Abstract
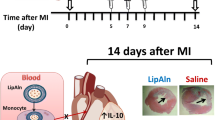
The innate immunity system plays a critical role in vascular repair and restenosis development. Liposomes encapsulating bisphosphonates (LipBPs), but not free BPs, suppress neointima formation following vascular injury mediated in part by monocytes. The objective of this study was to elucidate the role of monocyte subpopulations on vascular healing following LipBP treatment. The potency- and dose-dependent treatment effect of clodronate (CLOD) and alendronate (ALN) liposomes on restenosis inhibition, total monocyte depletion, and monocytes subpopulation was studied. Rats subjected to carotid injury were treated by a single IV injection of LipBPs at the time of injury. Low- and high-dose LipALN treatment (3 and 10 mg/kg, respectively) resulted in a dose-dependent effect on restenosis development after 30 days. Both doses of LipALN resulted in a dose-dependent inhibition of restenosis, but only high dose of LipALN depleted monocytes (−60.1 ± 4.4%, 48 h post injury). Although LipCLOD treatment (at an equivalent potency to 3 mg/kg alendronate) significantly reduced monocyte levels (72.1 ± 6%), no restenosis inhibition was observed. The major finding of this study is the correlation found between monocyte subclasses and restenosis inhibition. Non-classical monocyte (NCM) levels were found higher in LipALN-treated rats, but lower in LipCLOD-treated rats, 24 h after injury and treatment. We suggest that the inhibition of circulating monocyte subpopulations is the predominant mechanism by which LipBPs prevent restenosis. The effect of LipBP treatment on the monocyte subpopulation correlates with the dose and potency of LipBPs.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Experimental and clinical data indicate that the innate immunity system and inflammation play a critical role in vascular repair and restenosis development [1,2,3,4]. The recruitment of circulating monocytes following percutaneous coronary intervention (PCI) contributes to neointimal hyperplasia and restenosis of the blood vessel [5, 6]. Previous studies of our group demonstrated that partial and transient depletion of circulating monocytes by IV administration of LipBPs (liposomes containing a bisphosphonate including clodronate, pamidronate, or alendronate) reduces neointimal hyperplasia and restenosis in animal models of vascular injury [7,8,9,10,11,12,13]. A systemic injection of LipBPs partially reduces circulating monocyte levels for several days, resulting in reduced monocyte accumulation at the injured site (following balloon or stent angioplasty), attenuating downstream signaling, and decreasing neointimal hyperplasia [7, 9, 10, 12]. The unique monocyte-targeting mechanism of action (free BPs are inactive since they are not engulfed by circulating monocytes) completely spares endothelial and smooth muscle cells. Since the BPs are highly hydrophilic and negatively charged, they do not cross cellular membranes (oral absorption is less than 1%) [14]. Thus, the small amount (if at all) of BPs released after phagocytosis by monocytes has no adverse effects on other tissues. Neutrophils, smooth muscle cells (SMC), endothelial cells (EC), lymphocytes, and hepatocytes are not inhibited by LipBP treatment [13]. The relative number of neutrophils in total WBC count following LipBP treatment is increased due to the inhibition of monocytes [10]. Since blood monocyte levels are fully recovered 5 to 7 days after injection, the relative number of neutrophils is increased, and other cells are not affected, and neither infection nor side effects have been observed in therapeutic doses of liposomal BPs.
In a recent prospective, randomized, multicenter, double-blind, placebo-controlled phase IIa clinical trial, a single IV injection of LipALN to stented patients resulted in insignificant difference between the treatment and placebo groups of the average late lumen loss [15]. Nevertheless, subgroup analysis revealed a statistically significant reduction in late lumen loss of 30% in the “inflammatory group,” a group characterized by a baseline monocyte count higher than the basal median value. Phase IIb (“BLADE”) of liposomal alendronate in stented patients is ongoing [16].
Recent research assigned monocytes into functionally distinguished subclasses demonstrating considerable heterogeneity with respect to their phenotype and function [17, 18]. Three subpopulations of monocytes have been identified in humans as (i) classical (CM/pro-inflammatory; CD14++CD16−), (ii) intermediate (IM; CD14++ CD16+), and (iii) non-classical monocytes (NCM/anti-inflammatory/patrolling; CD14++CD16++) [17, 19]. Elevation of IM, 1, 2, and 7 days after PCI, has been found to be predictive of unfavorable cardiovascular outcomes [20]. In addition, a positive correlation has been documented between the level of circulating NCM 12 days after stent implantation and in-stent late gain loss [21]. It should be noted that the effect of LipBPs on monocyte subpopulations has not been previously reported. The aforementioned heterogeneity of monocytes has been observed in mice [22], rats [23], and pigs [24] with notable similarities among these species. To the best of our knowledge, there are no reports concerning monocyte subpopulations in animal models of restenosis. The aim of this study was to assess the extent of monocyte depletion as well as modulation of their subpopulations following treatment with LipBPs in the rat model of restenosis [7, 8, 25].
Materials and methods
Preparation of liposomal bisphosphonates
BP liposomes were prepared by the modified thin-film hydration method [9, 26, 27] with 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC, Lipoid, Ludwigshafen, Germany), the negatively charged lipid, distearoyl phosphatidylglycerol (DSPG, Lipoid), and cholesterol (Sigma-Aldrich, Israel). Liposomes were prepared at a molar ratio of 3:1:2, respectively. The lipids were dissolved in t-butanol and were lyophilized overnight. The lyophilized cake was hydrated with an aqueous solution containing 200 mM alendronate (ALN) or clodronate (CLOD) and then rotated in 70 °C for 15 min at 90–95 rpm. The suspension was then extruded in a thermo-barrel extruder (Lipex Biomembranes, Vancouver, Canada), through polycarbonate membranes between 0.8 and 0.2 μm. In order to remove un-encapsulated drug, the obtained liposomes were passed through a Sephadex G-50 column and eluted in pH 7.2 PBS. The liposomes were filter-sterilized (0.22 μm). Lipids and drug content of the liposomes was determined by HPLC as described previously [28, 29], and liposome size and ζ-potential were determined by dynamic light scattering at room temperature following 1:100 dilution with PBS (Zetasizer Nano ZSP, Malvern Instruments, UK).
Vascular injury model
The protocol for this study was approved by the Institutional Committee for Animal Care and Use of the Hebrew University of Jerusalem. Male Sabra rats weighing 300–400 g were purchased from Harlan Laboratories (Jerusalem, Israel). Animals were fed standard laboratory chow and tap water ad libitum. The rat carotid injury model was performed as described previously [7, 8, 11, 25, 27, 30, 31]. In short, the left common carotid artery was denuded of endothelium by the intraluminal passage of a 2F balloon catheter (Baxter, Irvine, CA, USA) introduced through the external carotid artery. The balloon was sufficiently distended with saline to generate a slight resistance and was passed three times.
Treatment groups
Treatments were conducted blindly, by researchers other than that performing the carotid injury. Rats were randomly treated with a single IV injection (via the tail vein) of LipALN10 (10 mg/kg; high-dose; n = 32), LipALN3 (3 mg/kg; low-dose; n = 21), LipCLOD10 (10 mg/kg), or saline (n = 31). Injections were conducted immediately after injury. In a separate arm of the study, treatment with LipALN in comparison to LipCLOD and saline was examined (n = 13 each).
Morphometric analysis
Arterial hyperplasia was assessed 1 month after injury/treatment as described previously [7, 8, 32,33,34].The arteries were perfusion-fixed in situ with 150 ml of 4% formaldehyde solution (pH 7.4), and the harvested arterial segments were embedded in paraffin. Rats’ arteries were cut at 8–10 sites (600 μm apart), and sections of 5–6 μm were mounted and stained with Verhoeff’s elastin stain [25]. Eight to 10 sections of each rat artery underwent computerized morphometric analysis (NIH ImageJ) by at least two researchers blinded to the treatment type (averaged data was used). All animals were analyzed, and no animal was excluded from analysis. The residual lumen, the area bounded by the internal elastic lamina (original lumen), and the area circumscribed by the external elastic lamina (total arterial area) were measured directly. The degree of neointimal thickening was expressed as the ratio between the area of the neointima and the original lumen (% stenosis) and as the ratio between the neointimal area to the area of the media (N/M).
Blood monocyte determination
Blood was drawn for FACS analyses at baseline and 1, 2, 7, and 14 days after treatment/injury. Blood specimens were drawn from the retro-orbital sinus by a capillary tube under isoflurane anesthesia (Minrad International, USA) and were placed in heparin tubes (Vacutainer, BD, USA). The total blood monocyte and monocyte subset ratio was measured by FACS. Blood samples (50 μl) were incubated with red blood cell lysing solution (ERYTHROLYSE, 1:20 dilution, AbD Serotec, UK) for 10 min at room temperature. The cells were washed and suspended in 1 ml FACS medium (PBS, 1% BSA, 0.02% sodium azide, 0.1% saponin). The cells were centrifuged at 8000 g for 1 min and then incubated for 30 min with Alexa Fluor 647-conjugated anti-ED1, and FITC-conjugated anti-CD43 (AbD Serotec, Oxford, UK), or the respective isotype-matched negative controls (mouse IgG1-Alexa Fluor 647 and igG1-FITC for ED1 and CD43, respectively; AbD Serotec, Oxford, UK). Total monocytes were classified as ED1high and their subsets as ED1highCD43high (NCM) and ED1highCD43low (CM). For method validation, rat blood samples (100 μl) were incubated for 30 min with an antibody mixture containing anti-CD4 (BD, USA), anti-ED1, and anti-CD43 (AbD Serotec, Oxford, UK). Data were acquired on an LSRII (BD Biosciences) and analyzed with FCS Express V3 software package.
Data analysis
Flow cytometry data are expressed as the mean ± standard error (SEM), and the restenosis analysis data as the mean ± standard deviation (SD). Comparisons among treatment groups were made by two-way analysis of variance (ANOVA) followed by Tukey test, and unpaired two-tailed t test when necessary. Differences were termed statistically significant at p < 0.05.
Results
We designed the liposomal formulations to have a similar physicochemical property in terms of drug/lipid ratio, size, and membrane charge for enabling proper comparisons (Table 1). LipALN and LipCLOD liposomes were formulated with the same lipids DSPC/Chol/DSPG (3:2:1, respectively), exhibiting similar properties including size (~200 nm), negative charge (~−24 mV), and drug concentration (5.2–5.8 mg/ml), and similar lipid concentration (~32 mg/ml). The liposomal formulations contained no detectable free, un-encapsulated drug.
Liposomal bisphosphonate and restenosis inhibition—dose and potency effects
Inhibition of restenosis by a single IV injection of LipBP at the time of vascular injury was determined after 30 days. Restenosis extent was assessed by both N/M ratio and % stenosis. Treatments with LipALN (3 and 10 mg/kg, “low-dose” and “high-dose,” respectively) resulted in a dose-dependent reduction of the N/M ratio (Fig. 1a). However, % stenosis was unaffected by the LipALN3 treatment (Fig. 1b), in contrast to animals treated with LipALN10 (from 38.6 ± 6.64 to 22 ± 5.65%, saline vs. LipALN10, respectively, p < 0.01). LipBP potency effect on restenosis following vascular injury was assessed by comparing treatments with LipCLOD10 to LipALN3. The dose of LipCLOD was selected to be three times higher than that of LipALN since the potency of LipALN was shown to be three to five times higher than that of LipCLOD in vivo [8, 10]. LipCLOD treatment did not affect the N/M ratio, nor did it change the % stenosis (Fig. 1c, d).
Effects of bisphosphonate dose and potency on restenosis in rats. Saline, liposomal clodronate (LipCLOD, 10 mg/kg), and liposomal alendronate (low-dose (3 mg/kg), LipALN3, or high-dose (10 mg/kg), LipALN10) were injected IV to Sabra rats at the time of vascular injury. Arterial morphometric analysis was performed 1 month after injury/treatment. a, c The ratio between the areas of neointima to media (N/M). b, d Neointima thickening expressed as the ratio between the area of the neointima and the original lumen (% stenosis). e, f Representative photomicrograph of Verhoeff’s tissue elastin staining of the arteries. Data is presented as the mean ± SD (n = 11–19). *p < 0.05, **p < 0.01, and ***p < 0.001, in comparison to the saline-treated group
Effect on monocytes and their subpopulations
The newly developed FACS method for monocyte subpopulation categorization in rats was based on the expression of ED1 (CD68) by all types of monocytes, and the differential expression of CD43 [35]. Monocyte subpopulations were classified as ED1highCD43low (classical monocyte, CM) or ED1highCD43high (non-classical monocyte, NCM). CMs (blue gated cells) are larger and contain more granules than NCMs (red gated cells; Fig. 2a, b). The method for monocyte and their subpopulation classification was validated using CD4 as a third antibody [36]. CMs express intermediate levels of CD4 whereas NCMs express high levels of CD4 (Fig. 2c, d).
Representative FACS images of rat peripheral blood monocyte and their subpopulation analyses. a Forward scattered (FCS) and size scattered (SSC) dot plot. b CMs (classical monocytes) were defined as ED1high CD43low (upper left quarter—blue gate), and NCMs (non-classical monocytes) were defined as ED1high CD43high (upper right quarter—red gate). c CD4 expression of ED1highCD43low-gated cells. d CD4 expression of ED1highCD43low-gated cells
Liposomal bisphosphonates and monocytes depletion—dose and potency effects
Monocyte levels were measured relative to baseline values (immediately prior to injury/treatment; Fig. 3). Monocyte count remained constant in both saline- and low-dose LipALN-treated rats at all tested time points. In contrast, high-dose LipALN treatment resulted in a significantly reduced monocyte level 48 h after injury (−60.1 ± 4.4%, p < 0.001), and 40% lower 7 days after injury (p < 0.05). Monocyte count, in all treatment groups, returned to baseline levels 14 days after injury. Despite the ineffectiveness of LipCLOD10 treatment on % stenosis (Fig. 1a, d), monocyte counts were significantly reduced 24 h after treatment with 72.1 ± 6% lower than those detected at baseline (p < 0.01). Hence, the effects of LipALN3 and LipCLOD10 on restenosis are not in correlation with their effects on monocyte levels.
Circulating monocyte levels in injured animals. Monocyte levels were measured in the blood before (baseline), and 1, 2, 7, and 14 days after injury/treatment by means of flow cytometry. Monocytes were defined as ED1-positive (CD68) cells and presented as percentage from individual baseline. Data is presented as the mean ± SEM; n = 11–19. *p < 0.05, **p < 0.01, and ***p < 0.001, in comparison to the saline-treated group
Effect of LipBP treatment on monocyte subpopulations
CM and NCM levels and their ratios are presented in Figs. 4 and 5, respectively. In saline-treated rats, the monocyte subpopulation ratio remained unchanged 1 and 2 days after injury, whereas NCM levels were elevated 7 days after injury (p < 0.05). Low- and high-dose LipALN treatment resulted in elevation of NCM subpopulation to 78 ± 9.9 and 83.2 ± 7.43%, respectively, and in a reduction of the CM/NCM ratio (p < 0.05 and p < 0.001, LipALN3 and LipALN10 vs. saline, respectively; Fig. 5a). It should be noted that LipALN10 treatment resulted in a more pronounced elevation in NCM subpopulation as well as reduction in the CM/NCM ratio, which was sustained up to 7 days after injury/treatment (p < 0.05, Figs. 4a and 5a). In contrast, LipCLOD10 treatment resulted in reduced NCM fraction 24 and 48 h after treatment and an increased CM/NCM ratio, 48 h after treatment (p < 0.01; Figs. 4b and 5b).
Monocyte subtypes in carotid-injured rats following treatment with liposomal bisphosphonates. Monocyte subtype levels were measured in the blood before (baseline) and 1, 2, 7, and 14 days after injury/treatment by means of flow cytometry. Levels of CM and NCM are presented in dark purple columns (CD43−) and in light purple columns (CD43+), respectively. Data is presented as the mean ± SD; n = 11–19. *p < 0.05, **p < 0.01 and ***p < 0.001, in comparison to baseline
The ratio of monocyte subtypes in carotid-injured rats following treatment with liposomal bisphosphonates. The ratio between CM (ED1highCD43low) and NCM (ED1highCD43low) was measured in the blood before and 1, 2, 7, and 14 days after injury/treatment by means of flow cytometry. Data is presented as the mean ± SD, n = 11–19. *p < 0.05, **p < 0.01, and ***p < 0.001, LipALN10 and LipCLOD10 vs. saline. # p < 0.05 and ## p < 0.01, LipALN3 vs. saline)
Discussion
It is well established that the anti-inflammatory effect of liposomal BPs in general, and restenosis inhibition in particular, stems from their effective depletion of circulating monocytes. Partial and transient reduction of blood monocyte levels following systemic LipBP administration leads to decreased monocyte infiltration to the injured artery. This results in the suppression of neointimal hyperplasia as demonstrated in rats and rabbits [8, 10,11,12,13]. The objective of the current study was to correlate between the dose and potency effect of LipBPs with their possible modulation of monocyte subtypes, following vascular injury in rats.
As expected, LipALN10 treatment resulted in a significant inhibition of depletion of monocytes and restenosis. However, certain queries have been raised in view of the data obtained: (i) Both doses of LipALN3 and LipALN10 (low-dose and high-dose) resulted in a dose-dependent inhibition of restenosis (Fig. 1a, c), yet only the high dose of LipALN caused monocyte depletion (Fig. 3). (ii) LipALN3 treatment resulted in a modest inhibition of restenosis (reduced N/M ratio and no effect on % stenosis, Fig. 1) but with no effect on monocyte depletion. In contrast to LipALN, LipCLOD treatment was found ineffective in restenosis inhibition (Fig. 1) yet significantly reduced monocyte levels (Fig. 3). Thus, there is no correlation between the extent of monocyte depletion and the degree of restenosis inhibition (see Table 2 summarizing LipBP effects on monocytes and restenosis).
LipALN and LipCLOD potency was previously studied in Raw264, J774 cell lines, and h-monocytes demonstrating that LipALN is at least two times more potent than LipCLOD in the inhibition of cell proliferation [10, 37]. The higher potency of LipALN was increased in vivo where a similar reduction of blood monocytes 24 h after injection was obtained by 3 and 15 mg/kg, LipALN and LipCLOD, respectively [10]. Therefore, a dose of 10 mg/kg LipCLOD was selected as comparable to 3 mg/kg LipALN. A possible explanation for the lack of correlation between LipALN and LipCLOD activities (Table 2) could be their different kinetics of action. LipALN causes earlier monocyte depletion (nadir at 24 h compared to a nadir of 48 h for LipCLOD). LiPALN also caused a longer and sustained depletion of monocytes compared to LiPCLOD (5 vs. 1 day following the nadir time point of depletion, respectively). Nevertheless, the different kinetics of reduced monocyte levels does not provide an explanation for the lack of LipCLOD10 effect on restenosis (Fig. 1), which has been demonstrated at higher dosages [7]. Another distinctive difference, which might explain the lack of correlation between LipALN and LipCLOD activities (Table 2), is their intracellular mechanism of action. Depending on the nature of the R(2) chain, CLOD (a non-nitrogen-containing BP) is metabolized to a cytotoxic analog of ATP [38], causing necrosis of monocytes/macrophages [10]. In contrast, ALN (a nitrogen-containing BP) inhibits the mevalonate pathway [38], causing apoptosis of monocytes/macrophages [10]. However, the above distinguishing mechanisms of action are insufficient to explain the miscorrelation between the effect on monocyte inhibition and restenosis inhibition observed. In addition (as noted above in point (i)), low-dose LipALN treatment resulted in partial inhibition of restenosis (Fig. 1), but did not affect monocyte levels at all examined time points (Fig. 3). Thus, there is no correlation between the extent of monocyte depletion and the degree of restenosis inhibition (Table 2).
In order to reconcile the above discrepancies, we sought to correlate the effect of LipBP treatment with their effect on monocyte subtypes. As mentioned above, there are three known subpopulations of monocytes in humans: (i) CM (CD14++CD16−), (ii) IM (CD14++ CD16+), and (iii) NCM (CD14++CD16++), with different functions [17, 19]. Murine monocyte subtypes are characterized by differential expression of Ly6C: Ly6Chigh(CM), Ly6Cint(IM), and Ly6Clow (NCM) [22]. Two monocyte subsets have been described in rats based on CD43 expression, CD43high monocyte (NCM), and CD43low monocytes (CM) [17, 18, 23]. It has been demonstrated that monocyte subpopulations in the rat can be determined by first using CD172 (a marker for monocytes) followed by CD43 [23, 39]. We have developed a method based on CD68 (as a marker for monocytes) followed by CD43. That CD68 is a well-defined marker for monocytes has been reported [40, 41]. To confirm that CD68 is a suitable marker for circulating monocytes, we stained the same blood samples with CD68 and CD172 antibodies and acquired comparable results (data not shown). In addition, size and granularity of the cells stained with CD68 antibody were typical to monocytes (Fig. 2). It is shown that the “patrolling/anti-inflammatory/non-classical” CD43high monocytes are CCR2 lowCX3CR1 high, and the “pro-inflammatory/classical” CD43low monocytes are CCR2 highCX3CR1 low. Our newly developed technique, based on a gentle perforation of the cell’s membrane, enabled the use of the intracellular CD68 for determining total monocyte number followed by CD43.
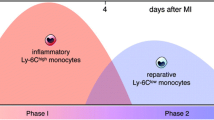
CMs are recruited during the first days after MI in mice while NCMs are recruited after 5 days in the second phase [42]. A sequential mobilization of CM and NCM to the injured heart, peaking at day 3 and day 5, respectively, has been described [43]. The “inflammatory” CMs induce phagocytosis and digestion of the damaged tissue, whereas NCMs (also termed patrolling monocytes) promote reparative activities by increasing myofibroblast accumulation, angiogenesis, and collagen deposition [2, 42]. Conversion from CM to NCN was suggested following inflammatory stimulation [44, 45]. It has been recently demonstrated in a murine injury model, induced by subcutaneous sponge implantation, that CMs recruited to the site of inflammation are slowly transitioned into repair macrophage [46]. In steady state, circulating CMs differentiate into NCMs [22]. Monocyte switch from CM to NCM is an important process that leads to resolution of the inflammatory process, and inhibition of this phenotypic change delayed proper resolution of inflammation and inhibited wound healing [47]. Inflammatory monocytes—but not the non-inflammatory subset—depend on the chemokine receptor CCR2 for localization to injured tissue. It has been shown that treatment with CCR2 siRNA prevents CM accumulation in sites of inflammation. The treatment attenuates their number in atherosclerotic plaques, reduces infarct size after coronary artery occlusion, prolongs normoglycemia in diabetic mice after pancreatic islet transplantation, and results in reduced tumor volumes and lower numbers of tumor-associated macrophages [48].
The key finding of our study is the apparent correlation between NCM level and restenosis inhibition (Table 2, and Figs. 4 and 5). Low- and high-dose LipALN treatment resulted in both elevation of NCM levels (24 h after injury/treatment) and restenosis inhibition. In contrast, LipCLOD treatment, which resulted in reduced NCM levels, had no effect on restenosis. Thus, we suggest that the effect of liposomal BP treatment on NCM levels correlates well with the inhibition of restenosis. The correlation suggested of reduced CM/NCM ratio and restenosis inhibition is in accord with reports in stented patients demonstrating a positive correlation between circulating NCM (patrolling) and in-stent restenosis [21]. Vascular injury leads to a complex inflammatory response, which is pivotal in the wound healing process [49]. It is plausible to assume that the inflammatory response is tightly controlled in order to avoid a chronic inflammatory response [50]. In the present work, we show that 3 and 10 mg/kg LipALN, not affecting and reducing total monocyte number, respectively, increases the ratio of patrolling monocytes (CD43high). The latter cells’ (NCM) major function is the resolution of inflammation [51]. Thus, LipALN administration reduces the inflammatory response, and consequently restenosis is inhibited.
The roles of CM/NCM have been explored in other cardiovascular pathologies. In a murine model of stroke, CMs are recruited from the bone marrow during the first 24 h after injury and differentiate into NCMs [52]. Indeed, the elevation of NCM following LipALN treatments was observed after 24 h (1 day prior to the nadir time point of monocyte depletion). Moreover, the opposing decrease of NCM levels in animals treated with LipCLOD occurred also after 24 h (the same time point of monocyte depletion). It is suggested that the elevated CM/NCM ratio found 48 h after injury in the LipCLOD-treated group delays healing, thus promoting restenosis development. In contrast, treatment with LipALN10 that reduces the CM/NCM ratio after 48 h results in significant inhibition of restenosis, and unchanged ratio (LipALN3) results in a modest inhibition (Table 2). This is corroborated with the findings of a recent study suggesting that the beneficial effect of remote ischemic pre-conditioning is due to elevated NCM with no changes in CM [53]. Studying the effect of LipBPs on monocyte subpopulations in other inflammatory-associated disorders (for example, cancer and endometriosis [11, 54]) could provide further insight into LipBP mechanism of action.
Conclusions
In this work, we have demonstrated for the first time the effect of LipBPs on blood monocyte subpopulations in a rat model of restenosis. Liposomal BPs inhibit restenosis by partial and transient depletion of circulating monocytes. The dose-dependent efficacy demonstrated by low- and high-dose LipALN treatments is attributed to the reduction in the CM/NCM ratio. Similarly, LipCLOD treatment, at a dose of equal potency to a low dose of alendronate, which resulted in elevated CM/NCM ratio, is in correlation to its lack of efficacy in restenosis inhibition.
References
Rogers C, Welt FG, Karnovsky MJ, Edelman ER. Monocyte recruitment and neointimal hyperplasia in rabbits. Coupled inhibitory effects of heparin. Arterioscler Thromb Vasc Biol. 1996;16(10):1312–8.
Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, et al. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science. 2007;317(5838):666–70.
Hansson GK. Atherosclerosis-an immune disease: the Anitschkov lecture 2007. Atherosclerosis. 2009;202(1):2–10.
Fuster JJ, Fernandez P, Gonzalez-Navarro H, Silvestre C, Nabah YN, Andres V. Control of cell proliferation in atherosclerosis: insights from animal models and human studies. Cardiovasc Res. 2010;86(2):254–64.
Toutouzas K, Colombo A, Stefanadis C. Inflammation and restenosis after percutaneous coronary interventions. Eur Heart J. 2004;25(19):1679–87.
Schober A, Weber C. Mechanisms of monocyte recruitment in vascular repair after injury. Antioxid Redox Signal. 2005;7(9–10):1249–57.
Danenberg HD, Fishbein I, Gao J, Monkkonen J, Reich R, Gati I, et al. Macrophage depletion by clodronate-containing liposomes reduces neointimal formation after balloon injury in rats and rabbits. Circulation. 2002;106(5):599–605.
Danenberg HD, Fishbein I, Epstein H, Waltenberger J, Moerman E, Monkkonen J, et al. Systemic depletion of macrophages by liposomal bisphosphonates reduces neointimal formation following balloon-injury in the rat carotid artery. J Cardiovasc Pharmacol. 2003;42(5):671–9.
Danenberg HD, Golomb G, Groothuis A, Gao J, Epstein H, Swaminathan RV, et al. Liposomal alendronate inhibits systemic innate immunity and reduces in-stent neointimal hyperplasia in rabbits. Circulation. 2003;108(22):2798–804.
Epstein-Barash H, Gutman D, Markovsky E, Mishan-Eisenberg G, Koroukhov N, Szebeni J, et al. Physicochemical parameters affecting liposomal bisphosphonates bioactivity for restenosis therapy: internalization, cell inhibition, activation of cytokines and complement, and mechanism of cell death. J Control Release. 2010;146(2):182–95.
Haber E, Afergan E, Epstein H, Gutman D, Koroukhov N, Ben-David M, et al. Route of administration-dependent anti-inflammatory effect of liposomal alendronate. J Control Release. 2010;148(2):226–33.
Cohen-Sela E, Rosenzweig O, Gao J, Epstein H, Gati I, Reich R, et al. Alendronate-loaded nanoparticles deplete monocytes and attenuate restenosis. J Control Release. 2006;113(1):23–30.
Gutman D, Golomb G. Liposomal alendronate for the treatment of restenosis. J Control Release. 2012;161(2):619–27.
Fazil M, Baboota S, Sahni JK, Ameeduzzafar AJ. Bisphosphonates: therapeutics potential and recent advances in drug delivery. Drug Deliv. 2015;22(1):1–9.
Banai S, Finkelstein A, Almagor Y, Assali A, Hasin Y, Rosenschein U, et al. Targeted anti-inflammatory systemic therapy for restenosis: the Biorest Liposomal Alendronate with Stenting sTudy (BLAST)—a double blind, randomized clinical trial. Am Heart J. 2013;165(2):234–40.
ClinicalTrials.gov. Biorest liposomal alendronate administration for diabetic patients undergoing Drug-Eluting Stent Percutaneous Coronary Intervention 2016 [updated 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT02645799?term=liposomes+alendronate&rank=2.
Ziegler-Heitbrock L. Monocyte subsets in man and other species. Cell Immunol. 2014;289(1–2):135–9.
Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5(12):953–64.
Moniuszko M, Bodzenta-Lukaszyk A, Kowal K, Lenczewska D, Dabrowska M. Enhanced frequencies of CD14++CD16+, but not CD14+CD16+, peripheral blood monocytes in severe asthmatic patients. Clin Immunol. 2009;130(3):338–46.
Zhou X, Liu XL, Ji WJ, Liu JX, Guo ZZ, Ren D, et al. The kinetics of circulating monocyte subsets and monocyte-platelet aggregates in the acute phase of ST-elevation myocardial infarction: associations with 2-year cardiovascular events. Medicine (Baltimore). 2016;95(18):e3466.
Liu Y, Imanishi T, Ikejima H, Tsujioka H, Ozaki Y, Kuroi A, et al. Association between circulating monocyte subsets and in-stent restenosis after coronary stent implantation in patients with ST-elevation myocardial infarction. Circ J. 2010;74(12):2585–91.
Sunderkotter C, Nikolic T, Dillon MJ, Van Rooijen N, Stehling M, Drevets DA, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172(7):4410–7.
Yrlid U, Jenkins CD, MacPherson GG. Relationships between distinct blood monocyte subsets and migrating intestinal lymph dendritic cells in vivo under steady-state conditions. J Immunol. 2006;176(7):4155–62.
Fairbairn CE, Sayette MA. The effect of alcohol on emotional inertia: a test of alcohol myopia. J Abnorm Psychol. 2013;122(3):770–81.
Golomb G, Fishbein I, Banai S, Mishaly D, Moscovitz D, Gertz SD, et al. Controlled delivery of a tyrphostin inhibits intimal hyperplasia in a rat carotid artery injury model. Atherosclerosis. 1996;125(2):171–82.
Epstein H, Gutman D, Cohen-Sela E, Haber E, Elmalak O, Koroukhov N, et al. Preparation of alendronate liposomes for enhanced stability and bioactivity: in vitro and in vivo characterization. AAPS J. 2008;10(4):505–15.
Epstein H, Berger V, Levi I, Eisenberg G, Koroukhov N, Gao J, et al. Nanosuspensions of alendronate with gallium or gadolinium attenuate neointimal hyperplasia in rats. J Control Release. 2007;117(3):322–32.
Aizik G, Waiskopf N, Agbaria M, Levi-Kalisman Y, Banin U. Golomb G. ACS Nano: Delivery of Liposomal Quantum Dots via Monocytes for Imaging of Inflamed Tissue; 2017.
Lang JK. Quantitative determination of cholesterol in liposome drug products and raw materials by high-performance liquid chromatography. J Chromatogr. 1990;507:157–63.
Holt AW, Tulis DA. Experimental rat and mouse carotid artery surgery: injury & remodeling studies. ISRN Minim Invasive Surg 2013;2013.
Fishbein I, Brauner R, Chorny M, Gao J, Chen X, Laks H, et al. Local delivery of mithramycin restores vascular reactivity and inhibits neointimal formation in injured arteries and vascular grafts. J Control Release. 2001;77(3):167–81.
Afergan E, Epstein H, Dahan R, Koroukhov N, Rohekar K, Danenberg HD, et al. Delivery of serotonin to the brain by monocytes following phagocytosis of liposomes. J Control Release. 2008;132(2):84–90.
Chan JM, Rhee JW, Drum CL, Bronson RT, Golomb G, Langer R, et al. In vivo prevention of arterial restenosis with paclitaxel-encapsulated targeted lipid-polymeric nanoparticles. Proc Natl Acad Sci U S A. 2011;108(48):19347–52.
Chan JM, Zhang L, Tong R, Ghosh D, Gao W, Liao G, et al. Spatiotemporal controlled delivery of nanoparticles to injured vasculature. Proc Natl Acad Sci U S A. 2010;107(5):2213–8.
Dijkstra CD, Dopp EA, van den Berg TK, Damoiseaux JG. Monoclonal antibodies against rat macrophages. J Immunol Methods. 1994;174(1–2):21–3.
Strauss-Ayali D, Conrad SM, Mosser DM. Monocyte subpopulations and their differentiation patterns during infection. J Leukoc Biol. 2007;82(2):244–52.
Makkonen N, Salminen A, Rogers MJ, Frith JC, Urtti A, Azhayeva E, et al. Contrasting effects of alendronate and clodronate on RAW 264 macrophages: the role of a bisphosphonate metabolite. Eur J Pharm Sci. 1999;8(2):109–18.
Lehenkari PP, Kellinsalmi M, Napankangas JP, Ylitalo KV, Monkkonen J, Rogers MJ, et al. Further insight into mechanism of action of clodronate: inhibition of mitochondrial ADP/ATP translocase by a nonhydrolyzable, adenine-containing metabolite. Mol Pharmacol. 2002;61(5):1255–62.
Abbondanzo SJ, Chang SL. HIV-1 transgenic rats display alterations in immunophenotype and cellular responses associated with aging. PLoS One. 2014;9(8):e105256.
Scriba A, Luciano L, Steiniger B. High-yield purification of rat monocytes by combined density gradient and immunomagnetic separation. J Immunol Methods. 1996;189(2):203–16.
Gilroy DW, Colville-Nash PR, McMaster S, Sawatzky DA, Willoughby DA, Lawrence T. Inducible cyclooxygenase-derived 15-deoxy(Delta)12-14PGJ2 brings about acute inflammatory resolution in rat pleurisy by inducing neutrophil and macrophage apoptosis. FASEB J. 2003;17(15):2269–71.
Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, et al. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204(12):3037–47.
Tsujioka H, Imanishi T, Ikejima H, Kuroi A, Takarada S, Tanimoto T, et al. Impact of heterogeneity of human peripheral blood monocyte subsets on myocardial salvage in patients with primary acute myocardial infarction. J Am Coll Cardiol. 2009;54(2):130–8.
Barendregt CS, Van der Laan AM, Bongers IL, Van Nieuwenhuizen C. Adolescents in secure residential care: the role of active and passive coping on general well-being and self-esteem. Eur Child Adolesc Psychiatry. 2015;24(7):845–54.
Ikejima H, Imanishi T, Tsujioka H, Kashiwagi M, Kuroi A, Tanimoto T, et al. Upregulation of fractalkine and its receptor, CX3CR1, is associated with coronary plaque rupture in patients with unstable angina pectoris. Circ J. 2010;74(2):337–45.
Crane MJ, Daley JM, van Houtte O, Brancato SK, Henry WL Jr, Albina JE. The monocyte to macrophage transition in the murine sterile wound. PLoS One. 2014;9(1):e86660.
Dal-Secco D, Wang J, Zeng Z, Kolaczkowska E, Wong CH, Petri B, et al. A dynamic spectrum of monocytes arising from the in situ reprogramming of CCR2+ monocytes at a site of sterile injury. J Exp Med. 2015;212(4):447–56.
Leuschner F, Dutta P, Gorbatov R, Novobrantseva TI, Donahoe JS, Courties G, et al. Therapeutic siRNA silencing in inflammatory monocytes in mice. Nat Biotechnol. 2011;29(11):1005–10.
Davis C, Fischer J, Ley K, Sarembock IJ. The role of inflammation in vascular injury and repair. J Thromb Haemost. 2003;1(8):1699–709.
Inoue T, Node K. Molecular basis of restenosis and novel issues of drug-eluting stents. Circ J. 2009;73(4):615–21.
Soehnlein O, Lindbom L. Phagocyte partnership during the onset and resolution of inflammation. Nat Rev Immunol. 2010;10(6):427–39.
Gliem M, Mausberg AK, Lee JI, Simiantonakis I, van Rooijen N, Hartung HP, et al. Macrophages prevent hemorrhagic infarct transformation in murine stroke models. Ann Neurol. 2012;71(6):743–52.
Chen L, Shao H, Zhou X, Liu G, Jiang J, Liu Z. Water-mediated cation intercalation of open-framework indium hexacyanoferrate with high voltage and fast kinetics. Nat Commun. 2016;7:11982.
Haber E, Danenberg HD, Koroukhov N, Ron-El R, Golomb G, Schachter M. Peritoneal macrophage depletion by liposomal bisphosphonate attenuates endometriosis in the rat model. Hum Reprod. 2009;24(2):398–407.
Acknowledgments
GG is grateful to the Woll Sisters and Brothers Chair in Cardiovascular Diseases.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol for this study was approved by the Institutional Committee for Animal Care and Use of the Hebrew University of Jerusalem.
Animal care
All institutional and national guidelines for the care and use of laboratories animals were followed.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Grad, E., Zolotarevsky, K., Danenberg, H.D. et al. The role of monocyte subpopulations in vascular injury following partial and transient depletion. Drug Deliv. and Transl. Res. 8, 945–953 (2018). https://doi.org/10.1007/s13346-017-0404-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-017-0404-5