Abstract
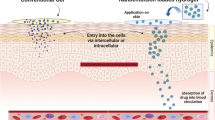
Adapalene (ADP), a topically administered antiacne drug, finds limitation due to poor penetration, limited localization, and associated incompatibility of photosensitization and skin irritation. To explicate an innovative and safe method for ADP administration and alleviating the associated limitations, solid lipid nanoparticles (SLN) of ADP have been fabricated and evaluated for efficacy in the present work. The SLN were prepared using pre-emulsion sonication method and incorporated into convenient topical dosage form, hydrogels. In vitro permeation studies of the hydrogels through HCS indicated gel containing ADP-SLN showed 2-fold more accumulation in skin layers as compared to conventional ADP gel. Rheological studies demonstrated ADP-SLN gel to possess pseudoplastic behavior, occlusion and hydration studies revealed permeation effectiveness of ADP-SLN gel over conventional ADP gel while primary skin irritation studies established safety of the ADP-SLN gel upon topical application. Hence, it was concluded that the studied ADP-SLN formulation with skin localizing ability may be a promising carrier for topical delivery of ADP.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Acne vulgaris, a long-term skin condition characterized by seborrhea, comedons, microcomedons, pustules, etc. is a common disorder affecting 80–85 % of teenagers in the world, leading to reduced self-esteem and depression in them. Acne involves abnormal proliferation and differentiation of keratinocytes following increased sebum production which acts as a medium for proliferation of gram-positive bacterium, Propionibacterium acnes, the latter leading to inflammatory response owing to bacterial antigens and cytokines [1–4].
Adapalene (ADP), 6-[3-(1-adamantyl)-4-methoxy-phenyl] naphthalene-2-carboxylic acid, is a third generation retinoid exhibiting keratolytic, anti-inflammatory, and antiseborrhoic action, hence is a choice of drug for treating acne. In addition to oral and topical retinoid therapy, the treatment for acne also involves oral and topical antibiotic therapy [5, 6]. Systemic and oral delivery of retinoids though being effective, are associated with severe complications such as embryopathy (cranio-facial, central nervous system, cardiovascular disorders), hepatosplenomagaly, and papilloedema while topical delivery is associated with instances like photosensitization and skin irritation, alarming need for an effective and safe topical therapy [8].
Various carriers such as nanoemulsions, nanosuspensions, mixed micelles, liposomes, polymeric nanoparticles, and nanoparticles based on solid lipids at room temperature have been developed to derive advantages such as increased bioavailability, controlled release, drug targeting, and increased stability [9, 10]. Solid lipid nanoparticles (SLNs) hold the promise as carrier to improve drug delivery to skin because these are composed of physiological and biodegradable lipids with low toxicity, and their small size and relatively narrow size distribution permit site-specific delivery to the skin, while occlusive effect leading to hydration and temporary opening of compact structure of stratum corneum facilitates drug penetration into the skin surface, allowing the lipid exchange between SLN and the outermost layers of stratum corneum [9–12]. Various studies have focused on the cosmetic and topical applications of SLN; SLNs as a topical carrier have been used for topical delivery of several drugs including clotrimazole and betamethasone 17-valerate and have reported to have potential to localize drug in skin [13, 14].
The purpose of this research was to incorporate ADP in SLN (ADP-SLN) and evaluate the consequent improvement in topical delivery when incorporated in gel matrix. It was believed that incorporating ADP into SLN would improve its permeability into the skin owing to the involvement of lipid carrier; additionally, the drug efficacy and local effect could be improved by epidermal restriction of the same [7–14]. Entrapment of ADP in SLN matrix would also minimize the contact of the drug with the skin, thereby reducing skin irritation and photosensitization instances and ensuring a control release in the skin [6, 12].
Experimental
Materials
ADP was received as a kind gift from Glenmark Pharmaceuticals Ltd., India. Cellulose acetate membranes were purchased from Hi Media, India. Carbopol 940 was purchased from Loba Chemie, India, and all other chemicals were procured from local sources.
Methods
Lipid selection
Lipid selection was done on the basis of maximum solubility of ADP in the test lipids. Various lipids such as Compritol 888 ATO, glyceryl monostearate, Precirol ATO 5, Gelucire, Emulcire 61, and stearic acid were used to study the solubility of the drug. Briefly, ADP (0.1 g) was dispersed under stirring in 100 mg melted lipids. Incremental addition of lipids was done until clear solution was obtained.
Surfactant system selection
The surfactant system was chosen on the basis of particle size (PS) and polydispersity index (PDI) of the dispersion obtained by utilizing various surfactant systems (Table 1). ADP/lipid (1:3) was melted at 70 °C, and 1 % of the lipid surfactant was added to the melt. Aqueous surfactant solution (2 %) maintained at 70 °C was used as a medium for emulsifying the lipid melt under stirring (35,000 rpm, 10 min; Omni International, USA). The resultant dispersion was evaluated for PS and PDI.
Optimization using experimental design
A Box-Behnken design containing 15 experimental runs was utilized to study the independent variables, viz., drug/lipid ratio, concentration of lipid-based surfactant, sonication time at three levels, and their effect on important dependent variables, viz., PS and % entrapment efficiency (EE). The levels for the drug/lipid ratio were 1:3, 1:7.5, and 1:10, concentration of lipid-based surfactant were 1, 3, and 5 % while the same for sonication time were 5, 10, and 15 min.
Preparation of ADP-loaded solid lipid nanoparticles
ADP-SLNs were prepared by using pre-emulsion probe sonication method. Lipid was heated to at least 10 °C above its melting point, and ADP was dissolved in the melt. Lipid surfactant was added to the lipid melt under manual stirring until clear melt was obtained. Aqueous surfactant solution (2 %) heated to temperature as that of lipid melt was added under stirring (35,000 rpm, 10 min; Omni International, USA) to the lipid phase to form pre-emulsion. The resultant pre-emulsion was further sonicated using probe sonicator (Sonics VCX750, USA), followed by cooling down to 2 to 4 °C in freezer to form ADP-SLN dispersion. The particle size of the resultant ADP-SLNs were analyzed for PS using Malvern Mastersizer 2000 MS (Malvern instruments, UK). For %EE, the ADP-SLN was centrifuged at 50,000 rpm for 20 min and the settled mass was collected, washed with chloroform, and analyzed spectrophotometrically (Jasco V-530, Japan) for content analysis by dissolving known amount of SLN into chloroform.
Optimization data analysis and response surface methodology validation
Optimization data analysis was done using Design Expert Software (version 7.1.6, Stat-Ease Inc., Minneapolis, MN), and the polynomial regression equations and 3D response surfaces were obtained. Finally, optimum formula with highest desirability value was picked, and the prognostic ability of mathematical model was tested by formulating the suggested optimum formulation and evaluation of response parameters.
Evaluation of the optimized SLN
X-ray diffractometry
X-ray scattering measurements were carried out using X-ray diffractometer (PW 3710, Philips Ltd.). A Cu Ka radiation source was used with the scanning rate (2 h min−1) of 5 °C per min. X-ray diffraction measurements were carried out on ADP-SLN, bulk ADP, and bulk lipid.
Fourier transform infrared study
A Jasco FTIR spectrophotometer (Jasco FTIR-401, Japan) was used for infrared analysis. About 1–2 mg of sample was mixed with dry potassium bromide, and the samples were examined at transmission mode over wave number range of 4000 to 400 cm−1. Samples used for FTIR analysis were the same as PXRD.
Preparation of ADP-SLN gel
Gels with different concentrations (0.3–1 %) were formulated and evaluated for rheological properties and for diffusion studies. Required quantity of carbopol 940 was dispersed in distilled water and allowed to hydrate under sonication for 4 to 5 h [15, 16]. Glycerol (10 % w/w) was added subsequently to the resultant gel as humectant. ADP-SLN equivalent to 100,000 units of ADP was incorporated in the above gel with gentle stirring, triethanolamine was added under gentle stirring, and the pH was adjusted to 6.0. The gel was allowed to stand overnight to remove the entrapped air.
Evaluation of ADP-SLN gel
Rheological study of gel
Cone and plate rheometer (Brookfield viscometer CAP 2000+2, India) was used to determine the shear stress versus shear rate for ADP-SLN gels. The aim of study was to assess the rheological behavior of SLN based semisolid formulations prepared with carbopol 940.To gain some insight into the influence of storage temperature and nature of lipid matrix, the shear stress variation was recorded at pre-defined shear rate from 0 to 1000 s−1 for the semisolid systems stored at three different temperatures (5, 25, and 40 °C).
Ex vivo skin penetration and localization study
Ex vivo skin penetration studies were performed with HCS from the hand region using Franz diffusion cell [17]. The cadaver skin was procured from B. J. Medical College, Pune, and after removing hair and subcutaneous fat, the tissue was punched to a disk of approximately 2.5-cm2 area, and this slice was mounted on the Franz diffusion cell. Phosphate-buffered saline (pH 7.4) maintained at 37 ± 2 °C served as receptor fluid. A small quantity (0.1 g) of the gel was applied to the skin surface. Serial sampling was performed at specified time intervals (1, 2, 3, 4, 5, 6, 7, 8, and 24 h) by removing the contents of the receptor compartment and replacing it with the fresh medium. The samples were analyzed using Jasco UV–VIS (Jasco V-530, Japan) spectrophotometer, and mean cumulative amount diffused Q (mg cm−2) at each sampling time points were calculated. The flux and the permeability coefficient for the gels were also calculated. At the end of 24 h, the amount of drug in the receptor compartment, the drug remaining on the skin and the drug concentration in the skin was determined by extraction into a suitable solvent, followed by spectrophotometric analysis using Jasco UV–VIS (Jasco V-530, Japan) spectrophotometer at 323 nm [17].
Ex vivo skin hydration studies
The skin hydrating effect of the selected SLN formulation was investigated ex vivo and compared with the conventional gel. The topical formulations were applied to the prepared HCS. After 24 h of gel application, skin was isolated, vertically sliced using microtome, and stained with carbol fuchsin. The slides were observed under optical microscope, and thickness of stratum corneum was observed. The photomicrographs were taken using optical microscope with image analyzer (Magnus MLX, India).
In vitro occlusion study
In vitro occlusion study was performed on formulated ADP-SLN gel. Two 100-ml beakers, one sample, and one reference, having approximately same dimensions, were filled with 50 ml water and covered with cellulose acetate membrane [18]. The cellulose membrane of sample beaker was applied with 500 mg ADP-SLN gel while the reference was used as such without applying anything. Both the beakers were stored at 40 °C and 75 % RH for 48 h. The occlusion factor F was calculated according to Eq. (1) after 6, 24, and 48 h.
An occlusion factor was calculated by the following formula:
where A is the water loss without sample (reference) and B is the water loss with sample (ADP-SLN gel).
Primary skin irritation study
Primary skin irritation study of selected formulation was performed using albino rabbits in accordance with guidelines of the Consumer Product Safety Commission [17]. Formalin was taken as positive control, and plain gel was used as negative control in the study. The study was approved by the Institutional Ethical Committee (CPCSEA/IAEC/PT-08/07-2K8).
Stability study
Stability studies of ADP-SLN and ADP-SLN gel involved 3-month storage of the formulation at accelerated stability conditions (i.e., 40 °C/75 %Rh) and observation of attributes (in case of gel) such as change in color, odor or consistency, presence of any precipitate or crystals, and for SLN, measurement of particle size, zeta potential, and entrapment efficiency.
Results and discussion
Lipid selection study
Of different lipids used, maximum solubility of ADP was observed in glyceryl monosterate (GMS) (0.3 g to solubilize 0.1 g of drug). The amount of Compritol 888 ATO, stearic acid, Precirol ATO 5, Emulcire 61, and Gelucire required to solubilize 0.1 g of ADP was >2, >3, 1, >3, and >2 g, respectively. The solubilizing potential coupled with already reported biocompatibility and acceptability of GMS for topical, peroral, and parenteral route favored its selection for the present study. The melting point of GMS and ADP being 60 and 319–327 °C, respectively, would lead a possible structure of ADP core surrounded by lipid in the SLN, leading to prolong release of drug [19, 20].
Surfactant system selection
Table 1 represents findings from different amalgamations of lipid- and water-based surfactant. Soya lecithin and other combinations of water-phase surfactants produced particles with high diameter and a high PDI value. The combination of Span 60 and Poloxamer 188, though, rendered smallest particle size but was eliminated owing to high PDI value. The combination of Span 60 and Tween 80 resulted in particles of diameter 320 nm and PDI of 0.210, and hence, this system was chosen to carry the study further. Tween as a long-chain surfactant provides aqueous phase stability to the formed emulsions while span provides the necessary stabilization for the lipidic phase into the continuous aqueous phase, thus leading to the formation of an emulsion system, of which low particle size and PDI are functional characteristics.
Optimization using experimental design
The experimental batches of Box–Behnken design are presented in Table 2. A three-factor, three-level design would require a total of 27 experimental runs without any repetitions and a total of 30 runs with three repetitions [20]. A Box–Behnken experimental design reduces the number of experiments to 15, hence was favored.
Optimization data analysis and response surface methodology validation
The formulations prepared as per the experimental design were evaluated, and the experimental results were evaluated using the Stat-Ease Design Expert.
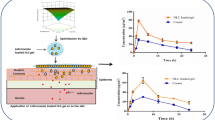
High F value (8487.51 and 18553.75 for PS and %EE, respectively), and P value less than 0.05 for PI and %EE models indicated good degree of significance. High R 2 values indicated good agreement between formulation variables and response parameters. The RSM for PS and %EE is presented in Fig. 1.
It is evident from the Fig. 1 that lipid at higher concentration contributes in building the particle size which may be due to increasing viscosity of the system owing to increased lipid concentration and resultant inefficiency of the surfactant system [21–23]. Span had a limited effect of reducing the particle size initially but with increasing concentration, dominance of its lipoic nature led to increase in particle size [22]. A limited effect of decrease in PS with increment in sonication time was also seen, which is due to the increased shear provided to break the globules in the pre-emulsion.
It is evident from the Fig. 2 that increase in the lipid concentration provides opportunity for the drug to disperse/solute in favorable medium rendering high %EE value. The same is observed in case of increasing span concentration which is attributed to its lipoid nature. The role of the sonication time is seen to contribute reduction of drug entrapment which is because lower particle size of globule leading to enhanced area for migration of drug to aqueous phase.
The solution provided by the Design Expert software on the basis of highest desirability value (0.986) was drug/lipid ratio (1:7.2875), concentration of Span 60 (2.45 %), and sonication time (11.12 min). To prove the reliability of the statistics, verification run was carried out further. The optimized formulation had an average PS of 170.41 nm and %EE of 91.099 % in response to the predicted values of 177 nm and 91.43 % by the software. The percentage error was −0.0369 and −0.011 % for PS and %EE, respectively.
Evaluation of the optimized SLN
X-ray diffractometry
The diffraction pattern of the SLN showed remarkable difference from those of the GMS and ADP. The same is presented in Fig. 2. It was confirmed that ADP existed in amorphous state in the ADP-SLN because of the disappearance of sharp peak of ADP in the diffraction pattern. It was also indicative that GMS in ADP-SLN was partially recrystallized or less ordered, causing increase in drug loading capacity.
FTIR study
The FTIR graphs for ADP-SLN, GMS, and bulk ADP are presented in Fig. 3. From the study, the characteristic peaks of drugs such as of alkene 2800–3000 cm−1, C=O 1600–1800 cm−1, lactone carbonyl stretch 1701 cm−1, carboxylate ion 1566–1546 cm−1, and C–OH stretch 1068 cm−1 disappeared and were replaced by the peak of GMS of H–OH (3300–3311 cm−1), COOH–OH stretch (2914–2848 cm−1), and C=O stretch (1730 cm−1) while remaining peaks also either shifted or replaced in the IR shown in Fig. 3 spectra of formulation. This established drug entrapment in lipid matrix.
Preparation of ADP-SLN gel
Rheological study of gel
Gels of ADP-SLN were prepared by using different concentrations of carbopol 940 (0.3–1 %), of which 0.4 % concentration was selected. The selection criteria for 0.4 % carbopol gel was based upon the consistency of gel, rheological pattern, drug release from the gel, skin hydration, and film-forming properties.
In order to study the influence of storage temperature and lipid matrix, the shear stress variation was recorded for semisolid system. Figure 4 shows the rheological behavior of 0.4 % carbopol 940 gel containing ADP-SLN kept at different temperature. The results were recorded after 1 week of storage at 5, 25, and at 40 °C, and pseudoplastic flow behavior was observed at all temperature conditions which meant ease of transport and storage at ambient temperatures.
Ex vivo skin penetration and localization study
The ex vivo penetration of ADP through HCS skin from SLN-based gel and conventional gel was calculated in terms of mean cumulative amount diffused at each sampling time point during time period of 24 h. Figure 5 represents comparative ex vivo release between ADP-SLN gel and marketed ADP gel. The flux and the permeability coefficient for the ADP-SLN gel was found to be 0.833 μg cm−2 h−1 and 0.095 cm h−1, respectively; the same for the conventional gel was found to be 4.791 μg cm−2 h−1 and 0.033 cm/h, respectively, indicating good permeability and epidermal localization of the SLN gel. The amount of ADP in the donor compartment localized into the HCS and in the receptor compartment over topical application of marketed ADP gel was found to be 52 ± 3.07, 25 ± 1.57, and 23 ± 1.97, respectively, in comparison to ADP-SLN gel, which was found to be 23 ± 2.82, 61 ± 2.36, and 16 ± 1.64, respectively.
Prolonged release of ADP from ADP-SLN can be attributed to embedment of ADP in the lipid matrix. It is believed that ADP might be getting transported in an encapsulated form (encapsulated in the SLN matrix) and while getting transported across the skin, expelled from SLN matrix as a consequence of polymorphic transitions occurring in the solid lipid [19].
Small particle size and lipoidal nature of the SLN attribute for the enhancement of penetration in the skin and its localization for longer period of time. Thus, drug localizing effect in the skin seems possible with novel colloidal particulate drug carriers such as SLN [19].
Ex vivo skin hydration studies
The purpose of this study was to investigate the comparative effect of SLN gel and plain gel on stratum corneum which directly links to moisturizing skin and drug penetration. The dark brown side layer in the photomicrographs represents the top layer of the skin, i.e., stratum corneum (Fig. 6). After application of the marketed gel, thickness of stratum corneum slightly changes, while application of the gel enriched with SLN showed a significant increase in the thickness of the stratum corneum, almost 3-fold as compared with the conventional gel and 3.5-fold compared with the untreated skin.
Because of the small particle size of SLN, it probably covers the surface of the skin, which reduces TEWL and evaporation of water from the skin, thus, increasing the moisture content of the skin and leading to increased thickness of the stratum corneum. This improved skin hydration was probably responsible for the increased drug penetration into the skin as observed in Ex-vivo penetration studies [19, 24].
In vitro occlusion study
The occlusion factor for the ADP-SLN gel was found to be 17.76, 35.09, and 39.49 % at 6th, 24th, and 48th hours, suggesting occlusive characteristics of SLN.
Due to the small particle size, the SLN form the compact structure in which the distance between two particles are so small that they prevent the water loss from the skin which further lead to skin hydration [18, 25]. Same phenomena explained by the occlusive study in which the water loss from beaker is prevented by the film of SLN on the cellulose acetate membrane. Due to this film, the water evaporation is reduced, which lead to little difference in beaker weight (as per formula) and more water content in SLN-applied beaker (sample) as compared to without any gel-containing beaker (reference) [9, 18].
Primary skin irritation study
Skin irritation test of ADP-SLN gel was performed on rabbit. The rabbits were kept under observation for 7 days. The observations were recorded as numerical score for each animal as follows:
-
0 = No visible reaction
-
1 = Mild erythema
-
2 = Intense erythema
-
3 = Intense erythema with edema
-
4 = Intense erythema with edema and vesicular erosion
After follow-up observations for 7 days, the formulation did not indicate any manifestation of skin irritation such as redness of skin or inflammation at site of application (erythema). Thus, it was concluded that the selected formulation was safe for topical application.
The safety demonstrated by the ADP-SLN upon topical application to rabbits can be attributed to the protection from direct contact offered by the lipid matrix of SLN [6, 12]. The SLN are believed to localize in the epidermal layers and sustain the release of ADP from their matrix, thus providing effective pharmacological effect with minimal or no irritation of photosensitization effect [17, 26].
Stability study
The ADP-SLN and ASP-SLN gel were found stable upon storage over the period of 3 months. No change in color, odor, or texture was observed, and also, no precipitate or drug crystals were seen upon storage. There were no significant changes observed in the particle size, zeta potential, and entrapment efficiency values of the SLN.
Conclusion
GMS-based SLN dispersions loaded with ADP, having low particle size, were prepared successfully using pre-emulsion sonication method. It was also studied that lipid and surfactant content plays a primary role in drug entrapment and particle size of the resultant SLN. PXRD and FTIR studies confirmed successful incorporation of ADP into the SLN. With gel-containing nanoparticulate dispersion, 2.5-fold concentration of ADP could be contained in skin layers. Also, ADP-SLN gel showed higher skin hydration and occlusion effect than that of a conventional gel, thus enabling grander drug accumulation in skin. The ADP-SLN gel also demonstrated no interaction with the skin in the primary irritation studies and was stable upon subjected to accelerated stability conditions, thus reinforcing safe and stable topical application of the ADP-SLN.
Abbreviations
- ADP:
-
Adapalene
- SLN:
-
Solid lipid nanoparticles
- ADP-SLN:
-
Adapalene-loaded solid lipid nanoparticles
- GMS:
-
Glyceryl monosterate
- PXRD:
-
Powder X-ray diffraction
- FTIR:
-
Fourier transform infrared
- HCS:
-
Human cadaver skin
- PS:
-
Particle size
- PDI:
-
Polydispersity index
- EE:
-
Entrapment efficiency
- RSM:
-
Response surface methodology
- TEWL:
-
Trans-epidermal water loss
References
Irby CE, Yentzer BA, Feldman SR. A review of ADP in the treatment of acne vulgaris. Drugs. 2004;43:421–4.
Kawashima M, Harada S, Loesche C, Miyachi Y. ADP gel 0.1% is effective and safe for Japanese patients with acne vulgaris: a randomized, multicenter, investigator-blinded, controlled study. J Dermatol Sci. 2008;49:241–8.
Sato T, Akimoto N, Kitamura K, Kurihara H, Hayashi N, Ito A. ADP suppresses sebum accumulation via the inhibition of triacylglycerol biosynthesis and perilipin expression in differentiated hamster sebocytes in-vitro. J Dermatol Sci. 2013;70:204–10.
Piskin S, Uzunali E. A review of the use of adapalene for the treatment of acne vulgaris. Ther Clin Risk Manag. 2007;3:621–4.
Muller RH, Mader K, Gohla S. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–78.
Santos MC, Mehnert W, Schaller M, Korting HC, Gysler A, Haberland A, et al. Drug targeting by solid lipid nanoparticles for dermal use. J Drug Target. 2002;10:489–95.
Shah KA, Date AA, Joshi MD, Patravale VB. Solid lipid nanoparticle of tretinoin: potential in topical delivery. Int J Pharm. 2007;345:163–71.
Leyden JJ, Shalita A, Thiboutot D, Washenik K, Webster G. Topical retinoids in inflammatory acne: a retrospective, investigator-blinded, vehicle-controlled, photographic assessment. Clin Ther. 2005;27:216–24.
Jenning V, Gysler A, Schafer-Korting M, Gohla SH. Vitamin A loaded solid lipid nanoparticles for topical use: occlusive properties and drug targeting to the upper skin. Eur J Pharm Biopharm. 2003;49:211–8.
Vyas SP, Khar RK. Nanoparticles. In: Vyas SP, Khar RK, editors. Targeted and controlled drug delivery-noval carrier systems. New Delhi: CBS; 2002. p. 331–8.
Wissing SA, Muller RH. The influence of solid lipid nanoparticles on skin hydration and viscoelasticity-in vivo study. Eur J Pharm Biopharm. 2003;56:67–72.
Maia CS, Mehnert W, Schafer-Korting M. Solid lipid nanoparticles as drug carriers for topical glucocorticoids. Int J Pharm. 2000;96:165–7.
Muller RH, Wissing SA, Souto EB, Barbosa CM. Development of a controlled release formulation based on SLN and NLC for topical clotrimazole delivery. Int J Pharm. 2004;278:71–7.
Jensen LB, Petersson K, Neilson HM. In-vitro penetration properties of solid lipid nanoparticles in intact and barrier-impaired skin. Eur J Pharm Biopharm. 2008;79:68–75.
Patel NA, Patel NJ, Patel RP. Formulation and evaluation of curcumin gel for topical evaluation. Pharm Dev Technol. 2009;14:83–92.
Bhalekar MR, Pokharkar V, Madgulkar A, Patil N, Patil N. Preparation and evaluation of miconazole nitrate-loaded solid lipid nanoparticles for topical delivery. AAPS Pharm Sci Technol. 2009;10(1):289–96.
Bhalekar MR, Upadhaya PG, Nalawade SD, Madgulkar AR, Kshirsagar SJ. Anti-rheumatic activity of Chloroquine-SLN gel on wistar rats using complete Freund’s adjuvant (CFA) model. Ind j rheumatol. 2015 (in press).
Muller RH, Radtke M, Wissing SA. Solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) in cosmetic and dermatological preparations. Adv Drug Deliv Rev. 2002;54:131–55.
Gohla S, Mader K, Muller RH. Solid lipid nanoparticles (SLN) for controlled drug delivery—a review of the state of the art. Eur J Pharm Biopharm. 2000;50:161–77.
Solanki AB, Parikh JR, Parikh RH. Formulation and optimization of piroxicam proniosomes by 3-factor, 3-level Box-Behnken design. AAPS Pharm Sci Technol. 2007;8:1–7.
Pachuau L, Mazumder B. A study on the effects of different surfactants on ethylcellulose microspheres. Int J PharmTechnol Res. 2009;1:966–71.
Gadhiri M, Vatanara A. Loading hydrophilic drug in solid lipid media as nanoparticles: statistical modeling of entrapment efficiency and particle size. Int J Pharm. 2012;424:128–37.
Yuan H, Huang LF. Solid lipid nanoparticles prepared by solvent diffusion method in a nanoreactor system. Colloids Surfaces B. 2008;61:132–7.
Wissing S, Muller RH. Solid lipid nanoparticles (SLN)—a novel carrier for UV-blockers, die pharmazie. 2001;56:783–786.
Wissing SA, Muller RH. The influence of the crystallinity of lipid nanoparticles on their occlusive properties. Int J Pharm. 2002;242:377–9.
Muller RH, Hommoss A, Pardieke J. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–84.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors report no declarations of interest.
Rights and permissions
About this article
Cite this article
Bhalekar, M., Upadhaya, P. & Madgulkar, A. Formulation and evaluation of Adapalene-loaded nanoparticulates for epidermal localization. Drug Deliv. and Transl. Res. 5, 585–595 (2015). https://doi.org/10.1007/s13346-015-0261-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13346-015-0261-z