Abstract
Insulin autoimmune syndrome (IAS) involves not only fasting hypoglycemia, but also postprandial hyperglycemia. In the present study, we hypothesized that glycated albumin (GA) levels and the GA/HbA1c ratio, which reflect fluctuations in plasma glucose levels, are elevated in IAS patients. Four IAS patients were enrolled in the present study. Thirty-two non-diabetic subjects matched for gender, age, and BMI were used as the control group. The fasting plasma glucose levels in the IAS patients were significantly lower than in the control group. However, the oral glucose tolerance test (OGTT) revealed impaired glucose tolerance or diabetes mellitus in all the IAS patients, and thus the OGTT 2-h plasma glucose levels were significantly higher than in the control group. The GA levels and the GA/HbA1c ratio in the IAS patients were significantly higher than in the control group, despite no significant difference in the HbA1c levels between the two groups. In one case in which IAS spontaneously went into remission, there was a significant correlation between anti-insulin antibodies and GA, but not HbA1c. Improvement in glucose fluctuations was observed by continuous glucose monitoring in another patient along with improvement in the clinical symptoms. Furthermore, anti-insulin antibodies, GA, and the GA/HbA1c ratio decreased, but HbA1c did not change significantly in three IAS patients along with the improvement in clinical symptoms. These results suggest that GA and the GA/HbA1c ratio are useful indicators for determining the level of disease activity in IAS patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Insulin autoimmune syndrome (IAS) is a disease in which a patient who has anti-insulin antibodies, even though he or she has not received an insulin injection, develops fasting hypoglycemia [1]. The serum of an IAS patient contains a large amount of insulin, most of which binds to the anti-insulin autoantibodies. When fasting, the patient may develop hypoglycemia caused by the liberation of insulin from the anti-insulin antibodies. However, it is known that insulin secreted after meals binds to anti-insulin antibodies in the serum, which hamper the action of insulin and thus induces postprandial hyperglycemia [2]. Discontinuing the administration of drugs and supplements that may induce IAS often leads to the spontaneous remission of IAS, which is accompanied by a decrease in anti-insulin antibodies.
HbA1c, an indicator of long-term glycemic control, mainly reflects average plasma glucose levels and to a far lesser extent reflects fluctuations in plasma glucose. On the other hand, glycated albumin (GA) is an indicator of intermediate glycemic control and reflects fluctuations in plasma glucose, as well as the average plasma glucose level [3]. In addition, the GA/HbA1c ratio is known to be an indicator that reflects fluctuations in plasma glucose [4].
It has been demonstrated that IAS patients experience increased glucose fluctuations by continuous glucose monitoring (CGM) analysis [5] because they develop postprandial hyperglycemia in addition to fasting hypoglycemia. We therefore hypothesized that the GA levels and the GA/HbA1c ratio, but not HbA1c levels, in IAS patients are elevated because GA reflects the fluctuations in plasma glucose. The following study aims to prove our hypothesis.
Patients and methods
Patients
Four IAS patients [two males and two females; mean age: 64.5 ± 25.0 years old; mean body mass index (BMI): 21.5 ± 4.8 kg/m2] were enrolled in the present study [6–9]. HbA1c and GA were simultaneously determined during the first visit (Table 1). Hypoglycemia was the primary reason that all the IAS patients visited the hospital. We defined the duration of IAS as the period between the first appearance of hypoglycemic symptoms and the first diagnosis day of IAS. The causes behind the onset of IAS in three patients were unknown. In one of these patients (case 3), it was suggested that IAS was associated with the supplements contained in methionine. A total of 228 Japanese subjects (158 males and 70 females) visited the Health Care Center at Kinki Central Hospital between 16 June and 7 July 2004 for health examinations [10]. All subjects had a 75-g oral glucose tolerance test (OGTT), and their glucose tolerance status was diagnosed according to the the World Health Organization criteria [11]. There were 212 non-diabetic subjects (145 males and 67 females) after the exclusion of 12 subjects with diabetes mellitus and 4 patients already being treated for diabetes mellitus. From these subjects, 32 non-diabetic subjects matched with the IAS patients for age, gender, and BMI were used as the control group (Table 2). The control group included 11 subjects with normal glucose tolerance (NGT), 14 subjects with impaired fasting glucose (IFG), and 7 subjects with impaired glucose tolerance (IGT). The study obtained the approval of the ethics committees of Kawanishi City Hospital and Kinki Central Hospital and was conducted with sufficient care in compliance with the spirit of the Declaration of Helsinki.
Laboratory methods
The blood tests were performed after the patients fasted overnight. OGTT was performed, and the plasma glucose levels and serum immunoreactive insulin (IRI) before, 30 min, 60 min, 120 min, and 180 min after the glucose load were determined. Serum C-peptide (C-peptide immunoreactivity; CPR) and the anti-insulin antibodies were not determined in the control group.
HbA1c, expressed as the National Glycohemoglobin Standardization Program value, was measured through the use of high-performance liquid chromatography. GA was determined by applying the enzymatic method using albumin-specific proteinase, ketoamine oxidase, and albumin assay reagent (Lucica GA-L; Asahi Kasei Pharma Co., Tokyo, Japan). The reference ranges of HbA1c were between 4.6 and 6.2%, and those of GA were between 11.7 and 16.0%. Serum IRI was determined by conducting an enzyme immunoassay using guinea pig anti-human insulin antibodies. Serum CPR was determined by conducting a chemiluminescent enzyme immunoassay (CLEIA) using mouse monoclonal antibodies for human CPR. Anti-insulin antibodies were determined by the radioimmunoassay-polyethylene glycol (RIA-PRG) method (Yamasa Co., Chiba, Japan).
Statistical analyses
All data are shown as mean ± SD. For statistical analysis, the unpaired Student’s t test and the paired Student’s t test were used to compare the two groups. Simple regression analyses were performed to assess the association between two variables with Pearson’s correlation coefficient with the StatView computer program (Version 5.0 for Windows, Abacus Concepts, Berkeley, CA). P-values <0.05 were considered statistically significant.
Results
The patients received a diagnosis of IAS from 3 days to 3 months after the onset of hypoglycemia (Table 1). Fasting IRI levels were high but individual differences were large. Anti-insulin antibodies were high in all the patients at greater than 80%. Only one patient (case 4) had a history of cardiovascular disease (cerebral infarction). In OGTT three patients showed a diabetic pattern, and one patient showed impaired glucose tolerance; no patients had NGT (Fig. 1; Table 1).
Plasma glucose and serum IRI (immunoreactive insulin) after the oral glucose tolerance test (OGTT) in the insulin autoimmune syndrome (IAS) patients. Plasma glucose (closed symbols) and serum IRI (open symbols) after OGTT in four IAS patients are shown (modified from [6–9]). a Case 1, b case 2, c case 3, d case 4
The fasting plasma glucose (FPG) levels in the IAS patients were significantly lower than in the control group (55 ± 25 mg/dl vs. 100 ± 9 mg/dl; P < 0.0001). The OGTT 2-h plasma glucose levels in the IAS patients were significantly higher than in the control group (214 ± 27 mg/dl vs. 124 ± 28 mg/dl; P < 0.0001). Although the serum CPR levels were slightly high at 3.1 ± 2.2 ng/ml, serum IRI levels were significantly higher at 2630 ± 3440 μU/ml. There was no significant difference in the HbA1c levels between the two groups (IAS; 5.9 ± 0.7% vs. control group: 5.6 ± 0.3%; P = 0.479). However, both the GA levels and the GA/HbA1c ratio in the IAS patients were significantly higher than in the control group (GA: 17.8 ± 4.2% vs. 14.6 ± 1.2%; P = 0.003, GA/HbA1c ratio: 3.01 ± 0.37 vs. 2.61 ± 0.18; P = 0.001) (Table 2).
Case 3 followed our advice to quit taking health supplements, leading to a decrease in anti-insulin antibodies and the subsequent disappearance of hypoglycemia [6]. The clinical course for case 3 is shown in Fig. 2. During the clinical course for case 3, while the HbA1c levels barely fluctuated, GA decreased together with the decrease in anti-insulin antibodies and serum IRI levels. There was a significant correlation between anti-insulin antibodies and GA, but not HbA1c (Fig. 3).
Long-term clinical course of case 3, including anti-insulin antibodies, serum IRI (immunoreactive insulin), HbA1c, and GA. Anti-insulin antibodies (Ins Ab; closed symbols), serum IRI (logarithmic transformation; open symbols) (a), HbA1c (closed symbols), and GA (closed symbols) levels (b) in case 3 are shown
After treatment of prednisolone to case 4, hypoglycemic symptoms disappeared [9]. When CGM data at 9 months after onset (dose of prednisolone: 2.5 mg per day) were compared with those at onset, the average glucose (AG) barely changed (Table 3). However, indicators of glycemic fluctuation [ΔBG (the difference between maximum blood glucose and minimum blood glucose) and SD value] were markedly decreased. At 30 months after onset (dose of prednisolone: 2.5 mg per day), anti-insulin antibodies decreased from 95.0% to 59.7%, and the GA/HbA1c ratio also decreased from 3.53 to 3.18.
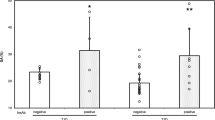
IAS-related examinations could be performed before (active phase) and after (remission phase) improvement in the clinical symptoms in three IAS patients. Along with improvement in the clinical symptoms, anti-insulin antibodies (91.0 ± 3.5% vs. 27.7 ± 28.1%; P = 0.049) decreased in all patients and GA (19.1 ± 3.9% vs. 16.5 ± 2.9%; P = 0.056) and the GA/HbA1c ratio (3.12 ± 0.36 vs. 2.75 ± 0.38; P = 0.002) also decreased (Fig. 4). However, HbA1c did not change significantly (6.1 ± 0.6% vs. 6.0 ± 0.3%; P = 0.603).
Anti-insulin antibodies, HbA1c, GA, and the GA/HbA1c ratio at active phase and remission phase in the IAS patients. Anti-insulin antibodies (a), HbA1c (b), GA (c), and the GA/HbA1c ratio (d) in active phase and remission phase were compared in 3 IAS patients [(closed circle) case 2, (closed square) case 3, and (closed triangle) case 4)]
Discussion
In the present study, we showed that the GA levels and the GA/HbA1c ratio in the IAS patients were significantly higher than in the control group, whereas there was no difference in the HbA1c levels between the two groups. Clinical improvements and decreases in anti-insulin antibodies and serum IRI levels were recognized in case 3, whose long-term clinical course we were able to observe. The GA levels in this patient also decreased to the reference range during the course of development, while the HbA1c levels did not change. There was a significant correlation between the anti-insulin antibodies and GA, but not HbA1c. Improvement in glucose fluctuations was observed by CGM analysis in another patient along with improvement in the clinical symptoms. Furthermore, along with improvement in the clinical symptoms, anti-insulin antibodies, GA, and the GA/HbA1c ratio decreased, but HbA1c did not change significantly in three IAS patients. These results suggest that GA is a useful indicator for determining the level of disease activity in IAS patients.
IAS is positioned as a disease that induces fasting hypoglycemia and is considered one of the causes of diabetes mellitus [11]. We performed OGTT in four IAS patients, all of whom were shown to have impaired glucose intolerance (one patient) or diabetes mellitus (three patients). The OGTT 2-h plasma glucose levels in the IAS patients were significantly higher than in the control group. Reports stated that the use of CGM led to the detection of both hypoglycemia and hyperglycemia in the daily lives of IAS patients [5]. In fact, these phenomena are observed in case 4 in the present study (Table 3).
HbA1c mainly reflects average plasma glucose levels, whereas GA reflects the fluctuations in plasma glucose as well as the average plasma glucose level [3]. Because the fluctuations in plasma glucose were significantly higher in IAS patients, we hypothesized that GA and the GA/HbA1c ratio were relatively high compared with HbA1c in IAS patients. The GA levels and GA/HbA1c ratio in the IAS patients were higher than in the control group, while there was no significant difference in HbA1c levels between the two groups. Along with improvement in the clinical symptoms, anti-insulin antibodies, GA, and the GA/HbA1c ratio decreased, but HbA1c did not change significantly. These results suggest that average plasma glucose levels in the IAS patients may not be different from the control group, while the fluctuations in plasma glucose in IAS patients may have increased. The above results may lead to the conclusion that the anti-insulin antibodies in IAS patients cause an increase in the fluctuations in plasma glucose without affecting average plasma glucose levels. The fluctuations in plasma glucose are known to be a greater risk factor for arteriosclerosis than average plasma glucose levels [12, 13]. For this reason, IAS patients with a high GA/HbA1c ratio, characterized by large fluctuations in plasma glucose, may develop arteriosclerosis. Although only one IAS patient was complicated with atherosclerotic disease (cerebral infarction) in the present study, it should be investigated further in the future.
Most IAS patients visit the hospital following the onset of hypoglycemic symptoms. For this reason, their symptoms need to be accurately distinguished from insulinoma, non-islet cell tumor hypoglycemia (NICTH), and other diseases that induce fasting hypoglycemia. Insulinoma and NICTH involve the persistent state of chronic hypoglycemia. Because of this, it seems that both the HbA1c and GA levels in a patient with either of these diseases may be low. While the HbA1c levels of the IAS patients in this study remained at the reference level, the GA levels and GA/HbA1c ratio were elevated. Therefore, the GA level or the GA/HbA1c ratio may be useful in distinguishing IAS from other diseases that induce hypoglycemia. These questions also need to be further examined in the future.
There are several limitations in the present study. First, the sample size was small. Second, we discussed the relationship between GA and glucose fluctuations detected by CGM in only one patient. Third, we also discussed pre- and post-treatment changes in the glucose fluctuations in only one patient. These questions, in addition to the knowledge obtained from the present study, need to be explored in a large number of IAS patients.
In conclusion, the GA levels and GA/HbA1c ratio in IAS patients were elevated reflecting the fluctuations in plasma glucose, and as anti-insulin antibodies decreased, the GA levels and the GA/HbA1c ratio also fell. These results suggest that GA and the GA/HbA1c ratio are useful indicators for determining the level of disease activity in IAS patients.
References
Hirata Y, Ishizu H, Ouchi N, Motomura S, Abe M, Hara Y, et al. Insulin autoimmunity in a case of spontaneous hypoglycemia. J Jpn Diabetes Soc. 1970;13:312–20.
Uchigata Y, Hirata Y, Iwamoto Y. Insulin autoimmune syndrome (Hirata disease): epidemiology in Asia, including Japan. Diabetol Int. 2010;1:21–5.
Koga M. Glycated albumin; clinical usefulness. Clin Chim Acta. 2014;433:96–104.
Ogawa A, Hayashi A, Kishihara E, Yoshino S, Takeuchi A, Shichiri M. New indices for predicting glycaemic variability. PLoS One. 2012;7:e46517.
Philippon M, Sejil S, Mugnier M, Rocher L, Guibergia C, Vialettes B, et al. Use of the continuous glucose monitoring system to treat insulin autoimmune syndrome: quantification of glucose excursions and evaluation of treatment efficacy. Diabet Med. 2014;31:e20–4.
Taniguchi J, Kumekawa M, Asahi N, Sato T, Ohno A, Ueki A, et al. A case of insulin autoimmune syndrome suspected of showing false positive results for insulin receptor antibody. J Jpn Diabetes Soc. 2009;52:449–55.
Nakatani Y, Banba N, Shimizu H, Momomegi N, Matsumura M, Hibi T, et al. A case of insulin autoimmune syndrome whose anti-insulin antibodies were improved by a small dose of glucocorticoid. J Jpn Diabetes Soc. 2010;54(Suppl 1):S210.
Deguchi A, Okauchi Y, Suehara S, Mineo I. Insulin autoimmune syndrome in a health supplement user: the effectiveness of cornstarch therapy for treating hypoglycemia. Intern Med. 2013;52:369–72.
Yoshino H, Kawakami K, Watanabe T, Yoshino G, Kohriyama K. A case of insulin autoimmune syndrome in an elder patient. Europ Geriatr Med. 2015;6:85–6.
Koga M, Otsuki M, Matsumoto S, Saito H, Mukai M, Kasayama S. Negative association of obesity and its related chronic inflammation with serum glycated albumin but not glycated hemoglobin levels. Clin Chim Acta. 2007;378:48–52.
Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–53.
The Decode study group. Glucose tolerance and mortality: comparison of WHO and American Diabetes Association diagnostic criteria. Lancet. 1999;354:617–21.
Tominaga M, Eguchi H, Manaka H, Igarashi K, Kato T, Sekikawa A. Impaired glucose tolerance is a risk factor for cardiovascular disease, but not impaired fasting glucose. The Funagata Diabetes Study. Diabetes Care. 1999;22:920–4.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Human rights and informed consent
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent or a substitute for it was obtained from all patients prior to their participation in this study.
About this article
Cite this article
Koga, M., Inada, S., Taniguchi, J. et al. High glycated albumin (GA) levels and the GA/HbA1c ratio in patients with insulin autoimmune syndrome. Diabetol Int 8, 199–204 (2017). https://doi.org/10.1007/s13340-016-0294-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13340-016-0294-7