Abstract
Background and Objective
Lithium, which is used to treat bipolar disorder, has a narrow therapeutic blood concentration range and quickly reaches clinically toxic levels. We performed a population pharmacokinetic analysis with a lithium tubular reabsorption model including urinary pH and investigated the relationship between blood lithium concentration and tremor as a side effect.
Methods
Routine clinical data, including 389 serum concentrations, were collected from 214 patients orally administered an adjusted amount of lithium carbonate. Pharmacokinetics were described using a one-compartment distribution model with first-order absorption and elimination. The fractions of the MID (Li+ + LiCO3−) and ION (2Li+ + CO32−) forms were calculated using the Henderson–Hasselbalch equation, and the influences of these fractions on clearance (CL) were evaluated. The rate of tremor development was analyzed using a logit model.
Results
Oral apparent CL (CL/F) was explained by nonrenal CL and renal CL, and renal CL was varied by the fractions of lithium forms influenced by urinary pH. The contribution of MID to CL was slightly larger than that of ION. The rate of tremor development was estimated to be more than 30% when the trough lithium concentration was greater than 1.26 mEq L−1.
Conclusion
Renal function and urinary pH are important indices in lithium treatment, so the serum concentration of lithium may be predicted based on the renal function and urinary pH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
We found that the blood lithium concentration of each patient can be predicted from the individual’s urine pH and renal function. Furthermore, the relationship between the expression of tremor (a side effect of lithium) and the blood lithium concentration was clarified. |
1 Introduction
In 1949, lithium was found to be effective against the manic phase of manic-depressive psychosis by Cade [1]. The efficacy ratio was subsequently reported to be approximately 70–80% in 1968 [2]. Lithium carbonate has since been used to treat bipolar disorder [3, 4], and its therapeutic effects were recently reconfirmed [5, 6]. Bipolar disorder is a psychiatric disease that repeats the depression and manic phases. However, the underlying disease mechanisms and validated pharmacological targets are yet to be clarified in detail. The current treatment strategy is mainly medical therapy that uses lithium carbonate as the first-choice drug and aims at relapse prevention, remission maintenance, and the enhancement of social and occupational functioning.
Administered lithium carbonate quickly dissociates in the body. Lithium ions (Li+) do not bind to plasma proteins or undergo metabolism; lithium is principally eliminated via renal excretion [7]. Lithium is filtered through the glomerular membrane depending on the blood flow rate in the kidney, and approximately 80% of the filtered lithium is then reabsorbed at the proximal tubule, Henle’s loop, and the distal tubule [8]. This reabsorption is competitively inhibited by sodium; sodium deficiency has been shown to promote lithium reabsorption and consequently causes an increase in serum lithium concentration [9]. The dissociation of lithium carbonate and the fraction of Li+ in urine are both influenced by pH, with the concentration of the ionic form increasing under acidic conditions. Since only Li+ is reabsorbed at the tubules, the extent of tubular reabsorption of lithium is also affected by urinary pH. However, dosing strategies for individual patients that account for the process of lithium tubular reabsorption have not yet been reported.
The target therapeutic range of the trough lithium concentration is narrow (0.4–1.0 mEq L−1) [10], and a high serum concentration exceeding this range has been shown to induce poisoning symptoms [11]. Clinical efficacy, safety, and side effects closely correlate with the serum concentration of lithium [12]. Furthermore, not only the effectiveness but also the degree of adverse drug reactions were found to be proportional to the trough lithium concentration, even within the therapeutic range [13]. Therefore, dosage adjustments of lithium based on the trough concentration are recommended.
Lithium carbonate induces a number of side effects in patients, including renal disorders, hypothyroidism, and weight gain [14]. However, the pharmacological and toxicological mechanisms of lithium have not yet been elucidated. A previous study reported that 13.9% of patients treated with lithium developed side effects [15], with tremor (4.1%) being the most frequent. A search for expression-related factors with a focus on only neurological symptoms such as tremors has not been conducted, even though lithium has been used for more than 50 years.
The study reported in the present paper had two aims. A population pharmacokinetic analysis was performed in order to investigate the effects of the tubular reabsorption of lithium and evaluate the influence of urinary pH variability on lithium clearance (CL). The relationship between serum lithium concentration and tremor as a side effect was also examined.
2 Patients and Methods
2.1 Ethics
The present study was performed in accordance with the Helsinki Declaration, after approval by the ethical review board of the University of Toyama (approval number: clinical 26–39). It was then approved by Yahata Kousei Hospital, Iizuka Hospital, and Fukuma Hospital. Written consent was obtained from all patients, and patient privacy and personal information were respected.
2.2 Patients and Measurement of Lithium Concentrations
Routine clinical data, including 389 serum lithium concentrations, were collected from 214 patients (91 males and 123 females). All patients received lithium carbonate for the treatment of psychiatric disorders, mainly bipolar disorder and schizophrenia, between December 2003 and August 2016 at three hospitals: Yahata Kousei Hospital, Iizuka Hospital, and Fukuma Hospital, Fukuoka, Japan. Patients receiving dialysis therapy and pregnant women were excluded. Lithium concentrations were measured using the ionic electrode method (at Yahata Kousei Hospital and Fukuma Hospital) or atomic absorption spectrometry (at Iizuka Hospital). The intra/interday coefficient of variation (CV) was less than 7% at all hospitals, and the lower limits of quantification were 0.05, 0.02, and 0.13 mEq L−1 at the three hospitals, respectively.
2.3 Population Pharmacokinetics
Population pharmacokinetic analysis was performed using the data for 214 patients. Population pharmacokinetic and exposure–response analyses were performed using the first-order conditional estimation with interactions (FOCE-I) method with the nonlinear mixed-effect modeling software NONMEM® version 7.3.0 (ICON Development Solutions, Gaithersburg, MD, USA). The entire procedure, including the execution of model runs, bootstrapping, the visual predictive check (VPC), and results management, was conducted in Wings for NONMEM, and a graphical analysis was performed using R (version 3.4.2). The pharmacokinetic model we used assumed a two-compartment distribution model for lithium with first-order absorption and elimination (ADVAN 13, TOL = 9). Pharmacokinetic parameters were oral apparent clearance (CL/F), the apparent volume of distribution compartment (VC/F), the virtual renal compartment focused on glomerulus and tubule (RGT) (VRGT), and the absorption half-life (t1/2,abs). The absorption rate constant (Ka) was calculated by dividing the natural logarithm of 2 by t1/2,abs. The between-subject variability in the pharmacokinetic parameter CL/F was modeled using a log-normal distribution, as shown below (Eq. 1):
Here, Pi is the pharmacokinetic parameter for the ith individual, PPOP is the population mean value of the parameters, and ηi is a normally distributed random variable with mean zero and variance ω2. Residual unidentified variability was modeled with the proportional error in the lithium concentration, as shown below (Eq. 2):
Here, Cij is the jth measured concentration in the ith subject and CPREDij is the predicted concentration based on the model. εCV is the proportional error model component with a mean of zero and a variance of σ2 for the concentration. In order to convert the data obtained on lithium dosages from milligrams of lithium carbonate into the mEq L−1 Li+ dose, we used Eq. 3 [16]:
The value of the objective function (OFV) obtained in the NONMEM® fitting routine was used to test the significance of various factors that influenced pharmacokinetic parameters. The difference in OFV (ΔOFV) noted in model comparisons was asymptotically distributed according to the chi-squared distribution, with the number of degrees of freedom (df) being equal to the difference in the number of parameters between the two models. The significance level was set at P < 0.05 (ΔOFV: 3.84). The stability of the model was assessed by confirming that the condition number, calculated as the ratio of the largest and smallest eigenvalues of the standard error variance–covariance matrix, was less than 1000 [35].
2.4 Dissociation Constants of Lithium Carbonate
Lithium carbonate has two dissociation constants (pKa): pKa1 = 6.2 and pKa2 = 9.7, which are calculated using the Henderson–Hasselbalch equation (Eq. 4) [17, 18]. [HA] is the molar concentration of an undissociated weak acid and [A−] is the molar concentration of a conjugate base.
Lithium carbonate dissociates in two steps, as shown below (Eq. 5):
Here, 2Li+ + CO32−, Li+ + LiCO3−, and Li2CO3 are denoted the ionic (ION) form, the middle (MID) form, and the molecular (MOL) form of lithium carbonate, respectively. Urine is on average weakly acidic (pH 6.0–6.5); however, the pH value actually varies in the interval 4.5–7.5 because the kidneys excrete excess acid or alkali in order to maintain a constant internal environment. The fraction of the MOL form was calculated as 0.6% at pH 7.5 and 1.9% at pH 8.0. In the present study, we assumed that only the ION and MID forms were involved in the process of glomerular filtration and tubular reabsorption, as shown in Fig. 1.
2.5 Lithium Tubular Reabsorption Model
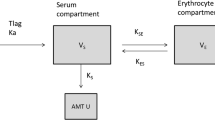
Lithium administered and transferred to the general circulation enters the renal tubules after undergoing glomerular filtration. Thereafter, a large proportion of the lithium is reabsorbed by the tubules and returned to the renal vein. Given this mechanism, we prepared a RGT compartment representing a kidney, and assumed a transition clearance of Q between the central and the RGT compartments (Fig. 2). A size factor (FSIZE) was applied to standardize the pharmacokinetic parameters assuming a standard total body weight (TBW) of 70 kg (Eq. 6) [19,20,21,22]. The allometric exponent (PWR) of FSIZE was fixed at 3/4 for CL/F and Q, and at 1 for VC/F and VRGT (Eq. 6):
Structure of the lithium tubular reabsorption model. a A two-compartment distribution model with first-order absorption and elimination. b Schematic diagram of the renal kinetics of lithium. Dp.o. oral administration, Ka absorption rate constant, F bioavailability, RGT virtual renal focused on glomerulus and tubule, VC, VRGT distribution volumes of the central and RGT compartments, Q intercompartmental clearance, QC-RGT, QRGT-C intercompartmental clearance from the central to the RGT and from the RGT to the central compartments, CL clearance, CLR renal clearance, RF renal function
Creatinine clearance (CLcr) was calculated using the Cockcroft–Gault formula [23] standardized to a TBW of 70 kg. Renal function (RF) was normalized to a standard CLcr (CLcrSTD) of 6 L h−1 70 kg−1 (100 mL min−1 70 kg−1), as shown below [24, 25] (Eq. 7):
RF was included in the model using a linear independent combination of renal and nonrenal CL parameters, as shown below (Eq. 8):
Here, CLNR is the nonrenal CL and CLR is the renal CL. CLR was the assumed sum of the ION form clearance (CLION) and the MID form clearance (CLMID), each multiplied by their fractions FION and FMID, respectively (Eq. 9):
FION and FMID were calculated using the Henderson–Hasselbalch equation incorporating urinary pH (UPH), as shown below (Eq. 10):
The pKa1 of lithium carbonate was 6.2. As for Q, CLR and renal function were incorporated in central to RGT Q (QC-RGT) assuming the glomerular filtration of lithium as shown in Eq. (11):
In contrast, the RGT-to-central Q (QRGT-C), assuming that the tubular reabsorption of lithium is fixed at 80% of QC-RGT, is given by (Eq. 12) [8]:
2.6 Model Evaluation
The parameter sensitivity of the final model was evaluated using two methods.
A nonparametric bootstrap was used to estimate uncertainty [26]. The final model was fitted repeatedly to 100 additional bootstrap datasets. The average, standard deviation (SD), relative standard error (%RSE), and 95% confidence intervals (CIs) were calculated from the empirical bootstrap distribution and compared with estimates from the original dataset.
A prediction-corrected visual predictive check (pcVPC) was used to check the distributions of the observed and predicted percentiles [27]. The VPC was evaluated by comparing the observed concentrations with the 90% percentile intervals (PIs) and 95% CIs simulated from the final parameters.
2.7 Exposure–Response Analysis
Exposure–response analysis was performed using data for 179 patients (70 males and 109 females) who were checked by each patient’s attending physician with regard to the presentation of tremor as a side effect. The presence of these symptoms was investigated simultaneously with blood collection. We evaluated the correlations between side-effect development and various factors, including serum lithium concentration, body weight, age, AST (aspartate aminotransferase), ALT (alanine aminotransferase), BUN (blood urea nitrogen), CLcr, and total dosage. The relationships between the factors and side-effect development were evaluated using exposure–response curves and a logit model in which the independent and dependent variables were the natural logarithm (ln) of the lithium concentration (x) and the probability of side effects (y), respectively. The logit model assumed that logit was proportional to the factor, which was the lithium concentration, including the between-subject variability modeled with the additive error of the logit, as shown below (Eq. 13):
Here, η is a normally distributed random variable with a mean of zero and a variance of ω2. We obtained a logistic curve and calculated the lithium concentrations corresponding to 30 or 50% probabilities of presenting side effects using (Eq. 14):
The uncertainty in the logit model was evaluated using a bootstrap resampling method from 300 additional datasets.
3 Results
3.1 Patients and Lithium Concentrations
A summary of the data for 214 patients is shown in Table 1, while Fig. 3 shows total serum lithium concentrations at various time points after the oral administration of lithium carbonate to patients. A previous study reported that the elimination half-life of lithium is 18–36 h [28], so our data did not include any measurements in the absorption phase despite the absence of any restriction on blood sampling times. The dose of lithium carbonate per day showed large intersubject variation because dosage adjustments based on trough concentrations were performed according to the attending physician’s judgment. A total of 177 measurements of urinary pH were obtained from 133 patients, and ranged between 5.0 and 8.5 in increments of 0.5. In the present study, this value was hypothesized to be 6.5 (median) in patients for whom urinary pH was not measured.
3.2 Population Pharmacokinetics Based on the Lithium Tubular Reabsorption Model
The pharmacokinetic model of lithium was a two-compartment distribution model with first-order absorption and elimination. When CLcr and urinary pH were incorporated into the clearance, the OFV was significantly reduced. We estimated MOL form clearance (CLMOL) similarly to CLION and CLMID but obtained a very small value (0.001 L h−1), and the OFV did not increase. Therefore, most of the CLR was contributed by the ION and MID forms. In the final model, Ka was fixed at 0.75 h−1 [29], and the CVs for the between-subject variability in CL/F and the CV for the residual unidentified variability were 22.9 and 25.7%, respectively. The condition number of the final model was 333, indicating that the model was stable. The final model parameters are shown below (Eq. 15):
3.3 Model Evaluation
Parameter estimates for the final model based on 100 bootstrap resamples are shown in Table 2. The final estimated values were very similar to the averages of the bootstrap distributions for all of the pharmacokinetic parameters, and fell within the empirical bootstrap 95th percentiles.
Model evaluation using pcVPC also confirmed that there was acceptable agreement between the observed data and the model-based simulated values (Fig. 4). The median of the observed values was within the 95% CI of the predicted values near the trough at about 12 h after administration.
Model evaluation using a prediction-corrected visual predictive check involving 100 simulated data sets. a Prediction-corrected scatterplot of measurements showing the 5th, 50th, and 95th percentiles. b pcVPC showing the 5th, 50th, and 95th percentiles for the observed and predicted values. Black dashed lines 5th and 95th percentiles of the predicted lithium concentrations, black solid line median predicted lithium concentration in 100 simulated subsets of the total dataset, gray-shaded areas 95% confidence intervals of the prediction percentiles, red circles observed lithium concentration, red dashed lines 5th and 95th percentiles of the observed lithium concentrations, red solid line median observed lithium concentration
3.4 Exposure–Response Analysis
A total of 304 serum lithium concentrations (median: 0.57 mEq L−1, 95th percentile interval: 0.22–1.14 mEq L−1) measured in 179 patients were available for the exposure–response analysis. Neurological symptoms such as tremor accounted for 31 points (10.2%).
In this analysis, no significant correlation between factors other than serum lithium concentration and side effect development was confirmed. The results of the logit model analysis to examine the relationship between serum lithium concentration and neurological symptoms such as tremor are shown in Fig. 5. The baseline, slope, and between-subject variability values were estimated as − 3.56, 2.15, and 0.779 (standard deviation), respectively. The odds ratio (OR) of the slope was calculated as 8.57, and the 95% CI of the OR was 1.80–40.71. The serum lithium concentrations corresponding to 30 and 50% probabilities of side-effect development were calculated as 1.26 and 1.66 mEq L−1, respectively.
Logistic regression of neurological symptoms (such as tremor) on lithium concentration. The symbols refer to the observed lithium concentrations in the patients with (top) or without (bottom) neurological symptoms. The continuous line represents the results of the logistic regression model. The dashed lines represent the 95th percentile interval of the logistic curve including between-subject variability. The gray lines represent the lithium concentrations corresponding to 30 and 50% probabilities of side-effect development. OR odds ratio of slope, CI confidence interval
The results for the parameter estimates of the logit model from 300 bootstrap resamples are shown in Table 2. Final estimated values were very similar to the averages of the bootstrap distributions for all parameters, and fell within the empirical bootstrap 95th percentiles.
4 Discussion
Lithium has recently been applied to treat not only bipolar disorder but also various psychiatric diseases. Its therapeutic serum concentration range is narrow and it quickly reaches clinically toxic levels, so dosage adjustments based on trough concentrations are recommended. Most patients with mental illnesses return home, precluding frequent blood concentration measurements. Accordingly, various methods of predicting the pharmacokinetics of lithium have been reported [16, 29,30,31,32,33]. In the present study, a model with the ability to predict the serum lithium concentration was developed following a population pharmacokinetic analysis of lithium, and the influence of urinary pH variability on lithium clearance was evaluated. Furthermore, the relationship between serum lithium concentration and tremor as a side effect was examined using an exposure–response analysis.
The clinical data collected showed that the lithium oral dosage varied because dosage adjustments based on the state of the patient and lithium levels were performed, and serum lithium concentration did not correlate with lithium dosage. No significant difference among institutions in patient background, lithium dosage, and underlying diseases was observed. Furthermore, there was no difference in serum lithium concentration between males and females. The population pharmacokinetics of lithium have previously been described using one- or two-compartment models [16, 29, 31,32,33]. The pharmacokinetic analysis in the present study was performed using a two-compartment distribution model with first-order absorption and elimination.
Lithium is mainly excreted renally, so nephropathic patients have high lithium concentrations. Therefore, CLcr was selected as a covariate of CL/F. The population mean values of CLNR and CLRGT were 0.512 and 0.572 L h−1, respectively, assuming that the urinary pH was 6.5 (the mode of this parameter). These results suggest that the renal excretion rate of lithium was approximately 55%. In contrast, approximately 45% of the lithium excretion was unaffected by renal function. The population mean values of CLION and CLMID were 0.549 and 0.584 L h−1, respectively, which suggest that lithium excretion was delayed under acidic conditions with a high proportion of Li+. However, the urinary pH was not measured in approximately 38% of the patients, so we assigned the mode value of the urinary pH to those patients. It was suggesting the current variances of the between-subject variability in a pharmacokinetic parameter might be underestimated. It was suggested that the lithium CL could be predicted by incorporating both renal function and urinary pH.
Lithium CL was calculated to be 1.08 L h−1 with a CLcr of 6 L h−1, a body weight of 70 kg, and a urine pH of 6.5, and was presumed to decrease with decreasing CLcr and body weight. Lithium CL was calculated as 1.56 L h−1 using a model reported by Yukawa et al. [31] and the median values of our data. Yukawa et al. [31] proposed a model which demonstrated that lithium CL decreases over 50 years of age. The mean age of the subjects in their analysis was 40.7 years, while that in the present study was 54 years, which explains the lower lithium CL in the present study. However, between-subject variability values were similar in our model and their model. The steady-state volume of distribution (VC + VRGT) in the present study was estimated to be 81.9 L, which is consistent with that reported previously [32].
The proportion of lithium tubular reabsorption (QRGT-C) to glomerular filtration (QC-RGT) was estimated to be approximately 0.8, and a significant ΔOFV was not obtained (not shown), so we fixed the value at 0.8 in the final model [8].
The bootstrap method was used to evaluate the accuracy and robustness of the general model. The median values obtained from the bootstrap procedure were similar to the parameter estimates from the original data set, and the 95% CIs overlapped with those of the original data set. These results indicate that the accuracy and robustness of the general model are acceptable. The final model was evaluated using the pcVPC method. The final model for lithium accurately predicted the concentration–time profile, particularly in the trough (around 12 h after administration), with most of the data observed to lie within the 95% PIs, closely reflecting the data distribution at individual time points. These results indicate that the model’s ability to predict serum lithium concentration was good.
The proportion of patients with side effects was 11.6% in the present study, which was nearly identical to the proportion (13.9%) reported previously for Japanese patients treated with lithium [15]. The development of side effects of lithium has been shown to correlate with lithium concentration, age, and daily dosage, whereas it was only correlated with lithium concentration in the present study. We evaluated this relationship using logistic regression. Prien et al. [12] reported that the probability of side effects when the serum lithium concentration was less than 1.4 mEq L−1 was 18%, whereas this value was 63% for concentrations of ≥ 1.4 mEq L−1. Similarly, the serum lithium concentrations associated with probabilities of 30 and 50% of developing neurological symptoms were calculated to be 1.26 and 1.66 mEq L−1, respectively, in the present study. This result supports the guidelines on general usage, which recommend that the dosage of lithium should be reduced when the trough lithium concentration is greater than 1.5 mEq L−1. Furthermore, neurological symptoms such as tremor were more likely to be seen in elderly patients or in patients with a lithium concentration of greater than 0.7 mEq L−1, as reported by Vesterqaard et al. [34]. In another study, the probability of neurological symptoms was found to be high if patients were older than 50 years [15]. In the present study there was no correlation between age and the development of tremor as a side effect, but the development of neurological symptoms such as tremor did appear to depend on the serum lithium concentration. However, no other factors beyond blood lithium concentration were correlated with neurological symptoms in our study.
Several limitations of the present study need to be discussed. First, the CLNR was estimated to be about 45% of the lithium CL. This result conflicts with a report that lithium is mainly excreted renally. The reason for this is unclear. Second, data on sodium concentrations and concomitant drugs were not collected, so the influence of these factors on the kinetics of lithium could not be examined. Third, the dispersion of the population mean values was wide. In the results from the bootstrap method, we obtained high relative standard error (%RSE) values for VRGT. This variability was considered to be caused by the elimination step of the final model. The mechanism of lithium excretion implies that it would have been more appropriate to have estimated the elimination process from the RGT compartment; however, prediction using the model incorporating renal elimination was difficult due to a lack of observations of the RGT compartment (not shown). Fourth, the variability of the urinary pH values was not considered in the present study. Urinary pH is known to be affected by various factors, including meals and medication. However, in the present study, we performed a population pharmacokinetic analysis which assumed that urinary pH did not fluctuate. Therefore, an analysis that accounts for the variation in urinary pH is needed.
5 Conclusions
In the present study, we performed a population pharmacokinetic analysis of lithium based on physiological mechanisms. Lithium clearance was related to tubular reabsorption, which was influenced by urinary pH; therefore, urinary pH is considered to be an important index in lithium treatments. Serum lithium concentrations may be predicted based on renal function and urinary pH. Furthermore, the results obtained demonstrate that neurological symptoms such as tremor correlated with serum lithium concentration, and the therapeutic index to prevent the development of such symptoms was presented.
References
Cade JF. Lithium salts in the treatment of psychotic excitement. Med J Aust. 1949;2:349–52.
Schou M. Lithium in psychiatric therapy and prophylaxis. J Psychiatr Res. 1968;6:67–95.
Grandjean EM, Aubry J-M. Lithium: updated human knowledge using an evidence-based approach. Part I: clinical efficacy in bipolar disorder. CNS Drugs. 2009;23:225–40.
Geddes JR, Miklowitz DJ. Treatment of bipolar disorder. Lancet. 2013;381:1672–82.
Smith LA, Cornelius V, Warnock A, Tacchi MJ, Taylor D. Pharmacological interventions for acute bipolar mania: a systematic review of randomized placebo-controlled trials. Bipolar Disord. 2007;9:551–60.
Yildiz A, Vieta E, Leucht S, Baldessarini RJ. Efficacy of antimanic treatments: meta-analysis of randomized, controlled trials. Neuropsychopharmacology. 2011;36:375–89.
Schou M. Pharmacology and toxicology of lithium. Annu Rev Pharmacol Toxicol. 1976;16:231–43.
Thomsen K, Olesen OV. Precipitating factors and renal mechanisms in lithium intoxication. Gen Pharmacol. 1978;9:85–9.
Thomsen K. Renal handling of lithium at non-toxic and toxic serum lithium levels. A review. Dan Med Bull. 1978;25:106–15.
Kanba S, Kato T, Terao T, Yamada K. Guideline for treatment of bipolar disorder by the Japanese Society of Mood Disorders, 2012. Psychiatry Clin Neurosci. 2013;67:285–300.
Schou M, Amdisen A, Trap-Jensen J. Lithium poisoning. Am J Psychiatr. 1968;125:520–7.
Prien RF, Caffey EM, Klett CJ. Relationship between serum lithium level and clinical response in acute mania treated with lithium. Br J Psychiatry. 1972;120:409–14.
Gelenberg A, et al. Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. N Engl J Med. 1989;321:1489–93.
McKnight RF, et al. Lithium toxicity profile: a systematic review and meta-analysis. Lancet. 2012;379:721–8.
Watanabe S. Side effects of antimanic drugs. Jpn J Neuropsychopharmacol. 1989;11:49–58.
Ebid AIM, Abd-allah DAT, Elhabiby MMM. Pharmacokinetics of lithium in Egyptian bipolar patients: dosage adjustment approach. Pharmacol Pharm. 2014;5:425–32.
Henderson L. Concerning the relationship between the strength of acids and their capacity to preserve neutrality. Am J Physiol. 1908;21:173–9.
Hasselbalch K. Die Berechnung der Wasserstoffzahl des Blutes aus der freien und gebundenen Kohlensäure desselben, und die Sauerstoffbindung des Blutes als Funktion der Wasserstoffzahl. Biochem Z. 1917;78:112–44.
Anderson BJ, Meakin GH. Scaling for size: some implications for paediatric anaesthesia dosing. Paediatr Anaesth. 2002;12:205–19.
Anderson BJ, Holford NHG. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–32.
Holford N, Heo YA, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102:2941–52.
Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24:25–36.
Cockcroft DW, Gault H. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41.
Mould DR, et al. Population pharmacokinetic and adverse event analysis of topotecan in patients with solid tumors. Clin Pharmacol Ther. 2002;71:334–48.
Matthews I, Kirkpatrick C, Holford N. Quantitative justification for target concentration intervention—parameter variability and predictive performance using population pharmacokinetic models for aminoglycosides. Br J Clin Pharmacol. 2004;58:8–19.
Parke J, Holford NHG, Charles BG. A procedure for generating bootstrap samples for the validation of nonlinear mixed-effects population models. Comput Methods Programs Biomed. 1999;59:19–29.
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO. Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J. 2011;13:143–51.
Thornhill DP. Pharmacokinetics of ordinary and sustained-release lithium carbonate in manic patients after acute dosage. Eur J Clin Pharmacol. 1978;14:267–71.
Turck D, Heinzel G, Luik G. Steady-state pharmacokinetics of lithium in healthy volunteers receiving concomitant meloxicam. Br J Clin Pharmacol. 2000;50:197–204.
Nielsen-Kudsk F, Amedisen A. Analysis of the pharmacokinetics of lithium in man. Eur J Clin Pharmacol. 1979;16:271–7.
Yukawa E, Nomiyama N, Higuchi S, Aoyama T. Lithium population pharmacokinetics from routine clinical data: role of patient characteristics for estimating dosing regimens. Ther Drug Monit. 1993;15:75–82.
Wing YK, Chan E, Chan K, Lee S, Shek CC. Lithium pharmacokinetics in Chinese manic-depressive patients. J Clin Psychopharmacol. 1997;17:179–84.
Findling RL, et al. First-dose pharmacokinetics of lithium carbonate in children and adolescents. J Clin Psychopharmacol. 2010;30:404–10.
Vesterqaard P, Poulstrup I, Schou M. Prospective studies on a lithium cohort. 3. Tremor, weight gain, diarrhea, psychological complaints. Acta Psychiatr Scand. 1988;78:434–41.
Feng Y, et al. Model-based clinical pharmacology profiling of ipilimumab in patients with advanced melanoma. Br J Clin Pharmacol. 2014;78:106–17.
Author information
Authors and Affiliations
Contributions
DY and YT contributed to the acquisition of data, analyzed and interpreted data, participated in the study design, and drafted the manuscript. CO analyzed and interpreted data and revised the manuscript. HK, HT, and HK contributed to the conception and design of the study and the interpretation of data. MS, SK, and HK contributed to serum concentration measurements and patient data sampling. All authors approved the final version to be published.
Corresponding author
Ethics declarations
Funding
This study was supported by a grant from SENSHIN Medical Research Foundation and Takeda Science Foundation.
Conflict of interest
The authors (Daichi Yamaguchi, Yasuhiro Tsuji, Miki Sonoda, Kenji Shin, Hiroko Kito, Chika Ogami, Hidefumi Kasai, Hideto To, and Hidetoshi Kamimura) declare no conflict of interest. All authors have completed the Unified Competing Interest form and declare that there was no support from any organization for the submitted work, no financial relationships with any organizations that may have an interest in the submitted work in the previous 3 years, and no other relationships or activities that may have influenced the submitted work.
Ethics approval
The present study was performed in accordance with the Helsinki Declaration after approval by the ethical review board of the University of Toyama (approval number: clinical 26-39). It was then approved by Yahata Kousei Hospital, Iizuka Hospital, and Fukuma Hospital.
Informed consent
Written consent was obtained from all patients, and patient privacy and personal information were respected.
Rights and permissions
About this article
Cite this article
Yamaguchi, D., Tsuji, Y., Sonoda, M. et al. Population Pharmacokinetics and Exposure–Response of Lithium Carbonate in Patients Based on Tubular Reabsorption Mechanisms. Eur J Drug Metab Pharmacokinet 44, 329–338 (2019). https://doi.org/10.1007/s13318-018-0536-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13318-018-0536-0