP XXIII) and was incubated at 37 °C for 10 h. At a predetermined time interval, a 20-μL portion of each solution was removed and the concentrations of drugs were analyzed by using HPLC.
HPLC analysis
The HPLC system consisted of a model 306 pump, a 151 variable UV detector, and a model 234 autoinjector from Gilson (Middleton, WI, USA). A Symmetry R18 column (Waters, Milford, MA, USA; 250 × 4.6 mm, 5 μm) with a guard column (Waters, 20 × 4.6 mm) was used. Samples prepared from each experiment were filtered through a membrane filter (0.45 μm). HPLC analysis was conducted at a flow rate of 1 mL/min using a mobile phase comprising acetonitrile and 10 mM pH 4.0 phosphate buffer (1.5:8.5, v/v). The eluate was monitored at 323 nm (for 5-ASA) and 260 nm (for SP and GSP) by a UV detector measuring the absorption with a sensitivity of AUFS 0.01. The retention times of 5-ASA, SP, and GSP were 7.1, 6.5, and 3.5 min, respectively.
Incubation of GSP with the contents of the small intestine and the cecum
A male SD rat (250–260 g) was sacrificed by CO2 and a midline incision was made. The contents of the proximal small intestine, the distal small intestine, and the cecum were collected separately and were suspended in pH 6.8 isotonic phosphate buffer to prepare a 20% suspension. The cecal contents were collected under nitrogen in a glove bag (Sigma). To microcentrifuge tubes, either GSP or SSZ in pH 6.8 isotonic phosphate buffer (0.5 mL, 1 mM) was added to the 20% (w/v) suspension (0.5 mL) and incubated at 37 °C under nitrogen (for the cecal contents). At appropriate time intervals, the samples were centrifuged at 10,000×g for 5 min. To 0.1-mL portions of the supernatants, 0.9 mL of methanol was added, followed by vortexing and then centrifuging at 20,000×g at 4 °C for 10 min. The concentrations of 5-ASA and SP in the supernatants were determined by using HPLC.
Animals
Seven-week-old male SD rats were purchased from Samtako Bio Korea (Kyeong-gi-do, South Korea) and housed in the animal care facility at Pusan National University, Busan, South Korea. Rats were grouped as follows: normal, DNBS control, and DNBS/drug-treated groups. Each group consisted of five rats. The SD rats were housed in the university animal facility at controlled temperature, humidity, and light conditions. The animal protocol used in this study was reviewed and approved by the Pusan National University—Institutional Animal Care and Use Committee (Approval number: PNU-2017-1525; Approval date: 2017-04-19) for ethical procedures and scientific care. All institutional and national guidelines for the care and use of laboratory animals were followed.
Induction of colitis
Colitis was induced by the method reported previously [20, 21]. Briefly, before induction of colitis, rats were starved for 24 h but had free access to water. The rats were lightly anesthetized with isoflurane. A rubber cannula (2 mm O.D.) was inserted rectally into the colon such that the tip was 8 cm proximal to the anus, approximately at the splenic flexure. DNBS dissolved in 50% (v/v) aqueous ethanol was instilled into the colon via the rubber cannula (35 mg/0.35 mL/rat).
Anti-colitic effects of drugs
To evaluate anti-colitic effects of drugs, each drug was suspended in 1 mL of phosphate-buffered saline (PBS, pH 7.4) and was administered via oral gavage using an oral zonde (Jungdo-BNP, Seoul, South Korea) and rats were divided into five groups where each group consisted of five rats and treated as follows: group 1 (normal group): oral gavage of 1 mL of PBS; group 2 (colitis group): oral gavage of 1 mL of PBS; group 3 (GSP20 group): oral gavage of GSP (20 mg/kg); group 4 (GSP40 group): oral gavage of GSP (40 mg/kg); group 5 (SSZ group): oral gavage of SSZ (30 mg/kg). In an independent experiment, the above experiment was performed with three groups: group 1 (normal): oral gavage of 1 mL of PBS; group 2 (colitis group): oral gavage of 1 mL of PBS; group 3 (SP group): oral gavage of SP (34.1 mg/kg). For evaluation of anti-colitic effects after rectal administration of drugs, SP (30 mM) or glycine (30 mM) suspended in pH 7.4 PBS (0.5 mL) was administered rectally to rats using a rubber cannula (2 mm O.D.). Rats were divided into five groups where each group consisted of five rats and treated as follows: group 1 (normal group): 0.5 mL of PBS; group 2 (colitis group): 0.5 mL of PBS; group 3 (SP group): 30 mL SP in 0.5 mL PBS; group 4 (glycine group): 30 mM glycine in 0.5 mL PBS; group 5 (SP plus glycine group): 30 mM SP plus 30 mM glycine in 0.5 mM PBS. Each drug was administered to rats once a day 72 h after induction of colitis and the rats were euthanized by CO2 after receiving the treatment for 7 days. Gross colonic damage score (CDS) was calculated according to the criteria set previously [20, 22]. The modified scoring system is as follows: 0, normal appearance; 1, localized hyperemia but no ulcer; 2, linear ulcers without significant inflammation; 3, 2- to 4-cm site of inflammation and ulceration; 4, serosal adhesion to other organs, 2- to 4-cm site of inflammation and ulceration; 5, stricture, serosal adhesion involving several bowel loops, < 4-cm site of inflammation and ulceration. Four independent observers blinded to the treatment carried out the CDS assessment. Using the distal colon (4 cm), myeloperoxidase (MPO) activity was measured as described previously [20]. One unit of MPO activity is defined as that degrading 1 μmol of peroxide per minute at 25 °C.
Plasma concentration of SP
Male SD rats were starved for 24 h, except for water. SP (150 mg/kg) or GSP (175 mg/kg) in PBS (1.0 mL) was administered by gavage. Blood samples were collected from the tail veins at appropriate time intervals and centrifuged at 4000×g for 10 min. To 0.1-mL portions of separated plasma, 1 M NaOH (0.01 mL) was added followed by the addition of ethyl acetate/dichloromethane (5:1, 0.3 mL), which was subjected to centrifugation at 4000 rpm for 5 min. The organic layers were isolated and evaporated and were dissolved in the mobile phase (0.1 mL). The concentration of SP in a 20-μL portion was determined by using HPLC [23].
Western blotting
To prepare tissue lysates of the inflamed distal colon, tissues (1 g) were disrupted and homogenized in 3 mL of ice cold RIPA buffer [50 mM Tris-HCl (pH 7.4), 1 mM EDTA, 0.7% Na deoxycholate, 1% NP-40, 150 mM NaCl, 0.3 μM aprotinin, 1 μM pepstatin, and 1 mM PMSF]. After incubation on ice for 30 min, the homogenates were centrifuged at 10,000×g at 4 °C for 10 min. Protein concentrations in the supernatants were determined using the BCA method. Tissue extracts were electrophoretically separated using 7.5 or 10% SDS-PAGE. COX-2 and iNOS proteins in the tissue homogenates were detected using a monoclonal anti-COX-2 antibody and an anti-iNOS (NOS-2) antibody (Santa Cruz Biotechnology). For cellular iNOS and COX-2 proteins, the proteins were detected in whole cell lysates obtained by using RIPA buffer. Signals were visualized using the Supersignal chemiluminescence substrate (Pierce, Rockford, IL, USA). Experiments were performed in duplicate and normalized with antibodies to α-tubulin or β-actin (Santa Cruz Biotechnology).
Cytokine-induced neutrophil chemoattractant-3 immunoassay
To measure cytokine-induced neutrophil chemoattractant-3 (CINC-3) in the inflamed tissues, the inflamed distal colon was homogenized in pH 6 potassium phosphate buffer, which was centrifuged at 10,000×g at 4 °C for 10 min. An appropriate volume of the supernatants was subjected to CINC-3 ELISA.
Data analysis
The results are expressed as mean ± standard error of the mean (SEM). One-way ANOVA followed by Tukey’s HSD test or the Mann-Whitney U test (for CDS) was used to test the difference between the data. Differences with α or P < 0.05 were considered significant.
Results
Oral gavage of GSP delivers SP to the large intestine of rats
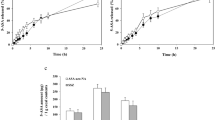
GSP was synthesized as shown in Fig. 1. Synthesis of GSP was verified by IR and 1H-NMR. In the IR spectra, a stretching band of the carbonyl group formed by the reaction between the carboxylic acid in glycine and the sulfonamide in SP was observed at 1732 cm−1 while the carbonyl stretching band in glycine was detected at 1604 cm−1. This band shift is ascribed to losing the zwitterionic property of glycine and coupling to the sulfonyl group in SP. 1H-NMR signals originated from the methylene in glycine were monitored as a multiplet between 3.4 and 3.6 ppm along with signals derived from SP without substantial change in chemical shift. We examined whether GSP could deliver SP to the large intestine in vitro and in vivo. The partition coefficient (PC) was measured as − 0.407 in an n-octanol/pH 6.8 buffer system. Glycine conjugation substantially lowered the PC of SP from 0.477 to − 0.407, indicating that the prodrug is more hydrophilic. When the prodrug was incubated with the small intestinal contents, and pH 1.2 and pH 6.8 buffers, SP was not detected in the media; moreover, there was no change in the level of the prodrug. On the contrary, GSP was converted to SP in the cecal contents, and the percent cleavage of the prodrug was about 50.5% at 6 h and 67.7% at 10 h (Fig. 2a). The conversion rate of GSP to SP was compared with SSZ (a colon-specific prodrug of 5-ASA). As shown in Fig. 2b, the conversion rates were similar. To further examine the delivery property, SP was detected in the cecum at appropriate time intervals after gavage of GSP. For comparison, the same experiment was performed with SSZ at an equimolar concentration. Since 5-ASA is metabolized to N-acetyl-5-ASA (NA-5-ASA) in the large intestinal tissue [11], NA-5-ASA was also measured in the cecum. As shown in Fig. 2c, 5-ASA and SP began to be detected in the cecum at 2 h after gavage of the prodrugs. Compared with 5-ASA released from SSZ, similar concentrations of SP were detected at 2, 4, and 6 h after gavage. NA-5-ASA was not detected under our experimental condition. Colonic delivery of a drug tends to reduce the systemic side effects by decreasing its systemic absorption [7]. To examine this, SP was monitored in the blood after oral gavage of GSP. For comparison, the same experiment was performed with SP. As shown in Fig. 2d, although substantial amount of SP was accumulated in the cecum after oral gavage of GSP, SP was barely detected in the blood. However, SP was detected up to about 28 μM after SP administration. These results suggest that GSP limits the systemic absorption of SP, thus likely minimizing unwanted effects owing to SP absorbed systemically.
GSP is colon-specific and limits systemic absorption of SP. a Glycylsulpiride (5 mM, GSP) was incubated with cecal and small intestinal contents suspended in pH 6.8 PBS (10%). At appropriate time intervals, concentration of sulpiride (SP) in the samples was determined by HPLC. b For comparison, incubation with 10% cecal contents was repeated with sulfasalazine (2 mM, SSZ). Male SD rats (250–260 g) were starved for 24 h but were provided water. c SSZ (30 mg/kg) or GSP (29 mg/kg, molar equivalent to 30 mg of SSZ) suspended in pH 7.4 PBS (1 mL) was administered to rats by oral gavage. The rats were sacrificed 2, 4, and 6 h after oral gavage and a midline incision was made to obtain the cecum. SP and 5-aminosalicylic acid (5-ASA) in the cecal contents were analyzed by HPLC. Drug concentrations are presented as mmol/kg cecal contents. d SP (150 mg/kg) or GSP (175 mg/kg, equivalent to 150 mg of SP) was administered to rats by oral gavage and blood was collected at predetermined intervals from the tail veins. SP in the blood was analyzed by HPLC. The data in a, b, c, and d represent mean ± SEM (n = 5)
Oral gavage of GSP, but not SP, improves CDS and reduces MPO activity in the inflamed colons
We next examined whether GSP is pharmacologically active against colitis. To assess this, GSP was administered via oral gavage once a day, 3 days after colitis induction by DNBS. The doses of GSP used were 20 mg/kg (equivalent to 17 mg/kg of SP) and 40 mg/kg (equivalent to 34.1 mg/kg of SP), which was determined based on anti-psychotic doses of SP [24]. The same experiment was performed with SSZ. The CDS and the MPO activity were determined after treatment with the prodrugs for 7 days. Photos of the distal colons are shown in Supplementary Data 1; GSP obviously alleviated colonic damage by DNBS-induced inflammation in a dose-dependent manner and was as effective as SSZ even at a low dose of GSP. The extent of colonic damage by DNBS-induced inflammation was scored according to the criteria set previously [20, 22]. As shown in Fig. 3a, while the DNBS control colon was severely damaged showing hemorrhagic destruction of the mucosa, stricture, and extensive serosal adhesion to other organs, GSP significantly alleviated the colonic injury, lowering the CDS, and no significant difference in therapeutic effect was observed between the GSP-treated and SSZ-treated groups. In parallel, Fig. 3b showed that GSP lowered MPO activity up to 70.3% (at 20 mg/kg) and 80.5% (at 40 mg/kg) of the DNBS control, which is close to the normal MPO level. SSZ reduced MPO activity to about 72%. We examined whether the anti-colitic effects of GSP were relevant to colonic delivery of SP; the experiments to assess anti-colitic effects were repeated with SP. As shown in Supplementary Data 2 (photos of the distal colons) and Fig. 3c (CDS), d (MPO), no significant anti-colitic effects were observed upon oral gavage of SP.
Oral gavage of GSP but not that of SP ameliorates rat colitis. a, b Glycylsulpiride (GSP, 20 and 40 mg/kg) or sulfasalazine (SSZ, 30 mg/kg) was administered to 2,4-dinitrobenzenesulfonic acid hydrate (DNBS)–induced colitis rats by oral gavage once a day and the rats were sacrificed 7 days after treatment with the drugs. a Colonic damage scores (CDS) were assigned for each group. *α < 0.05 vs. DNBS control. b Using the distal colon (4 cm), myeloperoxidase (MPO) activities were measured. *P < 0.05 vs. DNBS control. c, d Sulpiride (SP, 34.1 mg/kg, equivalent to 40 mg/kg GSP) was treated as in a followed by euthanasia of rats. c CDS were assigned for each group. *α < 0.05 vs. DNBS control. d Using the distal colon (4 cm), MPO activities were measured. *P < 0.05 vs. DNBS control. The data in a, b, c, and d represent mean ± SEM (n = 5)
GSP, but not SP, decreases the levels of inflammatory mediators in the inflamed colons
To further scrutinize anti-colitic effects, the levels of inflammatory mediators were measured in the inflamed colon after oral gavage of GSP and SSZ. As shown in Fig. 4a, b, consistent with MPO and CDS results obtained after oral gavage of drugs, GSP effectively decreased levels of inflammatory mediators, iNOS, COX-2 (Fig. 4c), and CINC-3 (Fig. 4d); this result was comparable to that obtained for SSZ. In addition, oral gavage of SP did not exhibit significant effects on the inflammatory mediators (Fig. 4c, d).
Oral gavage of GSP but not that of SP decreases levels of inflammatory mediators in the inflamed colons. a, b Glycylsulpiride (GSP, 20 and 40 mg/kg) or sulfasalazine (SSZ, 30 mg/kg) was administered to 2,4-dinitrobenzenesulfonic acid hydrate (DNBS)–induced colitis rats by oral gavage once a day and the rats were sacrificed 7 days after treatment with the drugs. Levels of inflammatory mediators, cyclooxygenase (COX)-2, and inducible nitric oxide synthase (iNOS) (a) and cytokine-induced neutrophil chemoattractant (CINC)-3 (b) were determined in the inflamed distal colons. The Western blot is representative of three independent experiments. c, d Sulpiride (SP, 34.1 mg/kg, equivalent to 40 mg/kg GSP) was treated as in a followed by euthanasia of rats. Levels of inflammatory mediators, COX-2 and iNOS (c) and CINC-3( d), were determined in the inflamed distal colons. The data in b and d represent mean ± SEM (n = 5). *P < 0.05 vs. DNBS control
Anti-colitic effects of GSP are primarily ascribed to SP released from the prodrug
Our data show that oral gavage of GSP, but not SP, is effective, suggesting that colonic delivery of SP is associated with the anti-colitic effects. To verify this argument, SP was administered rectally, mimicking the therapeutic situation by colonic delivery of SP, to colitic rats. Since GSP is converted to SP and glycine in the large intestine and glycine is reported to have an anti-inflammatory activity [19], the same experiment was performed with glycine and glycine plus SP. As shown in Supplementary Data 3 (photos of the distal colons) and Fig. 5a (CDS), b (MPO), c (COX-2 and iNOS), d (CINC-3), SP substantially improved all the inflammatory indices in the inflamed colons while glycine showed mixed effects. Glycine neither alleviated CDS nor lowered MPO activity significantly. At molecular levels, glycine weakly attenuated the levels of CINC-3 and iNOS while COX-2 level was lowered substantially. However, the anti-inflammatory effects of the combined treatment with SP and glycine were not significantly different from those of SP alone.
The anti-colitic effects of GSP are primarily ascribed to SP released from the prodrug. Sulpiride (SP, 30 mM) and/or glycine (Gly, 30 mM) in pH 7.4 PBS buffer (500 μL) were administered rectally to 2,4-dinitrobenzenesulfonic acid hydrate (DNBS)–induced colitis rats once a day and the rats were sacrificed 7 days after treatment with the drug. a Colonic damage scores (CDS) were assigned for each group. *α < 0.05 vs. DNBS control. b Using the distal colon (4 cm), myeloperoxidase (MPO) activities were measured. *P < 0.05 vs. DNBS control, n.s. not significant. Levels of inflammatory mediators, cyclooxygenase (COX)-2, and inducible nitric oxide synthase (iNOS) (c) and cytokine-induced neutrophil chemoattractant (CINC)-3 (d) were determined in the inflamed distal colons. The Western blot in c is representative of three independent experiments. The data in a, b, and d represent mean ± SEM (n = 5). *P < 0.05 vs. DNBS control, n.s. not significant
Discussion
We examined whether SP, an antagonist of dopamine receptors used for the treatment of psychosis and depression, elicited beneficial effects in the treatment of colonic inflammation. For this purpose, GSP was synthesized to deliver SP to the large intestine, expecting therapeutic advantages by colonic delivery such as efficient treatment of colonic inflammation and reduced systemic side effects [7]. GSP delivered SP to the large intestine without significant systemic absorption of SP and exerted anti-colitic effects in a DNBS-induced rat colitis model; these effects were comparable to those obtained for SSZ, a colon-specific 5-ASA prodrug currently being used in clinics.
After oral gavage of GSP and SSZ, SP and 5-ASA were detected in the cecum, whose concentrations were found to be similar at 2, 4, and 6 h. This observation is in line with in vitro data showing that conversion of GSP to SP occurred only in the cecal contents, the rate of which was comparable to that of conversion of SSZ to 5-ASA. Along with low PC and chemical stability of GSP, the above data suggest that GSP traverses the stomach and the small intestine without significant biochemical loss, and the colonic delivery efficiency of GSP is equivalent to that of SSZ. Our data showing that N-acetyl-5-ASA, a large intestinal metabolite of 5-ASA [11], was not detected in the cecum validate the direct comparison of 5-ASA with SP in the cecum as a way to assess colonic delivery efficiencies of SSZ and GSP.
GSP is very likely to limit the systemic absorption of SP. This is indicated by the fact that SP was barely detected in the blood after oral gavage of GSP while oral gavage of SP afforded about 28 μM SP in the blood. SP administered orally is mostly absorbed systemically in the stomach and small intestine and its bioavailability is 30% [25]. This pharmacokinetic property of SP verifies that the limited systemic absorption of SP is ascribed to the colonic delivery of the drug. Since SP is an anti-psychotic agent and can cause systemic side effects including extrapyramidal syndromes and hyperprolactinemia [26], limiting its systemic absorption is an important issue in the therapeutic switching of SP to an anti-colitic agent. In addition, considering that long-term SSZ therapy can elicit serious side effects, such as agranulocytosis and hypospermia, which is caused by systemic absorption of sulfapyridine produced from SSZ in the large intestine [10], limited systemic absorption strengthens the therapeutic advantage of GSP as an anti-colitic agent.
GSP is effective in attenuating DNBS-induced rat colitis. This was indicated by the data showing that GSP improved colonic damage and reduced MPO activity in the inflamed colons. Moreover, GSP effectively decreased inflammatory mediators in the inflamed colons. Overall anti-colitic effects of GSP were comparable with those of SSZ. For now, it is not clear whether anti-colitic effects of GSP are mediated by blocking dopamine receptors in the inflamed colon as oral gavage of SP at 34.1 mg/kg (equivalent to 40 mg/kg of GSP), sufficient to exert anti-dopaminergic effects [13], did not exhibit significant anti-colitic effects. Given that colonic delivery of a drug tends to achieve much greater concentration in the colon than conventional dosage forms of the drug [7], GSP delivers an SP content sufficient to antagonize dopamine receptors in the inflamed tissues. Therefore, the possibility that the duration of antagonistic action and SP concentration acting on dopamine receptors may be different between colonic delivery and conventional delivery of SP, which results in therapeutic differences in experimental colitis, cannot be excluded. It is also possible that SP may exert anti-inflammatory effects via a pharmacological mechanism other than dopamine receptor blockage, given that colonic concentration of SP can reach millimolar level, which cannot be achieved with conventional delivery of SP.
Our data showing that rectal glycine lowered the levels of inflammatory mediators, especially COX-2, suggest that glycine (delivered to the large intestine along with SP) may contribute to the anti-colitic effects of GSP as suggested in a previous paper [19]. However, it is likely that the therapeutic contribution of SP is much greater than that of glycine (in the anti-colitic effects of GSP) in our experimental conditions. Our data showed that rectal glycine was not significantly effective (except for lowering COX-2) and combined rectal treatment with SP and glycine showed no significant difference in anti-colitic effects compared with that obtained with SP alone. Further investigation is required to reveal anti-colitic pharmacology of SP delivered to the large intestine.
Although GSP is not significantly superior to SSZ in anti-colitic efficacy, it may still have advantage over SSZ in the treatment of IBD, which shares symptoms with irritable bowel syndrome [27]. SP can soothe the irritable colon, thus relieving abdominalgia [28, 29].
Collectively, GSP, delivering SP specifically to the large intestine, effectively ameliorates DNBS-induced rat colitis with limited systemic absorption of SP. Thus, colon-targeted SP may be therapeutically switched to an anti-colitic drug.
References
Podolsky DK. Inflammatory bowel disease. N Engl J Med. 2002;347(6):417–29. https://doi.org/10.1056/NEJMra020831.
Bielefeldt K, Davis B, Binion DG. Pain and inflammatory bowel disease. Inflamm Bowel Dis. 2009;15(5):778–88. https://doi.org/10.1002/ibd.20848.
Hugot JP, Zouali H, Lesage S, Thomas G. Etiology of the inflammatory bowel diseases. Int J Color Dis. 1999;14(1):2–9.
Ko JK, Auyeung KK. Inflammatory bowel disease: etiology, pathogenesis and current therapy. Curr Pharm Des. 2014;20(7):1082–96.
Lofberg R. Review article: medical treatment of mild to moderately active Crohn’s disease. Aliment Pharmacol Ther. 2003;17(Suppl 2):18–22.
Lee Y, Kim H, Kim W, Yoon JH, Jeong SH, Jung Y. Colon-specific delivery of celecoxib is a potential strategy to improve toxicological and pharmacological properties of the selective Cox-2 inhibitor: implication in treatment of familiar adenomatous polyposis. J Drug Target. 2012;20(6):524–34. https://doi.org/10.3109/1061186X.2012.693498.
Jung Y, Kim YM. What should be considered on design of a colon-specific prodrug? Expert Opin Drug Deliv. 2010;7(2):245–58. https://doi.org/10.1517/17425240903490401.
Sinha VR, Kumria R. Colonic drug delivery: prodrug approach. Pharm Res. 2001;18(5):557–64.
Chourasia MK, Jain SK. Pharmaceutical approaches to colon targeted drug delivery systems. J Pharm Pharm Sci. 2003;6(1):33–66.
Peppercorn MA. Sulfasalazine. Pharmacology, clinical use, toxicity, and related new drug development. Ann Intern Med. 1984;101(3):377–86.
Klotz U, Maier K, Fischer C, Heinkel K. Therapeutic efficacy of sulfasalazine and its metabolites in patients with ulcerative colitis and Crohn’s disease. N Engl J Med. 1980;303(26):1499–502. https://doi.org/10.1056/NEJM198012253032602.
Kim W, Lee Y, Jeong S, Nam J, Lee S, Jung Y. Colonic delivery of celecoxib is a potential pharmaceutical strategy for repositioning the selective COX-2 inhibitor as an anti-colitic agent. Arch Pharm Res. 2015;38(10):1830–8. https://doi.org/10.1007/s12272-015-0602-y.
Mucci A, Nolfe G, Maj M. Levosulpiride: a review of its clinical use in psychiatry. Pharmacol Res. 1995;31(2):95–101.
Mansi C, Savarino V, Vigneri S, Perilli D, Melga P, Sciaba L, et al. Gastrokinetic effects of levosulpiride in dyspeptic patients with diabetic gastroparesis. Am J Gastroenterol. 1995;90(11):1989–93.
Desai JK, Parmar NS. Gastric and duodenal anti-ulcer activity of sulpiride, a dopamine D2 receptor antagonist, in rats. Agents Actions. 1994;42(3–4):149–53.
Kawano M, Takagi R, Kaneko A, Matsushita S. Berberine is a dopamine D1- and D2-like receptor antagonist and ameliorates experimentally induced colitis by suppressing innate and adaptive immune responses. J Neuroimmunol. 2015;289:43–55. https://doi.org/10.1016/j.jneuroim.2015.10.001.
Terai H, Shimahara M. Chronic tongue ulceration resolved by sulpiride: a report of two cases. Clin Exp Dermatol. 2009;34(5):e40–2. https://doi.org/10.1111/j.1365-2230.2008.03110.x.
Arakawa T, Watanabe T, Tanigawa T, Tominaga K, Fujiwara Y, Morimoto K. Quality of ulcer healing in gastrointestinal tract: its pathophysiology and clinical relevance. World J Gastroenterol. 2012;18(35):4811–22. https://doi.org/10.3748/wjg.v18.i35.4811.
Lee S, Lee Y, Kim W, Nam J, Jeong S, Yoo JW, et al. Evaluation of glycine-bearing celecoxib derivatives as a colon-specific mutual prodrug acting on nuclear factor-kappaB, an anti-inflammatory target. Drug Des Devel Ther. 2015;9:4227–37. https://doi.org/10.2147/DDDT.S88543.
Kim W, Nam J, Lee S, Jeong S, Jung Y. 5-Aminosalicylic acid azo-linked to procainamide acts as an anticolitic mutual prodrug via additive inhibition of nuclear factor kappaB. Mol Pharm. 2016;13(6):2126–35. https://doi.org/10.1021/acs.molpharmaceut.6b00294.
Barone M, Chain F, Sokol H, Brigidi P, Bermudez-Humaran LG, Langella P, et al. A versatile new model of chemically induced chronic colitis using an outbred murine strain. Front Microbiol. 2018;9:565. https://doi.org/10.3389/fmicb.2018.00565.
Yano H, Hirayama F, Kamada M, Arima H, Uekama K. Colon-specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: alleviation of systemic side effect after oral administration. J Control Release. 2002;79(1–3):103–12.
Walash MI, Kh Sharaf El-Din MM, El-Enany NM, Eid MI, Shalan SM. Simultaneous determination of sulpiride and mebeverine by HPLC method using fluorescence detection: application to real human plasma. Chem Cent J. 2012;6:13. https://doi.org/10.1186/1752-153X-6-13.
Dong E, Nelson M, Grayson DR, Costa E, Guidotti A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc Natl Acad Sci U S A. 2008;105(36):13614–9. https://doi.org/10.1073/pnas.0805493105.
Bressolle F, Bres J, Faure-Jeantis A. Absolute bioavailability, rate of absorption, and dose proportionality of sulpiride in humans. J Pharm Sci. 1992;81(1):26–32.
Rossi F, Forgione A. Pharmacotoxicological aspects of levosulpiride. Pharmacol Res. 1995;31(2):81–94.
Abdul Rani R, Raja Ali RA, Lee YY. Irritable bowel syndrome and inflammatory bowel disease overlap syndrome: pieces of the puzzle are falling into place. Intest Res. 2016;14(4):297–304. https://doi.org/10.5217/ir.2016.14.4.297.
Lanfranchi GA, Bazzocchi G, Marzio L, Campieri M, Brignola C. Inhibition of postprandial colonic motility by sulpiride in patients with irritable colon. Eur J Clin Pharmacol. 1983;24(6):769–72.
Komarov FI, Rapoport SI, Ivanov SV, Kharaian LV, Kolesnikov DB, Kurikov AV. Sulpiride treatment of irritable colon syndrome. Klin Med (Mosk). 2000;78(7):22–6.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2018R1D1A3B07045694).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The animal protocol used in this study was reviewed and approved by the Pusan National University—Institutional Animal Care and Use Committee (Approval number: PNU-2017-1525; Approval date: 2017-04-19) for ethical procedures and scientific care. All institutional and national guidelines for the care and use of laboratory animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 434 kb)
Rights and permissions
About this article
Cite this article
Kim, D., Kim, W., Jeong, S. et al. Therapeutic switching of sulpiride, an anti-psychotic and prokinetic drug, to an anti-colitic drug using colon-specific drug delivery. Drug Deliv. and Transl. Res. 9, 334–343 (2019). https://doi.org/10.1007/s13346-018-00599-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-018-00599-7