Abstract
Pythium ultimum is an important soil-borne pathogen that causes damping-off and root rot in a large number of plant species. We developed a loop-mediated isothermal amplification (LAMP) method for the rapid and accurate detection of P. ultimum. A target gene encoding a spore wall protein was identified from the P. ultimum genome using a comparative genomics approach. The LAMP assay efficiently amplified the target DNA at 64 °C for 60 min, and its specificity and sensitivity were evaluated. Of the pathogen isolates tested, positive results were obtained only with P. ultimum isolates. The detection limit of the LAMP method was 1 pg μL−1 DNA, which is 1000 times more sensitive than conventional PCR. Moreover, this method was successfully used to detect P. ultimum DNA extracted from infected plant tissues, and the detection limit for P. ultimum in plants was nearly the same as for in vitro samples. Consequently, our LAMP assay enables the rapid, specific, and sensitive detection of P. ultimum DNA from pure cultures, as well as from infected plant tissues.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Pythium is a harmful genera of plant pathogens found worldwide. More than 120 Pythium species have been identified, and most of them are soil-borne and saprophytic plant pathogens with broad host ranges (Hendrix and Campbell 1973; Martin and Loper 1999). They usually attack young feeder roots and seedlings, and cause a variety of diseases, including pre- and post-emergence damping-off, and seed, root, and fruit rot (Larkin et al. 1995).
Pythium ultimum is a ubiquitous soil-borne pathogen and one of the most pathogenic Pythium species (Martin and Loper 1999). It is common in forests, grasslands, ponds, and agricultural ecosystems worldwide. P. ultimum attacks a large number of plant species, including soybean, wheat, corn, potato, and many other economic crops. It can infect the seeds, seedlings, roots, and lower stem parts of plants, resulting in damping-off and root rot. P. ultimum is also homothallic, meaning that a single isolate can mate with itself to complete the sexual stage (Francis and St Clair 1993). P. ultimum oospores can survive for extended periods in soil, initiating infections in roots or seeds. Control measures for P. ultimum caused diseases remain insufficient.
Pathogen detection is a fundamental part of disease control. The traditional method used for the identification and discrimination of Pythium species is the microscopic inspection of morphological characteristics (Pettitt et al. 2002). However, this method requires professional skills and is time-consuming, making it impractical for large-scale sample analysis. In recent years, molecular detection methods based on the polymerase chain reaction (PCR) have been developed. Conventional and real-time PCR assays have been widely used for the specific diagnosis of more than 20 plant-pathogenic Pythium species (Klemsdal et al. 2008; Schroeder et al. 2013). For example, Cullen et al. (2007) designed specific primers that target the internal transcribed spacer (ITS) region and detected P. ultimum using a conventional PCR assay (Cullen et al. 2007). However, PCR-based methods require experienced technicians and expensive special equipment (Morisset et al. 2008). Consequently, they may not be suitable in the field or resource-limited laboratories.
Loop-mediated isothermal amplification (LAMP) is a relatively novel technique that amplifies target DNA with high specificity, rapidity and simplicity (Notomi et al. 2000). This technique uses Bst DNA polymerase, which uses strand displacement activity to achieve amplification. LAMP assays use four to six primers that recognize the corresponding regions of target DNA (Notomi et al. 2000). The advantage of LAMP is that amplification occurs in approximately 1 h under isothermal conditions. Therefore, simple incubators such as a heating block or water bath are sufficient for DNA amplification. LAMP amplification results in the accumulation of 109 copies of target DNA, making it more effective than PCR. The amplified products can be detected using multiple methods, of which gel electrophoresis is the most accurate and generates typical ladder-like banding patterns. The reaction products can also be detected visually by adding different dyes, including SYBR green, hydroxy naphthol blue (HNB), or calcein, which can be seen with the naked eye (Mori et al. 2001; Goto et al. 2009). These advantages make LAMP appealing for use in rapid, easily performable pathogen detection assays compared with other diagnostic methods. To date, this technique has been successfully applied to the detection of fungi (Niessen and Vogel 2010), bacteria (Rigano et al. 2010), oomycetes (Dai et al. 2012), and viruses (Fukuta et al. 2003).
This study developed a LAMP assay for the detection of P. ultimum and evaluated it using inoculated plant samples. This method should be useful for the early and accurate diagnosis of P. ultimum so that timely plant protection measures can be instituted.
Materials and methods
Isolates used in the LAMP assay
The isolates of Pythium ultimum, other Pythium spp., Phytophthora spp., and fungi (Table 1) used in this study were originally collected from different provinces in China, and were maintained at the Department of Plant Pathology, Nanjing Agricultural University (Nanjing, China). Pythium and Phytophthora isolates were grown on V8 agar, whereas the fungal isolates were cultured on potato dextrose agar medium at 25 °C for at least 3 days before extracting DNA. Mycelia were harvested, collected and stored at −20 °C. Mycelial DNA extraction was performed using a modified hexadecyl trimethylammonium bromide (CTAB) method (Murray and Thompson 1980).
Target selection and LAMP primer design
The rDNA ITS sequences of P. ultimum (GenBank accession number: D86515), P. splendens (AB796308), P. spinosum (AJ233457), and P. helicoides (AB108026) were obtained from the GenBank database. The genome sequences of six publically available Pythium species, including P. ultimum, Pythium aphanidermatum, Pythium arrhenomanes, Pythium irregulare, Pythium iwayamai, and Pythium vexans, were obtained from the Pythium Genome Database (http://pythium.plantbiology.msu.edu), and used to perform a comparative genomics analysis. Using all of the P. ultimum genes as the query, BLAST searches against another five Pythium genomes were performed (E-value cutoff: 1E-5), and genes with no hits were treated as unique to P. ultimum. Subsequently, these unique genes were compared with P. ultimum genes using the BLAST tool (E-value cutoff: 1E-5) to obtain single-copy genes. Due to the limited Pythium genome sequences available, these unique single-copy genes were compared against the NCBI database, and genes without significant similarities (E-value cutoff: 1E-20) to any known sequence in the NCBI database were retained as candidate diagnostic targets for P. ultimum. The LAMP primers were designed using PrimerExplorer V4 software (http://primerexplorer.jp/elamp4.0.0). The design of the target for the SW-1 primer set is illustrated in Fig. 1. Five primers, F3 (5′-CAACTGGAAAAGCAAGCGG-3′), B3 (5′-CCGAAGAACTGTGTCCGC-3′), FIP (5′-GAGCCAGACGGGCCAGTATCAAGTTACAGTGGCGTTGTCA-3′), BIP (5′-TCTCTGTTGCTCGACTGGAGGGTTCCACCTCCTGTAAGACCT-3′), and F-Loop (5′-GCTTGCTCCAGTACGAATGC-3′), were used to detect P. ultimum.
LAMP reaction
The LAMP reaction was performed in a 25 μL mixture that included 2.5 μL of 10× ThermoPol buffer, 1.6 μM each of FIP and BIP primers, 0.2 μM each of F3 and B3 primers, 0.8 μM F-Loop primer, 0.8 M betaine, 6 mM MgSO4, 8 U of Bst DNA polymerase, 1.4 mM dNTPs, 0.2 mM HNB, and 2 μL of target DNA. The reaction mixture was incubated at 64 °C for 60 min in a standard water bath. The LAMP products were visualized by adding HNB before amplification. Samples that turned blue were considered to be positive, while samples that remained violet were negative. In addition, the LAMP products were electrophoresed in 2% agarose gels and then stained with ethidium bromide for visualization, and positive reactions resulted in a ladder-like banding pattern. The specificity and sensitivity tests were repeated at least three times.
PCR reaction
PCRs were conducted using the method of Kong et al. (2003). The PCR reaction mixture contained 2 μL of DNA temples, 2.5 μL of the 10× PCR buffer, 2.5 μL each of the 10 μM forward and reverse primers, 2 μL of the 2 mM dNTP solutions, 0.1 μL (5 U/μl) of Taq polymerase, and 13.4 μL sterile nanopure water in a total volume of 25 μL. PCR was carried out in a DNA thermal cycler (PCR Thermal Cycler Dice, TP600, Takara, Japan) with initial denatureation at 96 °C for 2 min, followed by 40 cycles of 94 °C for 30 s, 65 °C for 45 s, 72 °C for 1 min, and a final extension at 72 °C for 10 min. Amplification products were confirmed by electrophoresis in 1% agarose geles, and stained with ethidium bromide for visualisation under UV light.
Detection of P. ultimum in infected plants
To evaluate the application of LAMP as a tool for the diagnosis of P. ultimum, wheat, soybean, cucumber, and tobacco plants were inoculated with P. ultimum mycelia. Each plant leaf was inoculated with a small piece of V8 agar cut from the edge of a 3-day-old P. ultimum colony. After 24 h, tissue surrounding the infected region was harvested for DNA extraction by the CTAB method. These DNA samples were evaluated using the developed LAMP system. A color change in the products was assessed with the naked eye. The experiment was repeated at least three times.
Results
LAMP specificity test
In total, 4 P. ultimum isolates, 17 isolates from other Pythium species, 4 Phytophthora isolates, and 3 fungal isolates (Table 1) were used to test the specificity of the LAMP reaction. Initially, LAMP primers based on the ITS sequence were tested, but these failed to distinguish P. ultimum from related Pythium species. Then, a comparative genomics approach was performed to identify a target sequence, and a gene encoding a spore wall protein (GenBank accession number: MF765461) was found to be specific for P. ultimum. LAMP primer SW-1 was generated based on this gene, and was used to test the specificity of the assay. Primer SW-1 resulted in positive reactions when tested with four P. ultimum isolates, and no positive reaction was observed for related Pythium spp. (Table 1). Negative reactions were also seen for the Phytophthora spp. and fungal isolates. These results were evaluated with both the naked eye and gel electrophoresis, and three replicates were performed. The results indicate that primer SW-1 had good specificity for the LAMP –based detection of P. ultimum.
Comparison of the sensitivity of LAMP and conventional PCR assays
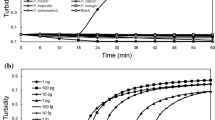
To determine the sensitivity of primer SW-1, ten-fold serial dilutions of P. ultimum genomic DNA were tested. The DNA template concentrations ranged from 10 ng μL−1 to 1 fg μL−1. As indicated by a color change and DNA amplification of the reaction products, the lowest limit of P. ultimum detection was 1 pg μL−1 DNA for the LAMP assay (Fig. 2a,b). In comparison, the detection limit of the conventional PCR assay using the outer primers F3 and B3 was 1 ng μL−1 DNA (Fig. 2c). This demonstrates that the LAMP assay has a wider dynamic range, with a sensitivity nearly 1000 times greater than that of conventional PCR for the detection of P. ultimum.
Successful detection in infected plants
To assess the reliability of our LAMP method, wheat, soybean, cucumber and tobacco leaves were inoculated with P. ultimum mycelia. At 24 h after inoculation, genomic DNA was extracted from the infected region and subjected to the LAMP reaction. Positive reactions were observed in all four infected plants, while negative reactions were seen in uninoculated plants (Fig. 3a).
Detection of P. ultimum in infected plants. Detection of LAMP products using HNB as a visual indicator. a For each plant, the left tube contained purified plant DNA and the right tube contained DNA from the infected region after inoculation with P. ultimum mycelia. b Investigation of the detection limit for P. ultimum DNA when mixed with 1 μL of soybean DNA. c Investigation of the detection limit for P. ultimum DNA when mixed with 1 μL of wheat DNA
To investigate the detection limit of the LAMP method for the pathogen in plants, we established the detection limit of purified P. ultimum DNA when mixed with a fixed amount of purified plant DNA. The target DNA in the LAMP reaction was 2 μL, which included 1 μL of plant DNA (50 ng μL−1) and 1 μL of P. ultimum DNA with concentrations ranging from 10 ng μL−1 to 1 fg μL−1. As shown in Fig. 3b, the detection limit was 10 pg μL−1 DNA for P. ultimum when mixed with soybean DNA. The lowest limit was 1 pg μL−1 DNA for P. ultimum when mixed with wheat DNA (Fig. 3c). These results suggest that the detection limit for P. ultimum in plants was nearly the same as for in vitro samples.
Discussion
Pythium ultimum is a ubiquitous soil-borne pathogen with a broad host range that causes a variety of diseases, including damping-off and root rot. The process of disease control requires high specificity and sensitivity in the detection of P. ultimum infection. In this study, we developed a rapid, sensitive, and reliable LAMP detection method for P. ultimum.
A key factor influencing the LAMP method is target selection. Most previous targets were based on ITS or housekeeping gene sequences (Fukuta et al. 2013; Takahashi et al. 2014). We initially selected the ITS sequence as the target, but the LAMP primer sets failed to show specificity for P. ultimum. We believe that the ITS sequences of closely related pathogens are too similar or even identical, and are not appropriate for distinguishing some pathogens. As increasing numbers of pathogens have been sequenced, bioinformatics tools have been widely used to identify targets for molecular diagnostics. A unique target identified by a comparative genomics pipeline was successfully applied to the LAMP detection of Xanthomonas arboricola pv. pruni (Buhlmann et al. 2013). In this study, we used a similar target identification method and the selected target was suitable for the diagnosis of P. ultimum.
Compared with conventional and real-time PCR assays, the LAMP assay is simple, rapid, specific, and sensitive, and it should become a new reliable method for the diagnosis of P. ultimum infection. The LAMP assay requires only a heating block or water bath instead of a thermal cycler to incubate the reaction mixture in approximately 1 h under isothermal conditions, and the reaction products can be detected visually. Our results show that the SW-1 primer set designed in this study successfully distinguished P. ultimum from related species. In addition, the sensitivity evaluation showed that the detection limit of LAMP was 1 pg μL−1, which is 1000 times more sensitive than conventional PCR. These results demonstrate the high specificity and sensitivity of the LAMP assay. We also tested the reliability of the LAMP method in infected plants, and positive reactions were seen in all infected samples, indicating that this method is realiable and suitable for the diagnosis of P. ultimum in the field.
In conclusion, we developed a visual LAMP assay for the diagnosis of P. ultimum. This LAMP method had good specificity, sensitivity, and accuracy, and was useful for the identification and detection of P. ultimum. This method should facilitate the early and accurate diagnosis of P. ultimum in the field so that timely plant protection measures can be initiated.
References
Buhlmann A, Pothier JF, Tomlinson JA, Frey JE, Boonham N, Smits THM, Duffy B (2013) Genomics-informed design of loop-mediated isothermal amplification for detection of phytopathogenic Xanthomonas arboricola pv. pruni at the intraspecific level. Plant Pathol 62:475–484
Cullen DW, Toth IK, Boonham N, Walsh K, Barker I, Lees AK (2007) Development and validation of conventional and quantitative polymerase chain reaction assays for the detection of storage rot potato pathogens, Phytophthora erythroseptica, Pythium ultimum and Phoma foveata. J Phytopathol 155:309–315
Dai TT, Lu CC, Lu J, Dong S, Ye W, Wang Y, Zheng X (2012) Development of a loop-mediated isothermal amplification assay for detection of Phytophthora sojae. FEMS Microbiol Lett 334:27–34
Francis DM, St Clair DA (1993) Outcrossing in the homothallic oomycete, Pythium ultimum, detected with molecular markers. Curr Genet 24:100–106
Fukuta S, Kato S, Yoshida K, Mizukami Y, Ishida A, Ueda J, Kanbe M, Ishimoto Y (2003) Detection of tomato yellow leaf curl virus by loop-mediated isothermal amplification reaction. J Virol Methods 112:35–40
Fukuta S, Takahashi R, Kuroyanagi S, Miyake N, Nagai H, Suzuki H, Hashizume F, Tsuji T, Taguchi H, Watanabe H, Kageyama K (2013) Detection of Pythium aphanidermatum in tomato using loop-mediated isothermal amplification (LAMP) with species-specific primers. Eur J Plant Pathol 136:689–701
Goto M, Honda E, Ogura A, Nomoto A, Hanaki KI (2009) Colorimetric detection of loop-mediated isothermal amplification reaction by using hydroxy naphthol blue. BioTechniques 46:167–172
Hendrix FF, Campbell WA (1973) Pythiums as plant pathogens. Annu Rev Phytopathol 11:77–98
Klemsdal SS, Herrero ML, Wanner LA, Lund G, Hermansen A (2008) PCR-based identification of Pythium spp. causing cavity spot in carrots and sensitive detection in soil samples. Plant Pathol 57:877–886
Kong P, Hong C, Jeffers SN, Richardson PA (2003) A species-specific polymerase chain reaction assay for rapid detection of Phytophthora nicotianae in irrigation water. Phytopathology 93:822–831
Larkin RP, English JT, Mihail JD (1995) Effects of infection by Pythium spp on root-system morphology of alfalfa seedlings. Phytopathology 85:430–435
Levesque CA, de Cock AW (2004) Molecular phylogeny and taxonomy of the genus Pythium. Mycol Res 108:1363–1383
Martin FN, Loper JE (1999) Soilborne plant diseases caused by Pythium spp: ecology, epidemiology, and prospects for biological control. Crit Rev Plant Sci 18:111–181
Mori Y, Nagamine K, Tomita N, Notomi T (2001) Detection of loop-mediated isothermal amplification reaction by turbidity derived from magnesium pyrophosphate formation. Biochem Bioph Res Co 289:150–154
Morisset D, Stebih D, Cankar K, Zel J, Gruden K (2008) Alternative DNA amplification methods to PCR and their application in GMO detection: a review. Eur Food Res Technol 227:1287–1297
Murray MG, Thompson WF (1980) Rapid isolation of high molecular weight plant DNA. Nucleic Acids Res 8:4321–4325
Niessen L, Vogel RF (2010) Detection of Fusarium graminearum DNA using a loop-mediated isothermal amplification (LAMP) assay. Int J Food Microbiol 140:183–191
Notomi T, Okayama H, Masubuchi H, Yonekawa T, Watanabe K, Amino N, Hase T (2000) Loop-mediated isothermal amplification of DNA. Nucleic Acids Res 28:E63
Pettitt TR, Wakeham AJ, Wainwright MF, White JG (2002) Comparison of serological, culture, and bait methods for detection of Pythium and Phytophthora zoospores in water. Plant Pathol 51:720–727
Rigano LA, Marano MR, Castagnaro AP, Do Amaral AM, Vojnov AA (2010) Rapid and sensitive detection of citrus bacterial canker by loop-mediated isothermal amplification combined with simple visual evaluation methods. BMC Microbiol 10:176
Schroeder KL, Martin FN, de Cock AWAM, Levesque CA, Spies CFJ, Okubara PA, Paulitz TC (2013) Molecular detection and quantification of Pythium species: evolving taxonomy, new tools, and challenges. Plant Dis 97:4–20
Takahashi R, Fukuta S, Kuroyanagi S, Miyake N, Nagai H, Kageyama K, Ishiguro Y (2014) Development and application of a loop-mediated isothermal amplification assay for rapid detection of Pythium helicoides. FEMS Microbiol Lett 355:28–35
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China [31501589] and Special Fund for Agro-scientific Research in the Public Interest [201503112].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Shen, D., Li, Q., Yu, J. et al. Development of a loop-mediated isothermal amplification method for the rapid detection of Pythium ultimum . Australasian Plant Pathol. 46, 571–576 (2017). https://doi.org/10.1007/s13313-017-0517-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-017-0517-9