Abstract
This study aimed to investigate the incidence and prognosis of postoperative complications after laparoscopic total gastrectomy (LTG) for gastric cancer (GC). We retrospectively enrolled 411 patients who underwent curative LTG for GC at seven institutions between January 2004 and December 2018. The patients were divided into two groups, complication group (CG) and non-complication group (non-CG), depending on the presence of serious postoperative complications (Clavien–Dindo grade III [≥ CD IIIa] or higher complications). Short-term outcomes and prognoses were compared between two groups. Serious postoperative complications occurred in 65 (15.8%) patients. No significant difference was observed between the two groups in the median operative time, intraoperative blood loss, number of lymph nodes harvested, or pathological stage; however, the 5-year overall survival (OS; CG 66.4% vs. non-CG 76.8%; p = 0.001), disease-specific survival (DSS; CG 70.1% vs. non-CG 76.2%; p = 0.011), and disease-free survival (CG 70.9% vs. non-CG 80.9%; p = 0.001) were significantly different. The Cox multivariate analysis identified the serious postoperative complications as independent risk factors for 5-year OS (HR 2.143, 95% CI 1.165–3.944, p = 0.014) and DSS (HR 2.467, 95% CI 1.223−4.975, p = 0.011). A significant difference was detected in the median days until postoperative recurrence (CG 223 days vs. non-CG 469 days; p = 0.017) between the two groups. Serious postoperative complications after LTG negatively affected the GC prognosis. Efforts to decrease incidences of serious complications should be made that may help in better prognosis in patients with GC after LTG.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is the fifth most common type of cancer and third leading cause of death worldwide [1, 2]. Surgical resection or gastrectomy is the most important treatment for GC, but the postoperative complications (incidence rates of 12.8–14.0% [3,4,5]) negatively affects the patients’ quality of life (QOL), subsequent treatment(s), and long-term survival [6, 7]. Several studies have confirmed the association of postoperative complications after gastrectomy for GC and poor oncological prognosis [8,9,10]. In 1994, laparoscopy-assisted distal end gastrectomy for early GC was introduced [11]; with advances in equipment and surgical techniques, laparoscopic gastrectomy (LG) has gradually been considered more often. In comparison to open gastrectomy, LG is associated with features such as feasibility, decreased surgical trauma, and a faster recovery. Further, many studies showed non-inferiority of LG to open gastrectomy for GC [12,13,14]. The impact of postoperative complications after LG for patients with GC remains controversial. In particular, laparoscopic total gastrectomy (LTG) requires a high degree of skill for performing the gastrectomy with systematic lymphadenectomy and post-resection reconstructions; the procedures are difficult and complex even for the experienced laparoscopic surgeons [15, 16]. Further, the rates of complications after LTG are also reportedly high (7.6–42.6%) [17,18,19], but information on the postoperative complications related adverse events (such as non-cancer-related deaths) is lacking. This study aimed to investigate the relationship between postoperative complications and long‐term survival in patients who underwent LTG for GC.
Methods
Patients
We retrospectively reviewed all the patients who underwent curative LTG for GC at seven institutions (Hokkaido University Hospital, Teine Keijinkai Hospital, Obihiro-Kosei General Hospital, Hokkaido Gastroenterology Hospital, Tonan Hospital, Kitami Red Cross Hospital, and Asahikawa City Hospital) between January 2004 and December 2018. All patients were diagnosed with GC using endoscopy, computed tomography (CT), or endoscopic ultrasound. The Japanese Classification of Gastric Carcinoma (JCGC) was used for tumor staging [20]. The primary indication for LTG was stage I GC based on the Japanese Society of Endoscopic Surgery (JSES) guidelines [21]; however, over time, we expanded the indication to include cases of advanced GC that could be curatively resected.
Data collection
Clinicopathological data, including age, sex, body mass index (BMI), American Society of Anesthesiologists physical status (ASA-PS), clinical stage, combined resection of other organs, lymph node dissection, and anastomosis method, were collected. Surgical outcomes, including operative time, estimated blood loss, postoperative complications, and length of postoperative hospital stay, were recorded. Patients were categorized either to a complication group (CG) or non-complication group (non-CG), depending on the presence of serious postoperative complications (≥ CD IIIa: Clavien–Dindo grade III or higher complications) [22, 23]. All patients provided informed consent, and the Hokkaido University Hospital Institutional Review Board approved the data collection and analysis (No. 016-0151). This study was performed in accordance with the principles of the Declaration of Helsinki.
Surgical procedure
Gastric procedure type (resection and reconstruction) was determined based on the experience and preference of a surgeon who was accredited through the Endoscopic Surgical Skill Qualification System of the JSES [21]. In cases where the operating surgeon lacked this qualification, a qualified surgeon supervised the surgery. The extent of lymph node dissection was determined based on the JGCA guidelines [20]. Patients who underwent D2 lymph node dissection with splenectomy and D2–No.10 lymph node dissection were included in D1+. Patients were divided into three groups based on the Clavien–Dindo postoperative complication classification grade [22, 23].
Postoperative follow-up
All patients were observed every 3 months after surgery. Hematological analysis (including the tumor marker analysis for carcinoembryonic antigen and carbohydrate antigen 19–9) was performed at each visit. Abdominal CT scans were performed every 6 months or when clinical recurrence was suspected. Gastrointestinal endoscopy was performed at 1, 3, and 5 years postoperatively. Based on this surveillance, data on the 5-year overall survival (OS; time from surgery to death for any reason or follow-up interruption) and disease-specific survival (DSS; time from surgery to death from GC, including operative mortality or follow-up interruption), and disease-free survival (DFS; time from surgery to death from GC, the first recurrence of GC, or follow-up interruption) were collected.
Statistical analysis
Pearson’s Chi-square test and Fisher’s exact probability test were performed for categorical variables. Mann–Whitney U tests were used to compare the clinicopathological characteristics for unpaired continuous variables between the two groups. Survival curves were estimated using the Kaplan–Meier method, and statistical differences were examined using the Wilcoxon test. A Cox proportional hazard regression model was used to determine the independent prognostic factors related to survival. Statistical significance was set at p < 0.05. Statistical analysis was performed using the JMP® 15 software (SAS Institute Inc., Cary, NC, USA).
Results
Clinical features and surgical outcomes of the study population
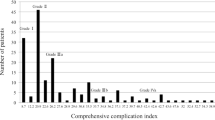
A total of 459 patients were screened; after applying the exclusion criteria (neoadjuvant therapy, cStage IV, resection of other organs, and a total number of harvested lymph nodes ≤ 15), 411 patients were finally included in the analysis. In this study, for accurate prognostic analysis, we included more than 16 lymph nodes dissected using the exclusion criteria [24]. CG and non-CG groups had 65 and 346 patients, respectively (Fig. 1). Table 1 shows the clinical characteristics and surgical outcomes of the patients. Among total patients, 284 (69.1%) and 127 (30.9%) were males and females, respectively, with a median age of 68 years (range 25–88) and median BMI of 23.0 kg/m2 (range 13.6–38.9). The ASA-PS was ≥ II in 286 (69.6%) patients, clinical JCGC stage was ≥ II in 187 (45.5%) patients, D2 lymphadenectomy was performed in 67 (16.3%) patients, median operation time was 330 (range, 123–762) min, median operative blood loss was 50 (range, 0–1940) mL, serious postoperative complications (≥ CD IIIa) occurred in 65 patients (15.8%), and median postoperative hospital stay was 13 (range 6–210) days.
Table 2 shows the clinicopathological characteristics of the study patients. No significant differences were observed in the age, BMI, ASA-PS, and clinical JCGC stage between the two groups. Female patients had significantly (p = 0.018) fewer complications than that of the male patients. Table 3 shows the surgical outcomes in patients of the two groups. The median operative time, blood loss, extent of lymph node dissection, method of esophagojejunostomy, and number of resected lymph nodes were not significantly different between the two groups; however, the median postoperative hospital stay was significantly (p < 0.001) longer in CG (34 days [range 8–210]) than that of the non-CG (12 days [range 6–43]). Further, the mortality was not observed in both the groups in 30 days postoperatively. Among the postoperative complications, esophagojejunostomy (EJS)-related complications (leakage or stenosis) were the most common (38.5%) (Table 4).
Prognosis
Table 5 shows the histological examination results of resected specimens; the pathological JCGC stage were similar (p = 0.729) in both the groups. The median follow-up periods for patients of the CG and non-CG were 36.7 (2–109.6 months) and 32.8 (1–139.2) months, respectively. During the follow-up period, postoperative recurrence was observed in 11 (16.9%) and 49 (14.7%) patients of the CG and non-CG, respectively, with no significance (p = 0.569). Further, the median days until recurrence was significantly (p = 0.017) shorter in CG (223 [range 60–1480] days) than that of the non-CG (469 [range 72–2289] days). For all the patients, the 5-year OS rate was 75.9%; 66.4 and 76.8% in the CG and non-CG, respectively. The Kaplan–Meier analysis for the OS indicated a significant (p = 0.001) difference between the two groups (Fig. 2a). The 5-year DSS and DFS rates were 80.3 and 75.3, 70.1 and 70.9, and 76.2 and 80.9% for all the patients, CG, and non-CG, respectively. The Kaplan–Meier analysis for DSS and DFS indicated significant (DSS, p = 0.011; DFS, p = 0.001) differences between the two groups (Fig. 2b, c).
Prognostic factors for OS, DSS and RFS
Table 6 shows the multivariate analysis conducted to assess the risk factors for OS, DSS, and DFS. In the OS, depth of tumor invasion (pT) and serious postoperative complications (≥ CD IIIa) were identified as the independent prognostic factors (pT: hazard ratio [HR] 0.194, 95% confidence interval [CI] 0.094–0.397], p < 0.001; ≥ CD IIIa: HR 2.143, 95% CI 1.165–3.944, p = 0.014). In DSS, pT, lymph node metastasis (pN), and serious postoperative complications (≥ CD IIIa) were identified as the independent prognostic factors (≥ CD IIIa: HR 2.467, 95% CI 1.223–4.975, p = 0.011; pT: HR 0.156, 95% CI 0.062–0.394, p < 0.001; pN: HR 2.289, 95% CI 1.088–4.814, p = 0.029). In DFS, age, sex, and pT were independent prognostic factors (age: HR 1.959, 95% CI 1.001–3.832, p = 0.004; sex: HR 2.033, 95% CI 1.193–3.463, p = 0.009; pT: HR 0.192, 95% CI 0.095–0.385, p < 0.001).
Discussion
This is the first multicenter retrospective study that compared the serious postoperative complications and long-term outcomes associated with LTG for GC; we found that serious complications after LTG had a significant negative impact on the GC prognosis.
Severe postoperative complications increase the treatment costs, prolong the hospital stay, and have a negative effect on patients’ QOL. The association of postoperative complications with the long-term survival has been suggested for malignant tumors, such as breast, colorectal, and periampullary cancers [25,26,27,28]. Higher local recurrence risk and worse long-term outcomes in patients with GC were also reported to be related to postoperative complications [29, 30]. Kubota et al. evaluated the prognostic significance of postoperative complications in patients with GC using the propensity score matching analysis and reported a significant and independent correlation between the infectious complications and decreased survival [29]. Tokunaga et al. also reported that postoperative intra-abdominal infectious complications (IaICs) in patients with GC predict a poor OS [8]. Hence, the presence of postoperative complications, especially infectious complications, in GC is significantly correlated with the disease recurrence and poor survival, and may be due to following reasons: (1) the inflammatory response, prolonged fasting conditions, and weight loss due to severe postoperative complications may result in immunosuppression. The cell-mediated immune response, particularly the cytotoxic T lymphocytes and natural killer cells, is compromised by systemic inflammation and surgical stress, promoting the immune escape of micrometastatic carcinoma cells [31, 32], (2) a large number of activated leukocytes and cytotoxic mediators such as interleukin-1 (IL-1), IL-6, and tumor necrosis factor-alpha released from the inflammatory response due to infection-related postoperative complications could accelerate the proliferation and invasion ability of residual cancer cells, which promotes the development of tumor recurrence and metastasis [33, 34], and (3) chemotherapy cannot be adequately administered.
Evans et al. [35] reported that laparoscopic surgery had little impact on the human immune function and induced a slight inflammatory reaction in elderly patients, which could effectively reduce the occurrence of cardiopulmonary complications. With the advancement of laparoscopic technology and accumulation of surgical experience, laparoscopic gastrectomy is attracting attention as an alternative surgical method for patients with GC, and the occurrence of trauma and postoperative complications is expected to decrease further in the future. Regarding the long-term oncological results in LTG for GC, the 2-year OS and DFS rates were comparable between LTG and open total gastrectomy in meta-analysis studies [36, 37]. These results demonstrated that the surgical method did not affect long-term survival rates. Further, Jia-Bin Wang et al. reported that laparoscopic gastrectomy for GC can improve the prognosis of patients with postoperative IaICs and is, therefore, recommended for patients at a high risk of IaICs [38]. At present, the prognostic benefit of reduced surgical invasiveness with laparoscopy is controversial. In the present study also, we observed significantly worse 5-year OS, DSS, and DFS in patients with complications after LTG for GC. Furthermore, previous studies have also reported that weight loss associated with gastrectomy for GC decreased the nutritional status, postoperative QOL, and compliance of S-1 adjuvant chemotherapy, and could have led to the poor survival [39, 40]. Indeed, body weight loss at 1 year after surgery was reported to be approximately 10 and 15% following distal and total gastrectomy, respectively [41,42,43]. Weight loss after total gastrectomy may occur through various mechanisms, such as the hyper-catabolism associated with inflammatory reactions due to surgical stress, reduced food intake owing to loss of reservoir function, and reduction in blood ghrelin level [44]. Furthermore, the postoperative complications related to EJS forces prolong fasting, resulting in poor nutritional status and QOL. The EJS stenosis increases the risk of aspiration pneumonia as well [45]. In the present study, the EJS-related complications were also the most common; it is necessary to establish simple EJS techniques that show less frequent postoperative complications.
Several recent studies have suggested that male gender is a risk factor for postoperative complications in bowel surgery [46]. Furthermore, male gender has also been associated with shorter overall survival and disease-free survival after anastomotic leakage, suggesting that the male risk factor deserves more attention [47, 48]. Our results in the present study showed that male gender was associated with more serious postoperative complications male (p = 0.018). The gender difference may be explained by a combination of anatomical differences and the recently shown hormonal differences that influence the intestinal microcirculation [49]. Without a clear biological explanation for these findings, this finding should be interpreted with caution.
Randomized controlled studies have established that adjuvant chemotherapy following gastrectomy has survival advantages as compared to gastrectomy alone [50, 51]. Thus, adequate delivery and completion of chemotherapy are necessary to obtain a survival benefit after curative gastrectomy for GC. Li et al. reported that the completion of multimodality therapy could extenuate the adverse influence of complications on the long-term survival of patients with locally advanced GC [52]. In the present study, no significant differences were observed in the number of patients receiving adjuvant chemotherapy between the two groups, but the median days until recurrence was significantly shorter in CG than that of non-CG); these findings indicate that postoperative complications could accelerate the proliferation and invasion ability of residual cancer cells. Moreover, the prognosis may have been worse in patients who were unable to complete multimodality therapy due to serious postoperative complications after LTG. The European Society for Medical Oncology (ESMO) suggests radical gastrectomy with free margins and an adequate lymphadenectomy, and if indicated along with perioperative neoadjuvant chemotherapy, as the standard of care in patients with advanced GC [53, 54]. Several recent studies demonstrated no difference in postoperative complication rates in gastric cancer patients who received neoadjuvant treatment compared with those undergoing operation first [55,56,57]. In Japan, no evidence of perioperative neoadjuvant therapy is reported for GC. For patients with high risk of postoperative complications, neoadjuvant approach may be more beneficial to improve the chemotherapeutic agent compliance.
This study had several limitations. First, this was a retrospective, observational, and non-experimental study. In addition, we included patients who underwent either laparo-assisted total gastrectomy (LATG) or totally laparoscopic total gastrectomy (TLTG); different results may have been obtained in an analysis with exclusion of patients who underwent LATG. Second, this study was conducted over a rather long period between 2004 and 2018, which may have resulted in historical biases in terms of the treatment strategies and perioperative management, affecting the short-term and prognostic outcomes after LTG. The detailed chemotherapy regimens administered after relapse and their impact were not known. Third, the surgical procedures and indication for GC were subjectively determined based on the experience of each surgeon. Fourth, shorter median follow-up time and underestimation of the survival differences between the two groups were possible. A well-designed randomized control trial is required to validate our findings.
Conclusions
Occurrence of serious postoperative complications after LTG showed a negative impact on the OS of the patients with GC. Strategies that decrease the complications after LTG are required for contributing towards better prognosis of patients with GC after LTG.
Data availability statement
The datasets generated and analyzed during the current study are available from the corresponding author on reasonable request.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A (2015) Global cancer statistics, 2012. CA Cancer J Clin 65(2):87–108. https://doi.org/10.3322/caac.21262 (Epub 2015 Feb 4 PMID: 25651787)
Ferlay J, Colombet M, Soerjomataram I, Parkin DM, Piñeros M, Znaor A, Bray F (2021) Cancer statistics for the year 2020: an overview. Int J Cancer. https://doi.org/10.1002/ijc.33588 (Epub ahead of print. PMID: 33818764)
Yasunaga H, Horiguchi H, Kuwabara K, Matsuda S, Fushimi K, Hashimoto H, Ayanian JZ (2013) Outcomes after laparoscopic or open distal gastrectomy for early-stage gastric cancer: a propensity-matched analysis. Ann Surg 257(4):640–646. https://doi.org/10.1097/SLA.0b013e31826fd541 (PMID: 23023204)
Kim HH, Han SU, Kim MC, Hyung WJ, Kim W, Lee HJ, Ryu SW, Cho GS, Song KY, Ryu SY (2014) Long-term results of laparoscopic gastrectomy for gastric cancer: a large-scale case-control and case-matched Korean multicenter study. J Clin Oncol 32(7):627–633. https://doi.org/10.1200/JCO.2013.48.8551 (Epub 2014 Jan 27 PMID: 24470012)
Yu J, Hu J, Huang C, Ying M, Peng X, Wei H, Jiang Z, Du X, Liu Z, Liu H, Li G, Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group (2013) The impact of age and comorbidity on postoperative complications in patients with advanced gastric cancer after laparoscopic D2 gastrectomy: results from the Chinese laparoscropic gastrointestinal surgery study (CLASS) group. Eur J Surg Oncol 39(10):1144–1149. https://doi.org/10.1016/j.ejso.2013.06.021 (Epub 2013 Jul 10 PMID: 23850088)
Fearon KC, Jenkins JT, Carli F, Lassen K (2013) Patient optimization for gastrointestinal cancer surgery. Br J Surg 100(1):15–27. https://doi.org/10.1002/bjs.8988 (Epub 2012 Nov 20 PMID: 23165327)
Yuan P, Wu Z, Li Z, Bu Z, Wu A, Wu X, Zhang L, Shi J, Ji J (2019) Impact of postoperative major complications on long-term survival after radical resection of gastric cancer. BMC Cancer 19(1):833. https://doi.org/10.1186/s12885-019-6024-3 (PMID: 31443699, PMCID: PMC6708212)
Tokunaga M, Tanizawa Y, Bando E, Kawamura T, Terashima M (2013) Poor survival rate in patients with postoperative intra-abdominal infectious complications following curative gastrectomy for gastric cancer. Ann Surg Oncol 20(5):1575–1583. https://doi.org/10.1245/s10434-012-2720-9 (Epub 2012 Oct 18 PMID: 23076557)
Kiuchi J, Komatsu S, Ichikawa D, Kosuga T, Okamoto K, Konishi H, Shiozaki A, Fujiwara H, Yasuda T, Otsuji E (2016) Putative risk factors for postoperative pneumonia which affects poor prognosis in patients with gastric cancer. Int J Clin Oncol 21(5):920–926. https://doi.org/10.1007/s10147-016-0987-8 (Epub 2016 May 12 PMID: 27173949)
Shimada H, Fukagawa T, Haga Y, Oba K (2017) Does postoperative morbidity worsen the oncological outcome after radical surgery for gastrointestinal cancers? A systematic review of the literature. Ann Gastroenterol Surg 1(1):11–23. https://doi.org/10.1002/ags3.12002 (PMID: 29863169, PMCID: PMC5881350)
Kitano S, Iso Y, Moriyama M, Sugimachi K (1994) Laparoscopy-assisted Billroth I gastrectomy. Surg Laparosc Endosc 4(2):146–148 (Erratum in: Surg Laparosc Endosc (2013) 23(5):480. PMID: 8180768)
Huscher CG, Mingoli A, Sgarzini G, Sansonetti A, Di Paola M, Recher A, Ponzano C (2005) Laparoscopic versus open subtotal gastrectomy for distal gastric cancer: five-year results of a randomized prospective trial. Ann Surg 241(2):232–237. https://doi.org/10.1097/01.sla.0000151892.35922.f2 (PMID: 15650632, PMCID: PMC1356907)
Lee SW, Nomura E, Bouras G, Tokuhara T, Tsunemi S, Tanigawa N (2010) Long-term oncologic outcomes from laparoscopic gastrectomy for gastric cancer: a single-center experience of 601 consecutive resections. J Am Coll Surg 211(1):33–40. https://doi.org/10.1016/j.jamcollsurg.2010.03.018 (Epub 2010 May 26 PMID: 20610246)
Liu D, Liang L, Liu L, Zhu Z, Liu S, Hu L, He Y, Fang Y, Wan X (2020) Short-term outcomes and prognosis of laparoscopy-assisted total gastrectomy in elderly patients with stomach cancer. Surg Endosc 34(12):5428–5438. https://doi.org/10.1007/s00464-019-07338-0 (Epub 2020 Jan 28 PMID: 31993813)
Ebihara Y, Okushiba S, Kawarada Y, Kitashiro S, Katoh H (2013) Outcome of functional end-to-end esophagojejunostomy in totally laparoscopic total gastrectomy. Langenbecks Arch Surg 398(3):475–479. https://doi.org/10.1007/s00423-013-1051-z (Epub 2013 Jan 25. PMID: 23354359, PMCID: PMC3597276)
Kitagami H, Morimoto M, Nakamura K, Watanabe T, Kurashima Y, Nonoyama K, Watanabe K, Fujihata S, Yasuda A, Yamamoto M, Shimizu Y, Tanaka M (2016) Technique of Roux-en-Y reconstruction using overlap method after laparoscopic total gastrectomy for gastric cancer: 100 consecutively successful cases. Surg Endosc 30(9):4086–4091. https://doi.org/10.1007/s00464-015-4724-6 (Epub 2015 Dec 23 PMID: 26701704)
Okabe H, Tsunoda S, Tanaka E, Hisamori S, Kawada H, Sakai Y (2015) Is laparoscopic total gastrectomy a safe operation? A review of various anastomotic techniques and their outcomes. Surg Today 45(5):549–558. https://doi.org/10.1007/s00595-014-0901-9 (Epub 2014 May 3 PMID: 24792009)
Liu F, Huang C, Xu Z, Su X, Zhao G, Ye J, Du X, Huang H, Hu J, Li G, Yu P, Li Y, Suo J, Zhao N, Zhang W, Li H, He H, Sun Y, Chinese Laparoscopic Gastrointestinal Surgery Study (CLASS) Group (2020) Morbidity and mortality of laparoscopic vs open total gastrectomy for clinical stage I gastric cancer: the CLASS02 multicenter randomized clinical trial. JAMA Oncol 6(10):1590–1597. https://doi.org/10.1001/jamaoncol.2020.3152 (PMID: 32815991; PMCID: PMC7441466)
Zheng C, Xu Y, Zhao G, Cai L, Li G, Xu Z, Yan S, Wu Z, Xue F, Sun Y, Xu D, Zhang W, Wan J, Yu P, Hu J, Su X, Ji J, Li Z, You J, Li Y, Fan L, Lin J, Lin J, Li P, Huang C (2021) Outcomes of laparoscopic total gastrectomy combined with spleen-preserving hilar lymphadenectomy for locally advanced proximal gastric cancer: a nonrandomized clinical trial. JAMA Netw Open 4(12):e2139992. https://doi.org/10.1001/jamanetworkopen.2021.39992 (PMID: 34928353; PMCID: PMC8689389)
Japanese Gastric Cancer Association (2011) Japanese gastric cancer treatment guidelines 2010 (ver 3). Gastric Cancer 14(2):113–123. https://doi.org/10.1007/s10120-011-0042-4
Japanese Society for Endoscopic Surgery (2014) Guidelines for the management of endoscopic surgery. Japanese Society for Endoscopic Surgery, Tokyo
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213. https://doi.org/10.1097/01.sla.0000133083.54934.ae (PMID: 15273542, PMCID: PMC1360123)
Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, Dindo D, Schulick RD, de Santibañes E, Pekolj J, Slankamenac K, Bassi C, Graf R, Vonlanthen R, Padbury R, Cameron JL, Makuuchi M (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196. https://doi.org/10.1097/SLA.0b013e3181b13ca2 (PMID: 19638912)
Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, Meyer L, Gress DM, Byrd DR, Winchester DP (2017) The eighth edition AJCC cancer staging manual continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin 67(2):93–99. https://doi.org/10.3322/caac.21388 (Epub 2017 Jan 17. PMID: 28094848)
Murthy BL, Thomson CS, Dodwell D, Shenoy H, Mikeljevic JS, Forman D, Horgan K (2007) Postoperative wound complications and systemic recurrence in breast cancer. Br J Cancer 97(9):1211–1217. https://doi.org/10.1038/sj.bjc.6604004 (Epub 2007 Oct 30. PMID: 17968426, PMCID: PMC2360477)
Kressner U, Graf W, Mahteme H, Påhlman L, Glimelius B (2002) Septic complications and prognosis after surgery for rectal cancer. Dis Colon Rectum 45(3):316–321. https://doi.org/10.1007/s10350-004-6174-4 (PMID: 12068187)
Artinyan A, Orcutt ST, Anaya DA, Richardson P, Chen GJ, Berger DH (2015) Infectious postoperative complications decrease long-term survival in patients undergoing curative surgery for colorectal cancer: a study of 12,075 patients. Ann Surg 261(3):497–505. https://doi.org/10.1097/SLA.0000000000000854 (PMID: 25185465)
Cho JY, Han HS, Yoon YS, Hwang DW, Jung K, Kim YK (2013) Postoperative complications influence prognosis and recurrence patterns in periampullary cancer. World J Surg 37(9):2234–2241. https://doi.org/10.1007/s00268-013-2106-6 (PMID: 23722466)
Kubota T, Hiki N, Sano T, Nomura S, Nunobe S, Kumagai K, Aikou S, Watanabe R, Kosuga T, Yamaguchi T (2014) Prognostic significance of complications after curative surgery for gastric cancer. Ann Surg Oncol 21(3):891–898. https://doi.org/10.1245/s10434-013-3384-9 (Epub 2013 Nov 20 PMID: 24254205)
Powell A, Coxon AH, Patel N, Chan D, Christian A, Lewis W (2018) Prognostic significance of post-operative morbidity severity score after potentially curative D2 gastrectomy for carcinoma. J Gastrointest Surg 22(9):1516–1527. https://doi.org/10.1007/s11605-018-3787-9 (Epub 2018 May 15. PMID: 29766446, PMCID: PMC6132392)
Lerut T, Moons J, Coosemans W, Van Raemdonck D, De Leyn P, Decaluwé H, Decker G, Nafteux P (2009) Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg 250(5):798–807. https://doi.org/10.1097/SLA.0b013e3181bdd5a8 (PMID: 19809297)
Roxburgh CS, Horgan PG, McMillan DC (2013) The perioperative immune/inflammatory insult in cancer surgery: time for intervention? Oncoimmunology 2(12):e27324. https://doi.org/10.4161/onci.27324 (Epub 2013 Dec 9. PMID: 24498571, PMCID: PMC3909539)
Saito T, Kurokawa Y, Miyazaki Y, Makino T, Takahashi T, Yamasaki M, Nakajima K, Takiguchi S, Mori M, Doki Y (2015) Which is a more reliable indicator of survival after gastric cancer surgery: postoperative complication occurrence or C-reactive protein elevation? J Surg Oncol 112(8):894–899. https://doi.org/10.1002/jso.24067 (Epub 2015 Oct 13 PMID: 26458724)
Okamura A, Takeuchi H, Matsuda S, Ogura M, Miyasho T, Nakamura R, Takahashi T, Wada N, Kawakubo H, Saikawa Y, Kitagawa Y (2015) Factors affecting cytokine change after esophagectomy for esophageal cancer. Ann Surg Oncol 22(9):3130–3135. https://doi.org/10.1245/s10434-014-4348-4 (Epub 2015 Jan 9 PMID: 25572684)
Evans C, Galustian C, Kumar D, Hagger R, Melville DM, Bodman-Smith M, Jourdan I, Gudgeon AM, Dalgleish AG (2009) Impact of surgery on immunologic function: comparison between minimally invasive techniques and conventional laparotomy for surgical resection of colorectal tumors. Am J Surg 197(2):238–245. https://doi.org/10.1016/j.amjsurg.2008.01.021 (Epub 2008 Jul 17 PMID: 18639228)
Chen XZ, Wen L, Rui YY, Liu CX, Zhao QC, Zhou ZG, Hu JK (2015) Long-term survival outcomes of laparoscopic versus open gastrectomy for gastric cancer: a systematic review and meta-analysis. Med (Baltim) 94(4):e454. https://doi.org/10.1097/MD.0000000000000454 (PMID: 25634185, PMCID: PMC4602964)
Shen H, Shan C, Liu S, Qiu M (2013) Laparoscopy-assisted versus open total gastrectomy for gastric cancer: a meta-analysis. J Laparoendosc Adv Surg Tech A 23(10):832–840. https://doi.org/10.1089/lap.2013.0152 (Epub 2013 Aug 27 PMID: 23980591)
Wang JB, Que SJ, Chen QY, Zhong Q, Liu ZY, Lin JX, Lu J, Cao LL, Lin M, Tu RH, Huang ZN, Lin JL, Zheng HL, Zheng CH, Li P, Huang CM, Xie JW (2021) Prognostic analysis of patients with intra-abdominal infectious complications after laparoscopic-assisted and open radical gastrectomy for gastric cancer - a propensity score-matching analysis. Surg Oncol 37:101583. https://doi.org/10.1016/j.suronc.2021.101583 (Epub 2021 May 14 PMID: 34087739)
Aoyama T, Yoshikawa T, Shirai J, Hayashi T, Yamada T, Tsuchida K, Hasegawa S, Cho H, Yukawa N, Oshima T, Rino Y, Masuda M, Tsuburaya A (2013) Body weight loss after surgery is an independent risk factor for continuation of S-1 adjuvant chemotherapy for gastric cancer. Ann Surg Oncol 20(6):2000–2006. https://doi.org/10.1245/s10434-012-2776-6 (Epub 2012 Dec 16 PMID: 23242818)
Aoyama T, Sato T, Maezawa Y, Kano K, Hayashi T, Yamada T, Yukawa N, Oshima T, Rino Y, Masuda M, Ogata T, Cho H, Yoshikawa T (2017) Postoperative weight loss leads to poor survival through poor S-1 efficacy in patients with stage II/III gastric cancer. Int J Clin Oncol 22(3):476–483. https://doi.org/10.1007/s10147-017-1089-y (Epub 2017 Feb 7 PMID: 28176023)
Davis JL, Selby LV, Chou JF, Schattner M, Ilson DH, Capanu M, Brennan MF, Coit DG, Strong VE (2016) Patterns and predictors of weight loss after gastrectomy for cancer. Ann Surg Oncol 23(5):1639–1645. https://doi.org/10.1245/s10434-015-5065-3 (Epub 2016 Jan 5. PMID: 26732274, PMCID: PMC4862874)
Takiguchi S, Miyazaki Y, Takahashi T, Kurokawa Y, Yamasaki M, Nakajima K, Miyata H, Hosoda H, Kangawa K, Mori M, Doki Y (2016) Impact of synthetic ghrelin administration for patients with severe body weight reduction more than 1 year after gastrectomy: a phase II clinical trial. Surg Today 46(3):379–385. https://doi.org/10.1007/s00595-015-1187-2 (Epub 2015 May 29 PMID: 26019019)
Kim JW, Jung SY, Cho JW, Kim BC, Chung KS, Yang DH (2014) Postoperative body mass index changes in gastric cancer patients according to reconstruction type: effectiveness of long jejunal bypass on weight loss in obese patients after distal gastrectomy. Indian J Surg 76(3):187–192. https://doi.org/10.1007/s12262-012-0651-0 (Epub 2012 Jul 8. PMID: 25177114, PMCID: PMC4141068)
Ryan AM, Healy LA, Power DG, Rowley SP, Reynolds JV (2007) Short-term nutritional implications of total gastrectomy for malignancy, and the impact of parenteral nutritional support. Clin Nutr 26(6):718–727. https://doi.org/10.1016/j.clnu.2007.08.013 (Epub 2007 Oct 18 PMID: 17949863)
Chen G, Wang J, Chen K, Kang M, Zhang H, Jin X, Lin L, Chen J (2021) Relationship between postoperative complications and the prognosis of gastric carcinoma patients who underwent surgical resection: a systematic review and meta-analysis. Cancer Control 28:10732748211011956. https://doi.org/10.1177/10732748211011955 (PMID: 34018400, PMCID: PMC8204457)
Kang SC, Kim HI, Kim MG (2016) Low serum albumin level, male sex, and total gastrectomy are risk factors of severe postoperative complications in elderly gastric cancer patients. J Gastric Cancer 16(1):43–50
Bell SW, Walker KG, Rickard MJ, Sinclair G, Dent OF, Chapuis PH, Bokey EL (2003) Anastomotic leakage after curative anterior resection results in a higher prevalence of local recurrence. Br J Surg 90(10):1261–1266. https://doi.org/10.1002/bjs.4219 (PMID: 14515297)
Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL (2004) Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg 240(2):255–259. https://doi.org/10.1097/01.sla.0000133186.81222.08 (PMID: 15273549; PMCID: PMC1356401)
Ba ZF, Yokoyama Y, Toth B, Rue LW 3rd, Bland KI, Chaudry IH (2004) Gender differences in small intestinal endothelial function: inhibitory role of androgens. Am J Physiol Gastrointest Liver Physiol 286(3):G452–G457. https://doi.org/10.1152/ajpgi.00357.2003 (Epub 2003 Oct 16 PMID: 14563675)
Sakuramoto S, Sasako M, Yamaguchi T, Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi Y, Imamura H, Higashino M, Yamamura Y, Kurita A, Arai K, ACTS-GC Group (2007) Adjuvant chemotherapy for gastric cancer with S-1, an oral fluoropyrimidine. N Engl J Med 357(18):1810–1820. https://doi.org/10.1056/NEJMoa072252 (Erratum. In: N Engl J Med (2008) 358(18):1977 PMID: 17978289)
Noh SH, Park SR, Yang HK, Chung HC, Chung IJ, Kim SW, Kim HH, Choi JH, Kim HK, Yu W, Lee JI, Shin DB, Ji J, Chen JS, Lim Y, Ha S, Bang YJ, CLASSIC trial investigators, (2014) Adjuvant capecitabine plus oxaliplatin for gastric cancer after D2 gastrectomy (Classic): 5-year follow-up of an open-label, randomised phase 3 trial. Lancet Oncol 15(12):1389–1396. https://doi.org/10.1016/S1470-2045(14)70473-5 (Epub 2014 Oct 15 PMID: 25439693)
Li SS, Udelsman BV, Parikh A, Klempner SJ, Clark JW, Roeland EJ, Wo JY, Hong TS, Mullen JT (2020) Impact of postoperative complication and completion of multimodality therapy on survival in patients undergoing gastrectomy for advanced gastric cancer. J Am Coll Surg 230(6):912–924. https://doi.org/10.1016/j.jamcollsurg.2019.12.038 (Epub 2020 Feb 6 PMID: 32035978)
Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D, Guidelines Committee ESMO (2016) Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 27(suppl 5):v38–v49. https://doi.org/10.1093/annonc/mdw350 (PMID: 27664260)
Rausei S, Boni L, Rovera F, Dionigi G (2013) Locally advanced gastric cancer: a new definition to standardise. J Clin Pathol 66(2):164–165. https://doi.org/10.1136/jclinpath-2012-201176 (Epub 2012 Dec 8 PMID: 23223567)
Badgwell B, Ajani J, Blum M, Ho L, Fournier K, Chiang YJ, Matamoros A, Das P, Mansfield P (2016) Postoperative morbidity and mortality rates are not increased for patients with gastric and gastroesophageal cancer who undergo preoperative chemoradiation therapy. Ann Surg Oncol 23(1):156–162. https://doi.org/10.1245/s10434-015-4643-8 (Epub 2015 Jun 10 PMID: 26059652)
Fuentes E, Ahmad R, Hong TS, Clark JW, Kwak EL, Rattner DW, Mullen JT (2016) Adjuvant therapy completion rates in patients with gastric cancer undergoing perioperative chemotherapy versus a surgery-first approach. J Gastrointest Surg 20(1):172–179. https://doi.org/10.1007/s11605-015-2954-5 (discussion 179 Epub 2015 Sep 22. PMID: 26394879)
Sun Z, Nussbaum DP, Speicher PJ, Czito BG, Tyler DS, Blazer DG 3rd (2015) Neoadjuvant radiation therapy does not increase perioperative morbidity among patients undergoing gastrectomy for gastric cancer. J Surg Oncol 112(1):46–50. https://doi.org/10.1002/jso.23957 (Epub 2015 Jul 14 PMID: 26179329)
Acknowledgements
The author is grateful to our colleagues (Drs. Hiroshi Kawase and Mamoru Miyasaka) and the interdisciplinary surgical team. We would like to thank Editage (www.editage.com) for English language editing.
Funding
No funding support was received for this study.
Author information
Authors and Affiliations
Contributions
Study conception and design: YE and SH. Acquisition of data: YE. Analysis and interpretation of data: YE and SH. Drafting of manuscript: YE. Critical revision of the manuscript: YE, NK, YM, KM, FN, TM, SO, and SH.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethics approval
Hokkaido University Hospital Institutional Review Board approved the data collection and analysis (No. 016-0151).
Consent to participate
All study participants provided informed consent, and the study design was approved by the appropriate ethics review board.
Consent to publish
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ebihara, Y., Kyogoku, N., Murakami, Y. et al. Relationship between laparoscopic total gastrectomy-associated postoperative complications and gastric cancer prognosis. Updates Surg 75, 149–158 (2023). https://doi.org/10.1007/s13304-022-01402-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-022-01402-6