Abstract
Rabies is an acute viral infection that causes encephalomyelitis in almost all warm blooded animals and is invariably fatal once the clinical signs appear. The present study was carried out to assess the effect of recombinant human interferon alpha (rhIFN α-2A) treatment on the survival of rabies infected mice and its correlation with cytokines expression. The gene expression of TNF-α and IL-6 was measured by SYBR Green Real Time PCR for two groups—“Pre-exposure” (mice were inoculated with rhIFN α-2A prior to rabies infection) and “Post-exposure” (mice were inoculated with rhIFN α-2A post rabies virus infection). Delayed mortality was observed in interferon treated infected groups. In addition, statistically significant decrease (P < 0.0001) in the expression of TNF-α and IL-6 was observed, both in the pre-exposure and post-exposure groups. These findings indicate that modulation of cytokine secretion using exogenous biologicals such as rhIFN may offer novel therapeutic approaches to treat diseases such as rabies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rabies is known since antiquity, yet the present situation is quite poignant. A highly effective and 100 % preventable post-exposure prophylaxis already exists [33], despite this, rabies continues to pose a significant health problem particularly in rural communities. In addition, once the clinical signs and symptoms appear, vaccination and immunoglobulin are ineffective and the disease invariably proves to be fatal. However, an interesting case of complete recovery of a symptomatic patient after experimental therapy of antivirals during drug-induced coma [12, 35] has opened up new avenue for effective therapeutics.
The use of synthetic immunomodulators such as Hepon [29], anti-viral drugs and interferon or interferon inducers against rabies has previously been reported in various animal models [10]. Bovine parainfluenza type 3 virus and Influenza virus vaccine have also demonstrated protection against rabies virus (RV) in rabbits [13] and hamsters [34] respectively. Investigators have previously demonstrated success against RV even with Polyriboinosinic–Polyribocytidylic acid (poly I:C) [13], a synthetic double stranded RNA and a potent interferon inducer [16]. The underlying mechanism of poly I:C is presumed to be stimulation of high levels of serum interferon [11], though it appears to be a poor inducer in man, thus limiting its use [6, 8]. However, Levy et al. have showed that high levels of serum interferon can be induced in primates on conjugation of poly I:C to poly-l-lysine and carboxymethylcellulose [16]. In addition, the effect of exogenous interferon treatment in RV infected mice or monkeys has also been investigated by many researchers [9, 10, 24, 32]. However, the mechanism behind the reported protective activity of exogenous interferon is not clear. Recently Tohamy et al., demonstrated that pre-treating rabies infected mice with interferon significantly reduced cytogenetic changes [31]. These reports formed the basis of the present study which is an attempt to investigate the role of recombinant human interferon alpha (rhIFN α-2A) on prognosis and expression of innate immunity cytokines during rabies infection.
Materials and methods
Animals, virus, interferon and antibodies
Three to four week old Swiss Albino mice were inoculated with CVS-11, a fixed RV strain. Roferon-A—recombinant human interferon alpha-2a (rhIFN α-2A) of 3 million IU (MIU), by Roche Pharmaceutical was also included in the study. Light Diagnostics™ Rabies Polyclonal DFA reagent (Goat IgG FITC Conjugate) specific as anti-RV nucleoprotein (N), reactive with all lyssaviruses; was obtained from Merck, Millipore, Inc.
Animal infection and tissue collection
Mice were divided into five groups of six mice each. Group1 (CVS)- mice were i.c inoculated with 10LD50 (in 30 µL) of CVS; Group 2 (Interferon)- mice were i.c inoculated with 30 µL of 3 × 105 IU/mL of interferon; Group 3 (Pre-exposure)- mice were primed with 30 µL of 3 × 105 IU/mL interferon followed by 30 µL of 10LD50 of CVS 24 h later; Group 4 (Post-exposure)- mice were i.c inoculated with 30 µL of 10LD50 of CVS and 24 h post infection (p.i), 30 µL of 3 × 105 IU/mL interferon was given; Group 5 (Control)- Phosphate buffered saline (PBS) inoculated mice served as controls. All the procedures were conducted in accordance with guidelines under animal protocols approved by the Institute Animal Ethics Committee and Committee for the Purpose of Control and Supervision of Experimental Animals (CPCSEA) guidelines. All groups of mice were observed daily twice for clinical findings. In virus infected groups, when moribund, mice were euthanized; brain tissues were harvested and stored at −80 °C until further processing. By day 7 p.i, mice from all the groups were sacrificed and brain tissues were harvested.
Direct fluorescent antibody test (FAT)
To detect the presence of rabies antigen in harvested tissues from Group 1, Group 3 and Group 4, impression smears were made and the slides were stained using the Rabies Polyclonal DFA reagent (Merck, Millipore, Inc.) as per CDC protocol for post-mortem diagnosis of rabies in animals [4]. Briefly, touch impression smears were made on glass slides from the harvested brain tissues and allowed to air dry. 1:20 diluted DFA reagent was added, enough to cover the smear area and incubated in humid chambers at 37 °C for 30 min in dark. The slides were then washed (3 × 10 min) with 1× PBS and air dried (in dark) and observed under a fluorescent microscope.
Total RNA extraction
Harvested mice brains were homogenized in PBS, centrifuged and supernatant was added to the AVL (lysis buffer) provided in the kit. Total RNA was extracted manually using a QIAamp® Viral RNA mini kit (Qiagen India Pvt. Ltd., New Delhi, India) as per the manufacturer’s instructions. The extracted viral RNA was stored in a −80 °C freezer until further processing.
Nested RT-PCR
To confirm the results of direct fluorescent antibody test, nested RT-PCR specific for N gene was performed on extracted RNA using a protocol by Nagaraj et al. with minor modifications [21] using gene specific primers (Table 1). The first-round PCR was performed using a one-step Reverse Transcriptase PCR (RT-PCR) kit (Qiagen) and the second-round PCR was carried out using a PCR Master Mix (Fermentas Inc., Maryland, USA) as per the manufacturer’s instructions. The PCR products were then run on agarose gel electrophoresis (1.5 % agarose gel infused with SybrSafe® Invitrogen dye for visualization); for the confirmation. The nested PCR product had a size of 762 bp.
Real-time SYBR Green PCR
Total RNA extracted (as described earlier) was also used for detection of innate cytokines namely TNF-α and IL-6 (Table 1) using Takara One Step SYBR® Ex Taq™ qRT-PCR Kit as per manufacturer’s instructions. Glyceraldehyde-3 phosphate dehydrogenase (GAPDH) was used as an endogenous reference gene. Briefly, 2 µL RNA template was added to 18 µL reaction mixture (as per manufacturer’s instructions) with 0.8 µM primer for each of the target genes. The cycling conditions used were: RT step 42 °C/300 s; RT inactivation 95 °C/10 s; 40 cycles of Denaturation, 94 °C/30 s; Annealing 54 °C (GAPDH), 50 °C (TNF-α), 55 °C (IL-6), for 45 s and Extension 72 °C/60 s; followed by melt curve analysis, 95 °C/60 s; increase in temperature from 60 °C to 95 °C at R-0.2 °C. Amplification, data acquisition and analysis were carried out by using ABI Step One instrument. Relative gene expression of cytokines was analysed using the comparative CT method (2−ΔΔCT method) [17, 28].
Statistical analyses
Results for each of the innate cytokine gene expression were expressed as mean ± SEM for each group under the study. Evaluation of the significances of the differences between the means of parameters was performed using one-way ANOVA and Tukey test. In all cases, P < 0.05 was considered significant. Graphs were plotted and statistical analyses were done using GraphPad Prism 5 Software (GraphPad Software, San Diego, CA).
Results
Clinical manifestations
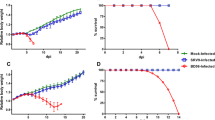
All the experimental groups under study were observed daily for clinical findings and mortality, if any. No clinical abnormality or mortalities were observed in group 2 (Interferon) and group 5 (control), considering which at the end of observation period; a complete necropsy was performed in all the animals following euthanasia. All tissues and organs were grossly checked but no abnormalities were noted. In RV inoculated groups i.e. group 1 (CVS), group 3 (Pre-exposure) and group 4 (Post-exposure) characteristic clinical changes were observed in mice in the form of ruffling of fur, toe walking, hunched back, hind limb paralysis, complete immobilization, tremors and finally death (Fig. 1). Signs in all three CVS infected groups began to appear on day 3 p.i. but hind limb paralysis was more pronounced by day 5 p.i. Interestingly, while complete immobilization was observed by day 6 p.i. in all three groups, it started culminating into death in group 1 and was more pronounced in group 3 by day 7 p.i. but no mortality was observed in group 4. Mice in post-exposure group continued to remain immobile and exhibited tremors till day 7 p.i.
Clinical manifestations recorded over the duration of experimentation in the animals a Ruffled fur, b Toe walking, c Hunched back, d Hind limb paralysis and e Complete immobilization; f shows survival chart of animals under study. Graphs depict number of animals v/s days post infection in experimental groups
Antigen detection
In addition to the clinical manifestations, characterization of central nervous system (CNS) invasion in all three CVS infected groups was ascertained by immunofluorescence (FAT) and conventional nested RT-PCR. FAT results were interpreted and graded based on staining intensity and distribution of antigen (Fig. 2a, b). It is important to emphasize that there appeared to be no difference in antigen distribution pattern or staining intensity in the FAT results between the pre-exposure and post-exposure groups. Immunofluorescence results were confirmed and found to be in consensus with results of nested PCR (Fig. 2c).
Detection of rabies nucleoprotein antigen by FAT using FITC tagged antibody. The presence of well-distributed, punctiform nucleoproteins showing apple green fluorescence was considered as a positive. a Normal mouse brain, b rabies infected mouse brain, c detection of rabies N gene using nested RT-PCR—Lane 1 Ladder, Lane 2 1477 bp product of the first round (outer), Lane 3 762 bp product of the second round (inner) of PCR
Effect on innate immunity cytokines
In order to assess the effect of rhIFN on expression of innate immunity cytokines, mRNA levels of TNF-α and IL-6 were determined using real time SYBR green PCR. It was observed that TNF- α and IL-6 levels were found to be upregulated between 1.9 and 7.9 fold in groups inoculated with only CVS or only IFN at 7 d.p.i. However, a 16 fold downregulation in the levels of TNF-α was observed in the pre-exposure group as oppose to the 4 fold decrease in post-exposure group. Similarly, an evident downregulation in IL-6 levels was observed in the interferon treated groups with a little over 2-fold downregulation in post-exposure group that exhibited delayed mortality (Fig. 3).
Results of fold change in expression for a TNF-α and b IL-6 are expressed as the mean ± SEM for each group. Evaluation of the significance of differences between the means of parameters was performed using the One way ANOVA and Tukey test. In all cases P < 0.05 was considered significant. (*P < 0.05; **P < 0.01; ***P < 0.0001)
Discussion
Interferon, has been known to modify the immune response of the CNS—behaviourally as well as neurophysiologically [5, 27] and our previous study has shown that exogenously administered rhIFN α can effectively modify cytokine expression in the brain [20]. Our results were similar to that reported by Tohamy et al., where pre treatment of mice with IFN, 24 h before infection delayed the onset of specific rabies signs [31]. Therefore, based on development of characteristic signs of illness in the pre-exposure and post-exposure groups, we speculated that priming with interferon in the pre-exposure group can lead to local production of cytokines and recruitment of lymphocytes. This in turn leads to an amplified cytokine response that aggravated on RV infection and hence may be responsible for intensifying the development of disease signs. This speculation was also based on an earlier report that suggests that RV induced pro-inflammatory response of IL-1α/β or TNF-α may activate the p55 TNF-α capable of recruiting lymphocyte leading to a cascade of cytokine response [30].
Upregulation in the levels of TNF-α in CVS infected group was as expected. Some previous reports indicate a positive correlation between RV infection and TNF-α expression [15, 22, 30]. Nuovo et al. have shown that TNF-α is expressed not by rabies infected cells but by the non-infected cells that are in close proximity of the virally infected cell [22]. We therefore hypothesize that rhIFN appears to somehow limit the infectivity of RV and therefore subsequently limit or suppress the expression of TNF-α in both pre-exposure and post-exposure group; which could be a possible explanation for the downregulation observed in both IFN treated RV infected groups. However, further immunohistochemical studies are warranted to investigate the extent of RV infection and inflammation as well as TNF-α localization. It follows that our results support the hypothesis put forward by Nuovo et al., that inhibition of cytokine expression may have some therapeutic potential. A 16 fold downregulation in the level of TNF-α observed in the pre-exposure group may be a result of the amplified immune response generated prior to infection suggesting that over-suppression may not always be beneficial. This is crucial since cytokine-mediated neuroprotection is concentration dependent and may get hampered when concentrations are either too low or too high [3]. Though, decrease in TNF- α levels has proven to be beneficial in several cases like Guillian–Barre syndrome (GBS), a disease often confused with the paralytic form of rabies [26]. In some other cases, inhibition or absence of TNF-α is known to decrease the severity of CNS autoimmune disease such as experimental allergic encephalomyelitis (EAE) [23].
A similar trend was observed with IL-6 expression though neuroprotective effects of IL-6 are found to be independent of TNF-α [3]. While a 4-7 fold upregulation was observed in groups administered with either interferon or CVS, an evident downregulation was observed in the post-exposure groups exhibiting relatively prolonged survival. These findings are interesting since lower IL-6 levels are associated with greater survival rates in RV infection. Delayed mortality in CVS infected mice deficient for the p55 Kd TNF-α receptor (p55TNFR−/−) is also found to be correlated with lower levels of IL 6 in the brain [2]. Decrease in mRNA expression of IL-2 and more pronounced IL-6 expression has also been reported in paws of dogs immunized with the anti-rabies vaccine (ARV) [25]. Our results are similar to a previous study that reported higher levels of IL-6 on day 7 p.i. in only rabies infected group as well as infected- vaccinated group as compared to control mice. However, lower levels of IL-6 were observed, together with greater survival rates in the group infected, vaccinated and treated with an immunomodulator such as Propionibacterium acnes [18, 19]. It is evident that though viral infection and in some cases vaccination too, both by themselves may be capable of stimulating the cytokine response, introduction of an immunomodulator in combination can significantly alter the gene expression, at times leading to even downregulation or inhibition that may prove beneficial to the host.
These findings suggest that the use of rhIFN alongwith vaccine can offer better protection post-exposure to the virus. This is similar to theory proposed by many researchers nearly a decade ago that recommend the use of vaccines in combination with exogenous interferon or an interferon inducer such as poly I:C as intervention post exposure to RV [1, 8, 34]. In addition, administration of booster doses of interferon may prove beneficial [9, 10]. Interferon therapy has also been employed to treat and successfully help recover a patient infected with West Nile Virus encephalitis [14]. Interferon treatment is also found to reduce the infectivity of vesicular stomatitis virus (VSV), another member of rhabdoviridae by inhibiting the transport of the envelope glycoprotein to the plasma membrane [7]. It is evident from the study that exogenous rhIFN α could not prevent the onset of rabies in either the pre or post exposure group but delayed mortality was observed in the latter suggesting that rhIFN holds some potential as candidate for post exposure intervention in rabies infection.
References
Baer GM, Moore SA, Shaddock JH, Levy HB. An effective rabies treatment in exposed monkeys: a single dose of interferon inducer and vaccine. Bull World Health Organ. 1979;57(5):807–13.
Camelo S, Lafage M, Lafon M. Absence of the p55 Kd TNF-alpha receptor promotes survival in rabies virus acute encephalitis. J Neurovirol. 2000;6(6):507–18.
Carlson NG, Wieggel WA, Chen J, Bacchi A, Rogers SW, Gahring LC. Inflammatory cytokines IL-1 alpha, IL-1 beta, IL-6, and TNF-alpha impart neuroprotection to an excitotoxin through distinct pathways. J Immunol. 1999;163(7):3963–8.
(CDC) CfDCaP. Protocol for postmortem diagnosis of rabies in animals by direct fluorescent antibody testing. A minimum standard for rabies diagnosis in the United States. Atlanta.
Dafny N, Prieto-Gomez B, Reyes-Vazquez C. Does the immune system communicate with the central nervous system? Interferon modifies central nervous activity. J Neuroimmunol. 1985;9(1–2):1–12.
Guggenheim MA, Baron S. Clinical studies of an interferon inducer, polyriboinosinic-polyribocytidylic acid [poly (I)-poly (C)], in children. J Infect Dis. 1977;136(1):50–8.
Hansen BD, Nara PL, Maheshwari RK, Sidhu GS, Bernbaum JG, Hoekzema D, et al. Loss of infectivity by progeny virus from alpha interferon-treated human immunodeficiency virus type 1-infected T cells is associated with defective assembly of envelope gp120. J Virol. 1992;66(12):7543–8.
Harmon MW, Janis B. Therapy of murine rabies after exposure: efficacy of polyriboinosinic-polyribocytidylic acid alone and in combination with three rabies vaccines. J Infect Dis. 1975;132(3):241–9.
Hilfenhaus J, Karges HE, Weinmann E, Barth R. Effect of administered human interferon on experimental rabies in monkeys. Infect Immun. 1975;11(5):1156–8.
Hilfenhaus J, Weinmann E, Majer M, Barth R, Jaeger O. Administration of human interferon to rabies virus-infected monkeys after exposure. J Infect Dis. 1977;135(5):846–9.
Ho M, Nash C, Morgan CW, Armstrong JA, Carroll RG, Postic B. Interferon administered in the cerebrospinal space and its effect on rabies in rabbits. Infect Immun. 1974;9(2):286–93.
Jackson AC. Update on rabies diagnosis and treatment. Curr Infect Dis Rep. 2009;11(4):296–301.
Janis B, Habel K. Rabies in rabbits and mice: protective effect of polyriboinosinic-polyribocytidylic acid. J Infect Dis. 1972;125(4):345–52.
Kalil AC, Devetten MP, Singh S, Lesiak B, Poage DP, Bargenquast K, et al. Use of interferon-alpha in patients with West Nile encephalitis: report of 2 cases. Clin Infect Dis. 2005;40(5):764–6.
Kuang Y, Lackay SN, Zhao L, Fu ZF. Role of chemokines in the enhancement of BBB permeability and inflammatory infiltration after rabies virus infection. Virus Res. 2009;144(1–2):18–26.
Levy HB, Baer G, Baron S, Buckler CE, Gibbs CJ, Iadarola MJ, et al. A modified polyriboinosinic-polyribocytidylic acid complex that induces interferon in primates. J Infect Dis. 1975;132(4):434–9.
Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) method. Methods. 2001;25(4):402–8.
Megid J, Peracolli MT, Curi PR, Zanetti CR, Cabrera WH, Vassao R, et al. Effect of vaccination and the immunomodulators “bacillus of Calmette-Guerin, avridine and Propionibacterium acnes” on rabies in mice. Comp Immunol Microbiol Infect Dis. 1998;21(4):305–18.
Megid J, Kaneno R, Nozaki CN, Brito CJ, Almeida MF. Increased interleukin-10 associated with low IL-6 concentration correlated with greater survival rates in mice infected by rabies virus vaccinated against it and immunomodulated with P. acnes. Comp Immunol Microbiol Infect Dis. 2004;27(6):393–411.
Mehta S, Mukherjee S, Balasubramanian D, Chowdhary A. Evaluation of neuroimmunomodulatory activity of recombinant human interferon alpha. Neuroimmunomodulation. 2014;21(5):250–6.
Nagaraj T, Vasanth JP, Desai A, Kamat A, Madhusudana SN, Ravi V. Ante mortem diagnosis of human rabies using saliva samples: comparison of real time and conventional RT-PCR techniques. J Clin Virol. 2006;36(1):17–23.
Nuovo GJ, Defaria DL, Chanona-Vilchi JG, Zhang Y. Molecular detection of rabies encephalitis and correlation with cytokine expression. Mod Pathol. 2005;18(1):62–7.
Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-α is neither necessary nor sufficient. J Immunol. 2007;178(11):7334–43.
Postic B, Fenje P. Effect of administered interferon on rabies in rabbits. Appl Microbiol. 1971;22(3):428–31.
Quaranta A, Siniscalchi M, Albrizio M, Volpe S, Buonavoglia C, Vallortigara G. Influence of behavioural lateralization on interleukin-2 and interleukin-6 gene expression in dogs before and after immunization with rabies vaccine. Behav Brain Res. 2008;186(2):256–60.
Radhakrishnan VV, Sumi MG, Reuben S, Mathai A, Nair MD. Circulating tumour necrosis factor alpha & soluble TNF receptors in patients with Guillain-Barre syndrome. Indian J Med Res. 2003;117:216–20.
Reyes-Vazquez C, Mendoza-Fernandez V, Herrera-Ruiz M, Dafny N. Interferon modulates glucose-sensitive neurons in the hypothalamus. Exp Brain Res. 1997;116(3):519–24.
Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3(6):1101–8.
Silin DS, Lyubomska OV, Ershov FI, Frolov VM, Kutsyna GA. Synthetic and natural immunomodulators acting as interferon inducers. Curr Pharm Des. 2009;15(11):1238–47.
Solanki A, Radotra BD, Vasishta RK. Correlation of cytokine expression with rabies virus distribution in rabies encephalitis. J Neuroimmunol. 2009;217(1–2):85–9.
Tohamy AA, Fahmy AM, Dkhil MA, Diab MSM. Protective role of interferon against cytotoxicity induced by rabies virus in mice. Afr J Biotechnol. 2010;9(7):1097–105.
Weinmann E, Majer M, Hilfenhaus J. Intramuscular and/or intralumbar postexposure treatment of rabies virus-infected cynomolgus monkeys with human interferon. Infect Immun. 1979;24(1):24–31.
WHO. Rabies vaccines. WHO position paper. The Weekly Epidemiological Record. 2007;82(49–50):425–435.
Wiktor TJ, Postic B, Ho M, Koprowski H. Role of interferon induction in the protective activity of rabies vaccines. J Infect Dis. 1972;126(4):408–18.
Willoughby RE Jr, Tieves KS, Hoffman GM, Ghanayem NS, Amlie-Lefond CM, Schwabe MJ, et al. Survival after treatment of rabies with induction of coma. N Engl J Med. 2005;352(24):2508–14.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehta, S., Roy, S., Mukherjee, S. et al. Exogenous interferon prolongs survival of rabies infected mice. VirusDis. 26, 163–169 (2015). https://doi.org/10.1007/s13337-015-0269-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13337-015-0269-5