Abstract
Wheat streak mosaic virus is an established major threat to wheat in North America and is newly identified in Australia. Three genetic sources of resistance were examined, Wsm1 (from an alien translocation), Wsm2 (from CO960293-2), and c2652 (selected in Canada). We report their effectiveness in the field when inoculated with an Australian WSMV isolate. Also included were advanced breeding lines with and without Wsm2 and a number of elite Australian cultivars. ELISA testing on individual plants indicated we achieved between 85% and 100% infection with WSMV in susceptible lines following artificial inoculation which reduced their yield by 22 to 44% and height by 19 to 51%. Kernel weight was significantly affected in some of the susceptible lines. All three sources of resistance (Wsm1, Wsm2, c2652) and Wsm2 derivatives protected wheat against infection despite repeated inoculation. Inoculated resistant plots were virtually disease free and suffered neither significant yield loss nor height reduction. National yield trials of the breeding derivatives showed no difference in yields between those with and without Wsm2 under non-WSMV conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Wheat streak mosaic virus (WSMV) (Genus Tritimovirus: Family Potyviridae), naturally transmitted by the wheat curl mite (WMC) Aceria tosichella Keifer, causes one of the most destructive viral diseases of wheat worldwide in both spring and winter wheat (Baley et al. 2001; Sharp et al. 2002; Seifers et al. 2006; Coutts et al. 2008a; Murray and Brennan 2009). Infected plants display symptoms of streaking and chlorosis of the leaves, may be severely stunted, and produce less grain which is almost always of lower quality (Finney and Sill 1963). The greatest losses occur when early infection so weakens plants that they suffer from poor head fertility and grain fill or fail to produce fertile heads altogether (Staples and Allington 1956; Atkinson and Grant 1967; Lanoiselet et al. 2008).
Until WSMV was recently reported in Australia (Ellis et al. 2003) and New Zealand (Lebas et al. 2009), wheat production in temperate Oceania was not thought to be at risk from losses due to this virus. With outbreaks of wheat streak mosaic (WSM) disease having been confirmed in areas of wheat production (Ellis et al. 2003; Coutts et al. 2008a, b), the absence of genetic resistance in Australian wheat cultivars has put the wheat industry at risk of major losses. Moreover, as wheat in Australia is mostly grown in rain-fed areas with frequent terminal drought, the finding that WSMV infection shortens roots and reduces water use efficiency (Price et al. 2010) adds urgency to the need to apply effective control measures. With its attendant foliar chlorosis and necrosis, WSM also reduces the quantity and quality of wheat grown for forage (Price et al. 2010). This effect is also of concern in Australia where, to an increasing extent in recent years, dual-purpose wheat is sown early in zones of higher rainfall to provide forage for sheep or cattle without loss of grain yield at harvest (Virgona et al. 2006). With the arrival of WSMV and the double threat it poses, farmers in these areas of higher rainfall are specifically advised to delay sowing in autumn until temperatures drop (GRDC Fact Sheet, Wheat Curl Mite, 2009). This severely limits the usefulness of dual purpose wheat sowings and profitable grain and graze options for farmers. In such a cropping regime the WCM vector is more likely to find the “green bridge” it needs to continue its life cycle and spread the virus. The early-sown crop is thus predisposed to a greater threat of infection from increased mite activity with the warmer conditions during early growth stages, and once infected the virulence is enhanced by the higher temperatures (Pfannenstiel and Niblett 1978; Chen 1985; Seifers et al. 1995, 2006, 2007). In sum, the stage is set for severe epidemics which affect both forage and grain production. In the absence of natural resistance in cultivated wheat, the Australian wheat industry is clearly at risk of major losses to WSMV.
There are two broad general strategies available to control WSM. One is to control the mite vector, the second is to enhance the host’s ability to tolerate or resist the virus infection itself. Mites cannot be controlled through chemical means as these are too toxic, therefore control can only be achieved through cultural practices. In the Great Plains region of the U.S.A., where winter wheat is sown in autumn, late sowing and the removal of volunteer wheat help prevent WCM from finding a green bridge and completing their life cycle (Velandia et al. 2010). The emergence of volunteers that provide the green bridge can never be perfectly controlled, so cultural practice will, at best, be only partially effective. Similar issues occur in certain regions of Australia where winter-habit wheat crops are sown in early autumn where higher temperatures dramatically increase the risk of WSMV infection. The vector can be controlled by introgressing one or more sources of genetic resistance to WCM feeding from an array of species related to wheat (Harvey et al. 1999). This approach has been shown to offer effective control for a time but suffers from the ability of mite vector populations to evolve rapidly from avirulence to virulence (Harvey et al. 1999).
The second strategy, to control losses from WSM by host genetic resistance to the virus itself, has been pursued since natural resistance to WSMV infection was first discovered in wild relatives of wheat. Resistance from Thinopyrum intermedium, were first identified by McKinney and Sando (1951), with stable translocations into wheat being developed by Lay et al. (1971). A translocation on the short arm of chromosome 4D was designated as Wsm1 (Friebe et al. 1992, 1996a). However, limitations arising from factors such as linkage drag (Friebe et al. 1996a, b) and temperature sensitivity (Pfannenstiel and Niblett 1978; Seifers et al. 1995, 2006, 2007) have hindered the deployment of such resistance in commercial cultivars. Recently, recombination of the translocation has reduced the linkage drag and the attendant yield decrease in the absence of virus infection (Friebe et al. 2009). One U.S. cultivar, Mace PI 615160 (unrelated to the cultivar Mace released by Australian Grains Technologies), has been released with Wsm1 resistance and improved agronomic performance (Graybosch et al. 2009). A second source of resistance (Wsm2), derived from the experimental wheat selection CO960293-2 (Haley et al. 2002), has been identified and mapped to chromosome 3B (Lu et al. 2011). The Wsm2 resistance was temperature-sensitive in its original source (Seifers et al. 2006) and in the first North American commercial cultivar, RonL (PI648020), in which it was deployed (Seifers et al. 2011). A second North American cultivar with Wsm2 resistance, Snowmass (PI658597) (Haley et al. 2011), has also been released. Deploying Wsm2 resistance may not necessarily be limited by temperature sensitivity as lines derived by repeated cycles of selection have been shown to sustain virus resistance at higher temperature (Fahim et al. 2011). A third source of resistance, effective at elevated temperature and designated “c2652”, has been identified in an experimental doubled haploid spring wheat line C2652, following repeated cycles of selection (Haber et al. 2005).
We report here the impact in the field of the Australian (ACT) isolate of WSMV on a number of wheats cultivars and how effectively these three sources of genetic resistance protect yield when challenged by repeated rigorous artificial inoculation.
Materials and methods
Plant material
The germplasm examined in this study fell into three categories: a) the specific lines used as sources of resistance; b) advanced breeding lines derived by crossing from one of the sources of resistance (CO960293-2, Wsm2); and c) an array of contemporary Australian cultivars now commonly grown in areas at risk from WSMV infection.
The specific lines tested as examples of three different sources of resistance were: i) a Wsm1-carrying 4Ai#2S.4DL translocation line designated Wsm1-CA740 (Wells et al. 1982); ii) two lines derived from CO960293-2 (CA743 and CA745) carrying Wsm2 resistance (Haley et al. 2002) and iii) c2652 resistance in line CA742, a WSMV resistant selection from within spring wheat line c2652 (Haber et al. unpublished).
Advanced breeding lines were derived from CO960293-2 (Wsm2) as follows: CO960293-2 was backcrossed to Superb (a high yield spring milling wheat of pedigree Grandin*2/AC Domain) or straight crossed to HY644 (a Fusarium Head Blight resistant high yield spring feed wheat). Two homozygous resistant derivatives were selected from each cross and used as the pollen donor to three Australian wheats, Sunstate, EGA Hume, or Yitpi. A number of lines were advanced without selection for resistance and F6 lines selected for yield trials. A suite of these lines were then screened in the glasshouse to identify resistant and susceptible lines. A number of elite Australian lines were used that are commonly grown in WSMV prone areas. Table 1 describes all lines.
Preparation of virus inoculum
An Australian field isolate of the virus was used to infect susceptible wheat. Leaves were weighed and blended with 0.02 m potassium phosphate buffer pH 7 (1 /10 w/v) in a blender. The homogenate was filtered through four layers of Miracloth® (Calbiochem, La Jolla, California, USA). Abrasive Celite (Spectrum Chemicals, Gardena, California, USA) was added at 2% w/v and the mixture was left on ice for 1 h. This was applied to the leaves of test plants using a spray gun (Fahim et al. 2010) with air pressure set at 270 kPa.
Glasshouse screening of germplasm
In the glasshouse experiment, six wheat plants of each accession were grown in 10 cm pots. The plants were doubly inoculated at the 2–3 leaf stage, with the prepared sap extracts from WSMV-infected leaf material. The plants were scored for symptoms at 14 dpi (days post inoculation) on a scale of 0–4 with 0 as healthy, 1 as mild with very few streaks, 2 as moderate with streaks that coalesce, 3 as severe with approximately 50% leaf area with streaks, 4 as the most severe or lethal symptoms where the streaks develop into chlorosis of more than 70% of leaf area. Leaf samples were collected from individual plants and assayed for the virus using ELISA (as detailed in Fahim et al. 2010). Two uninoculated plants per line were included as healthy controls.
WSMV field trial
A field trial was conducted in the winter growing season of 2009 at Ginninderra Experimental Station, Canberra (35°12′2.59″S, 149°5′22.74″E). The experiment was designed in a complete randomised block design with two replicates of each line and treatment arranged in two blocks. A total of 19 genotypes were grown in 4.9 m long by 0.8 m wide plots with each consisting of 3 rows (approximately 50 seed sown per plot). There was a 54 cm gap between plots and a 1 m gap at the end of each plot. The field trial was sown on 10th of July 2009 and harvested on December 21, 2009.
In the field experiment, half of the plots were inoculated with WSMV the at 2–3 leaf stage on the morning of 23rd of August using a spray gun, again on the 1st of September and finally on the 21st of September. The youngest fully expanded leaf with virus like symptoms was collected on 6th of November (6 weeks after the last inoculation). Relative incidence of WSMV was calculated on all plots by sampling ten plants at random and assaying using ELISA (Fahim et al. 2010).
The trial was sprayed with fungicide (Tilt250E) at late tillering according to the manufacturer’s instructions (Syngenta Crop Protection, Guelph, Canada). The trial was grown under rainfed conditions, however due to a dry spell in October, four allocations of irrigation of approximately 3 mm each were added at weekly intervals.
A maturity score (Zadoks et al. 1974) was taken when most of the lines were at anthesis. Prior to harvest, height measurements (cm) were taken as was a harvest index (HI) cut. This cut measured 0.5 m × 0.8 m and was taken randomly across the width of each plot by cutting at ground level. The tillers were bundled together, dried and weighed for total biomass. Heads and grain yield per m2 were recorded and HI calculated. The remainder of each plot was harvested with a single row harvester and total grain yield recorded along with grain weight from the HI cut. Kernel weight (from 250 grains) and hectolitre weight were calculated on a subsample from each plot.
Uninoculated yield trials
A total of 29 advanced derivatives of the Wsm2 crosses into Australian cultivars were included in breeding trials in 2008. Briefly, these lines were included as unreplicated entries in “percentage replicated” designed trials (Cullis et al. 2006) conducted in nine high rainfall sites, covering four States of Australia. The earliest maturing lines were grown in SW Western Australia, the mid-maturing lines in trial in SE New South Wales and the latest maturing lines were grown in trial in SE Victoria and SE South Australia. All trials were run by commercial enterprises that base their agronomy on “best farmer practice” for the specific region. Kalyx Agriculture (Carlisle WA) ran three trials in Western Australia (Kojonup, Mount Barker and Gibson). Agritech Crop Research Pty Ltd (Young NSW) ran trials in New South Wales (Cowra, Monteagles and Wallendbeen). Southern Farming Systems (Newtown Vic) ran trials in Mininerra and Inverleigh and Mackillop Farm Management Group Inc. (Naracoorte SA) ran a trial at Conmurra.
Statistical analysis
Genstat (v13) was used for spatial analysis of the uninoculated yield trials and also to conduct two-way ANOVA with and without interactions on all measured parameters in the WSMV inoculated trial. Significant differences between WSMV treatment of lines were identified at α = 0.05 when the differences between means of the measured parameter were greater than the least significant difference (LSD).
Results
Resistance in glasshouse
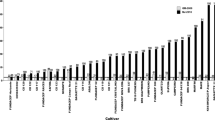
The presence of the Wsm1 resistance gene was inferred using molecular markers (Talbert et al. 1996; Fahim et al. 2011). However no suitable markers were available for Wsm2 and c2652, therefore the presence of the respective resistance genes in progeny lines were inferred using the established glasshouse bioassay to identify the resistance phenotype. All original sources of resistance held up to WSMV infection in the glasshouse. Testing of sets of F6 Australian derived sibling lines showed that 13 were either homozygous for resistance (Wsm2) and 13 were homozygous for susceptibility (wsm2), with three having an intermediate average ELISA level because both resistant and susceptible plants were amongst the six tested individuals of those lines (Fig. 1). From these glasshouse studies, uniformly resistant and susceptible sibling lines were chosen for the inoculated field trial. Lines were chosen that had a uniform disease reaction whilst also ensuring all backgrounds were represented.
Resistance in the field
Spray inoculation of field plots resulted in excellent uniform infection and efficiency of inoculation was almost as good as in the glasshouse. The ELISA performed on leaf samples showed 80 to 100% infection in all inoculated susceptible genotypes. All of the inoculated plots of resistant lines had very low or zero infection (Table 2). Neither visual inspection of plots nor ELISA detected virus infections in any un-inoculated plots, regardless of whether they contained resistance genes or not. This indicated the successful establishment of infection in the treated plots, lack of migration into untreated plots, and lack of viruliferous mites during the experiment.
A two-way ANOVA was used to analyse the growth and yield components by genotype and WSMV treatment (Table 3). There were significant genotype differences in all yield and growth components, while WSMV inoculation had significant effects on five of the components. Table 3 summarises the yield components which were affected by inoculation, and Table 4 shows which lines contributed to the significant results.
The yield trial suffered from a mid-late season drought (October) and despite attempts at irrigation, the trial average was approximately 1 tonne ha-1. However, we still saw significant yield effects of WSMV in many of the susceptible lines (Fig. 2). All lines carrying resistance genes Wsm1, Wsm2 or c2652 had yields that were unaffected by the disease, despite the highly effective inoculation procedure.
The disease reactions of the Australian derived sibling lines observed in the glasshouse were also very evident in the field trial. There was no loss of yield on average for the five Wsm2 resistant lines (range 6% loss to 5% increase for individual genotypes), however, total grain yields of all lines not carrying resistance were reduced in the presence of disease, and for most of these lines the reduction was significant (P < 0.05). All five Australian wheat cultivars showed a reduction of yield ranging from 29% to 44%. The susceptible sibling lines also had reduced yields, ranging from 22% to 44%.
The harvest index was significantly affected by WSMV inoculation. Total biomass was reduced by inoculation, whereas the number of heads per m2 was not. The lines that were significantly different for HI parameters were generally susceptible although one resistant selection (HRZ07.0433) had a higher harvest index and another (HRZ07.0485) had higher total biomass in the uninoculated plots.
WSMV had no significant effect on the height of resistant lines whereas it significantly (P < 0.05) stunted Sunbrook, Preston and Wedgetail. Wedgetail was most severely affected (51% reduction), followed by Preston (31%), Sunbrook (30%), Chara (20%) and Yitpi (19%). Grain size was affected by WSMV infection in Chara and two of the HRZ susceptible lines; kernel weight was significantly reduced in inoculated plots. WSMV had no effect on maturity score or hectolitre weight.
To assess whether linkage drag might be a problem for the Wsm2 resistance, we grouped the germplasm into four pools for comparisons: 1) the unadapted resistant sources, 2) the lines from elite Australian backgrounds with introgressed Wsm2, selected for resistance; iii) the susceptible sibling lines of pool 2; and 4) the adapted Australian parents. Each pool was analysed with and without the virus treatment (Fig. 3). The un-inoculated resistant sources (pool 1) which were not in adapted backgrounds yielded significantly less than any of the other un-inoculated pools. The other three un-inoculated pools did not differ significantly from each other. When crossed into adapted germplasm, Wsm2 protects against yield losses from inoculated WSMV, as seen in the comparison of the resistant pool against its susceptible pool counterpart and also has comparable yield in the absence of virus to the cultivars and the susceptible sibling lines (Figs. 2 and 3).
Average yield (t/Ha) of lines grouped as follows: Resistance sources, the four sources of resistant germplasm; Resistant Breeder Lines, the five derived lines carrying Wsm2; Susceptible Breeder Lines, the five derived lines without Wsm2; Australian varieties, the five susceptible Australian varieties. Means and standard errors of means are shown
The lack of yield penalty was further explored by yield trialing of the 26 homozygous Australian derived sibling sets of lines segregating for Wsm2. In 13 yield trials in 2008, lines carrying the Wsm2 resistance averaged 79% of the site mean yields, whilst the non-Wms2 lines averaged 82%. There was no significant difference between these averages. This showed that Wsm2 carrying derivatives had no yield penalty compared to their siblings in the absence of wheat streak mosaic disease.
Discussion
We had previously documented in glasshouse trials that inoculating susceptible wheat lines with WSMV adversely affected their growth, while resistant lines derived from Wsm1, Wsm2 and c2652 remained unaffected (Fahim et al. 2011). The current report extends the analysis and confirms that Wsm1, Wsm2 and c2652 are also effective in the field to protect against yield losses.
Earlier studies conducted in controlled temperature cabinets had also shown that there were differences in the temperature sensitivity of the resistances expressed in derivatives from the three different sources (Fahim et al. 2011; Seifers et al. 1995, 2006, 2007). All were similarly effective up to 20°C against the ACT isolate of WSMV. At temperatures above 20°C, the resistance expressed by line CA740, which carried the Wsm1 gene on a 4Ai#2S.4DL translocation, was ineffective. This response against the ACT isolate was similar to that reported earlier against the Sidney81 isolate from Kansas (Seifers et al. 1995). The resistance derived from CO960293-2 (now designated Wsm2) was similarly temperature-sensitive in most lines, but one selection expressed effective resistance at 26°C. Finally, the tested lines that derived their resistance from c2652 expressed resistance that was effective at temperatures as high as 28°C.
With the current field study we established that lines that were susceptible in the glasshouse also suffered major yield losses to WSMV in the field, and lines that expressed resistance to WSMV were protected from yield losses in the field. Wsm1 was shown by Sharp et al. (2002) to confer significant yield protection in the field over two years; the average yield was 80% compared to uninoculated plots, whereas sibling lines without Wsm1 when inoculated yielded on average only 26% of the uninoculated plots. Divis et al. (2006) also reported the effectiveness of Wsm1 against infection and yield loss in the field. Seifers et al. (2006, 2007) likewise showed that both Wsm1 and Wsm2 were effective in the field at protecting yield against North American isolates of the virus. This current study extends these findings to the warmer field conditions of Australia and shows that Wsm1 and Wsm2 confer total protection against infection and yield loss using an Australian isolate, WSMV-ACT. This is also the first demonstration that resistance derived from a new high temperature effective resistance, c2652, is completely effective under field conditions.
The five cultivars we tested were all susceptible to WSMV and along with the five susceptible siblings from Australian derived material, all experienced substantial losses (Table 4, Fig. 2). Among the tested cultivars, Wedgetail illustrates most clearly the potential for losses and, correspondingly, the benefits of conferring effective genetic resistance. As a winter wheat with high grain quality which is grown in environments where it is also sown early and used as winter forage, it is vulnerable to becoming infected at early plant growth stages if large populations of viruliferous WCM are present, for example following a mild summer with moisture and appropriate green bridges. This heightened risk of WSMV infection causing losses of both forage and grain yield has undermined confidence in the valuable dual-purpose application of this cultivar.
Infection with WSMV induces an array of changes that interact to reduce biomass, grain yield and quality. The manner and extent of these interactions vary with the earliness of infection, intensity of inoculation, host genotype and environmental conditions. In this study we observed, for example, that virus infection stunted plants of the cultivar Sunbrook but did not reduce plot yield significantly. In the cultivar Wedgetail, by contrast, infection reduced height (a proxy for biomass) and yield dramatically, but in sufficiently similar measure that the HI parameter was not changed significantly. The cultivars Preston and Yitpi experienced proportionally greater losses of grain yield than biomass, reducing their HI. Foliar symptoms that range from light chlorotic streaking and mosaic to severe chlorosis and necrosis affect the volume and quality of wheat as forage. Severe infection stunts growth and retards development, producing fewer tillers and delayed or deminished anthesis and seed set (Martin and Harvey 1992; Tatineni et al. 2010), followed by poor seed filling (Weise 1987). When a high proportion of plants in a field are infected at an early growth stage, these effects combine to reduce yields substantially. For example, in a two-year field study for seven cultivars in Oklahoma, Hunger et al. (1992) estimated that WSMV infection reduced fertile tillers and grain yield by as much as 75% and 87%, respectively.
The expression of a resistance gene may protect against losses from virus infection but may be associated with other genes that contribute to reduced yields in the absence of infection. This effect of linkage drag hindered the deployment of the Wsm1 resistance gene in its original form (Sharp et al. 2002); though studies that have examined more recently developed Wsm1 lines suggest the linkage drag may be overcome (Baley et al. 2001; Divis et al. 2006). In the present study, Wsm2 derivatives in adapted backgrounds showed no signs of linkage drag in the absence of virus (Fig. 3) which bodes well for the deployment of this resistance in new cultivars. That assessment for c2652 will have to wait for backcross derivatives in adapted backgrounds to be developed.
Resistance conferred by Wsm1, Wsm2 and c2652 were effective in protecting against WSMV under field condition. However, these WSMV resistant wheat lines are still susceptible to other viruses such as BYDV and HVP (Gieck et al. 2007; Seifers et al. 2009, 2011). Therefore, it would be of great value to stack resistance genes against these viruses into high yielding cultivars. Continuing evolution of virus populations may also bring about shifts from avirulence to virulence in interactions with any single resistance gene. This highlights the importance of pyramiding genes for resistance to WSMV along with those that protect against other important diseases.
References
Atkinson TG, Grant MN (1967) An evaluation of streak mosaic losses in winter wheat. Phytopathology 57:188–192
Baley GJ, Talbert LE, Martin JM, Young MJ, Habernicht DK, Kushnak GD, Berg JE, Lanning SP, Bruckner PL (2001) Agronomic and end-use qualities of Wheat streak mosaic virus resistant spring wheat. Crop Sci 41:1779–1784
Chen ST (1985) Environmental factors affecting the resistance of certain agrotricums and their derivatives against Wheat Streak Mosaic Virus. Dissertation Abstracts International, B (Sciences and Engineering) 45: 3680B
Coutts BA, Hammond NEB, Kehoe MA, Jones RAC (2008a) Finding Wheat streak mosaic virus in south-west Australia. Aus J Agr Res 59:836–843
Coutts BA, Strickland GR, Kehoe MA, Severtson DL, Jones RAC (2008b) The epidemiology of Wheat streak mosaic virus in Australia: case histories, gradients, mite vectors, and alternative hosts. Aus J Agr Res 59:844–853
Cullis BR, Smith AB, Coombes NE (2006) On the design of early generation variety trials with correlated data. J Agr Biol Envir St 11:381–393
Divis LA, Graybosch RA, Peterson CJ, Baenziger PS, Hein GL, Beecher BB, Martin TJ (2006) Agronomic and quality effects in winter wheat of a gene conditioning resistance to Wheat streak mosaic virus. Euphytica 152:41–49
Ellis MH, Rebetzke GJ, Chu P (2003) First report of Wheat streak mosaic virus in Australia. Plant Pathol 52:808
Fahim M, Dove H, Kelman WM, Ayala-Navarrete L, Larkin PJ (2010) Does grazing of infected wheat by sheep result in salivary transmission of Wheat streak mosaic virus? Crop Pasture Sci 61:247–254
Fahim M, Mechanicos A, Ayala-Navarette L, Haber S, Larkin PJ (2011) Resistance to Wheat streak mosaic virus in Australia – a survey of resources and development of markers. Plant Pathol. doi:10.1111/j.1365-3059.2011.02542.x
Finney KF, Sill WH (1963) Effects of 2 virus diseases on milling and baking properties of wheat grain and flour and on probable nutritive value of forage wheat. Agron J 55:476–478
Friebe B, Mukai Y, Gill BS, Cauderon Y (1992) C-banding and in situ hybridization analyses of Agropyron intermedium, a partial wheat x Ag. intermedium amphiploid and 6 derived chromosome addition lines. Theor Appl Genet 84:899–905
Friebe B, Gill KS, Tuleen NA, Gill BS (1996a) Transfer of Wheat streak mosaic virus resistance from Agropyron intermedium into wheat. Crop Sci 36:857–861
Friebe B, Jiang J, Raupp WJ, McIntosh RA, Gill BS (1996b) Characterization of wheat-alien translocations conferring resistance to diseases and pests: Current status. Euphytica 91:59–87
Friebe B, Qi LL, Wilson DL, Chang ZJ, Selfers DL, Martin TJ, Fritz AK, Gill BS (2009) Wheat-Thinopyrum intermedium recombinants resistant to Wheat streak mosaic virus and Triticum mosaic virus. Crop Sci 49:1221–1226
Gieck SL, Hamm PB, Clough GH, David NL (2007) High plains virus: An emerging disease of sweet corn in the Columbia basin of Oregon and Washington. Phytopathology 97:S167–S168
Graybosch RA, Peterson CJ, Baenziger PS, Baltensperger DD, Nelson LA, Jin Y, Kolmer J, Seabourn B, French R, Hein G, Martin TJ, Beecher B, Schwarzacher T, Heslop-Harrison P (2009) Registration of 'Mace' hard red winter wheat. J Plant Registrations 3:51–56
GRDC Fact Sheet: Wheat Curl Mite, 2009 <http://www.grdc.com.au/uploads/documents/GRDC_WheatCurlMite_4pp.pdf>
Haber S, Gilbert J, Comeau A (2005) Stress-directed selection identifies lines of spring wheat with enhanced resistance to Fusarium head blight and other diseases. Proceedings 4th Canadian Workshop on Fusarium Head Blight, Ottawa Congress Centre, Ottawa, Ontario, Canada, November 1–3, 2005
Haley SD, Martin TJ, Quick JS, Seifers DL, Stromberger JA, Clayshulte SR, Clifford BL, Peairs FB, Rudolph JB, Johnson JJ, Gill BS, Friebe B (2002) Registration of CO960293-2 wheat germplasm resistant to Wheat streak mosaic virus and Russian wheat aphid. Crop Sci 42:1381–1382
Haley SD, Johnson JJ, Peairs FB, Stromberger JA, Heaton EE, Seifert SA, Kottke RA, Rudolph JB, Martin TJ, Bai GH, Chen XM, Bowden RL, Jin Y, Kolmer JA, Seifers DL, Chen MS, Seabourn BW (2011) Registration of 'Snowmass' wheat. J Plant Registrations 5:87–90
Harvey TL, Seifers DL, Martin TJ, Brown-Guedira G, Gill BS (1999) Survival of wheat curl mites on different sources of resistance in wheat. Crop Sci 39:1887–1889
Hunger RM, Sherwood JL, Evans CK, Montana JR (1992) Effects of planting date and inoculation date on severity of Wheat streak mosaic virus in hard winter wheat-wheat cultivars. Plant Dis 76:1056–1060
Lanoiselet VM, Hind-Lanoiselet TL, Murray GM (2008) Studies on the seed transmission of Wheat streak mosaic virus. Australas Plant Path 37:584–588
Lay CL, Wells DG, Gardner WS (1971) Immunity from Wheat streak mosaic virus in irradiated agrotricum progenies. Crop Sci 11:431–432
Lebas BSM, Ochoa-Corona FM, Alexander BJR, Lister RA, Fletcher JDF, Bithell SL, Burnip GM (2009) First report of Wheat streak mosaic virus on wheat in New Zealand. Plant Dis 93:430
Lu HJ, Price J, Devkota R, Rush C, Rudd J (2011) A dominant gene for resistance to Wheat streak mosaic virus in winter wheat line CO960293-2. Crop Sci 51:5–12
Martin T, Harvey T (1992) Field screening procedure for resistance to wheat streak mosaic virus. Cereal Res Commun 20:213–215
McKinney HH, Sando WJ (1951) Susceptibility and resistance to the wheat streak-mosaic virus in the genera Triticum, Agropyron, Secale, and certain hybrids. Plant Dis Rep 35:476–479
Murray GM, Brennan JP (2009) Estimating disease losses to the Australian wheat industry. Australas Plant Path 38:558–570
Pfannenstiel MA, Niblett CL (1978) The nature of the resistance of agrotricums to Wheat streak mosaic virus. Phytopathology 68:1204–1209
Price JA, Workneh F, Evett SR, Jones DC, Arthur J, Rush CM (2010) Effects of Wheat streak mosaic virus on root development and water-use efficiency of hard red winter wheat. Plant Dis 94:766–770
Seifers DL, Martin TJ, Harvey TL, Gill BS (1995) Temperature sensitivity and efficacy of Wheat streak mosaic virus resistance derived from Agropyron Intermedium. Plant Dis 79:1104–1106
Seifers DL, Martin TJ, Harvey TL, Haber S, Haley SD (2006) Temperature sensitivity and efficacy of Wheat streak mosaic virus resistance derived from CO960293 wheat. Plant Dis 90:623–628
Seifers DL, Martin TJ, Harvey TL, Haber S (2007) Temperature-sensitive Wheat streak mosaic virus resistance identified in KS03HW12 wheat. Plant Dis 91:1029–1033
Seifers DL, Martin TJ, Harvey TL, Fellers JP, Michaud JP (2009) Identification of the wheat curl mite as the vector of Triticum mosaic virus. Plant Dis 93:25–29
Seifers DL, Martin TJ, Fellers JP (2011) Occurrence and yield effects of wheat infected with Triticum mosaic virus in Kansas. Plant Dis 95:183–188
Sharp GL, Martin JM, Lanning SP, Blake NK, Brey CW, Sivamani E, Qu R, Talbert LE (2002) Field evaluation of transgenic and classical sources of Wheat streak mosaic virus resistance. Crop Sci 42:105–110
Staples R, Allington WB (1956) Streak mosaic of wheat in Nebraska and its control. University of Nebraska, Lincoln. Agriculture Experiment Station Research Bulletin
Talbert LE, Bruckner PL, Smith LY, Sears R, Martin TJ (1996) Development of PCR markers linked to resistance to Wheat streak mosaic virus in wheat. Theor Appl Genet 93:463–467
Tatineni S, Graybosch RA, Hein GL, Wegulo SN, French R (2010) Wheat cultivar-specific disease synergism and alteration of virus accumulation during co-Iinfection with Wheat streak mosaic virus and Triticum mosaic virus. Phytopathology 100:230–238
Velandia M, Rejesus RM, Jones DC, Price JA, Workneh F, Rush CM (2010) Economic impact of Wheat streak mosaic virus in the Texas high plains. Crop Prot 29:699–703
Virgona JM, Gummer FAJ, Angus JF (2006) Effects of grazing on wheat growth, yield, development, water-use and nitrogen-use. Aust J Agr Res 57:1307–1319
Weise MV (1987) Wheat streak mosaic. Compendium of Wheat Diseases, 2nd edn. American Phytopathological Society, St. Paul. MN, pp 80–81
Wells D, Kota R, Sandhu H, Gardner W, Finney K (1982) Registration of one disomic substitution line and five translocation lines of winter wheat germplasm resistant to Wheat streak mosaic virus (Reg. No. GP 199 to GP 204). Crop Sci 22:1277–1278
Zadoks JC, Chang TT, Konzak CF (1974) A decimal code for the growth stages of cereals. Weeds Res 14:415–421
Acknowledgements
The authors thankfully acknowledge Megan Hemming and Maryse Bourgault CSIRO, for critically reviewing the manuscript. The first author thankfully acknowledges AusAID for financial assistance as PhD studentship.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fahim, M., Larkin, P.J., Haber, S. et al. Effectiveness of three potential sources of resistance in wheat against Wheat streak mosaic virus under field conditions. Australasian Plant Pathol. 41, 301–309 (2012). https://doi.org/10.1007/s13313-012-0125-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13313-012-0125-7