Abstract
Siewert III cancers were classified as esophageal cancers by the TNM 7th edition (TNM7), while being defined as gastric cancers by the new TNM 8th edition (TNM8). Aim of this study was to compare previous and present TNM classifications of Siewert III. From 2000 to 2015, 309 patients with Siewert III adenocarcinoma were treated at ten high-volume centers, belonging to the GIRCG (Italian Research Group for Gastric Cancer). We retrospectively analyzed overall survival according to TNM classifications: gastric TNM8 was compared with either gastric TNM7 or esophageal TNM7. Median number of lymph nodes harvested was 31 (interquartile range 22–44). Agreement between gastric TNM7 and TNM8 was very good (weighted kappa 92.3%, IC 95% 90.3–94.1%). Accordingly, stage migration was observed in 54 of 309 patients (17.5%), with 12 patients upstaged (3.9%) and 42 downstaged (13.6%). Cox models including either gastric TNM7 or TNM8 achieved similar goodness-of-fit and c-index. Differences were much larger, when shifting from esophageal TNM7 to gastric TNM8: the agreement was much lower (weighted kappa 69.1%, 65.2–73.2%), with 196 of 309 patients (63.4%) downstaging. The corresponding Cox model presented the lowest goodness-of-fit and discrimination ability. Gastric TNM7 and TNM8 were largely superimposable, so that stage migration was minor and prognostic significance was similar. At variance, stage migration was substantial when shifting from esophageal TNM7 to TNM8. Moreover, survival models with esophageal TNM7 presented the worst goodness-of-fit and the lowest discrimination ability. This further supports placing Siewert III among gastric cancers, as done in TNM8.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Esophagogastric (EGJ) junction cancers had different definitions overtime, going back and forth from being defined as either esophageal or gastric cancers. TNM 7th edition classified them all as esophageal cancers [1]. This choice, initially supposed to solve the problem of definition, created a huge debate about the topic instead. If everybody considered Siewert I as an esophageal cancer and most considered Siewert II as well as an esophageal cancer, very few agreed to define Siewert III as such. This argument was so strong that Siewert III was generally considered a gastric cancer invading the esophagus by consensus conferences and guidelines [2, 3]. Siewert type III cancers are those of the proximal stomach invading the EGJ, with tumor epicenter from 2 to 5 cm below the EGJ, according to the classification of EGJ adenocarcinoma first proposed by Siewert [4]. The new TNM 8th ed. [5]. introduced some relevant changes, first of all the definition of anatomic boundary between esophagus and stomach: tumors involving the EGJ with tumor epicenter no more than 2 cm into the proximal stomach are classified as esophageal cancers. EGJ tumors with their epicenter located more than 2 cm into the proximal stomach are classified as gastric cancers, similar to Siewert’s original definition of Siewert III. Hence, Siewert III are gastric cancers again. Is any problem solved? Actually issues are not limited to definition and, as done by other authors for gastric cancer [6,7,8], in the present study we aimed at comparing TNM 7th ed. and TNM 8th ed. in terms of overall survival. To make things a little more complicated, TNM 8th ed. for gastric cancer (gastric TNM8) has to be compared with TNM 7th ed. for esophageal cancers (esophageal TNM7), which was supposed to be used till December 2017, and TNM 7th ed. for gastric cancers (gastric TNM7), which was frequently used by most centers.
Methods
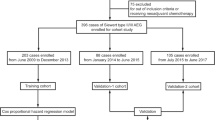
From 2000 to 2015, 309 patients with Siewert III adenocarcinoma were treated at 10 high-volume centers for Upper G.I. surgery, belonging to the GIRCG (Italian Research Group for Gastric Cancer). All patients were treated with surgical radical intent, patients undergoing palliative surgery were excluded. Both patients undergoing upfront surgery and receiving induction or perioperative chemotherapy were included. Induction treatment included different protocols of only preoperative chemotherapy, while perioperative regimes had chemotherapy both prior and after surgery.
Different surgical operations were performed by different centers, but mostly surgery consisted in total gastrectomy and distal esophagectomy together with D2 abdominal and distal mediastinal lymphadenectomy, possibly with a solo abdominal access. Patients were followed-up regularly after surgery with protocols that differed in the various centers. Pathologic examinations reported grading, Lauren histotype, R status, and number of resected and positive lymph nodes.
All patients were staged with esophageal TNM7, gastric TNM7 and gastric TNM8. The main differences between esophageal TNM7 and gastric TNM7 cancers concerned T4 patients: in esophageal TNM7, T4a are those cancers invading pleura, pericardium and diaphragm, while in gastric TNM7 T4a cancers invade serosa (visceral peritoneum). T4b cancers in gastric TNM7 invade adjacent structures, irrespective of resectability of the cancer, while in esophageal TNM7, the tumor invades unresectable adjacent structures, such as aorta, vertebral body, or trachea. This created relevant issues to define T4 patients using esophageal TNM7.
In the comparison between gastric TNM7 and TNM8; T, N and M categories did not change, except pN3, which was subdivided into N3a (7–15 positive lymph nodes) and N3b (≥ 16 positive lymph nodes). This change was responsible for various stage migrations.
Statistical analyses
When a class had less than 20 subjects, it was joined to adjacent class(es).
Agreement between different TNM editions was evaluated by weighted Cohen’s kappa with equally spaced weights, whose confidence interval was computed by bootstrap.
Survival curves were estimated by the Kaplan–Meier method and compared by the log-rank test or by the test for trend, as appropriate.
Prognostic value of different stage classifications was tested by Cox regression models, controlling for sex, age, Lauren histology and number of excised nodes, and by stratifying by centre. When comparing non-nested Cox models, the best model was considered that with the lowest Akaike’ Information Criterion (AIC), which takes into account both the goodness-of-fit of the model and its parsimony. Discrimination ability was evaluated by Harrell’s C discrimination index.
Results
Demographics
In the present series median age was 70 (range 23–91) and 223 patients (72%) were males. Of the 309 patients treated, 225 (72.8%) underwent upfront surgery, while 84 (27.2%) underwent multimodal treatments and surgery: 47 and 37 underwent induction and perioperative chemotherapy, respectively.
The most common surgical resection was total gastrectomy and distal esophagectomy (n = 255, 82.5%) together with D2 abdominal lymphadenectomy (n = 212, 68.6%). Distal mediastinal lymphadenectomy was performed in 140 patients (46%). R0 was achieved in 267 patients (86.4%). Median number of lymph nodes harvested was 31 (interquartile range 22–44), while the median number of positive nodes was 4 (0–9).
Eighty-six patients with upfront surgery (38.2%) underwent subsequent adjuvant chemotherapy.
Median follow-up was 36 months (interquartile range 14–69 months).
Stage distribution and migration
When comparing gastric TNM7 and gastric TNM8, only stages IIIA, IIIB and IIIC changed (Table 1). In detail, 42 IIIA TNM7 patients remained IIIA TNM8, while 2 upstaged to IIIB TNM8 (5%). Among the 50 patients classified as IIIB by TNM7, 30 IIIB TNM7 patients remained IIIB TNM8, 10 patients downstaged to IIIA TNM8 (20%), while another 10 upstaged to IIIC TNM8 (20%). The largest variation occurred in the stage IIIC of TNM7 classification, where most patients (32/54 = 59%) were downstaged to IIIB, while 22 IIIC TNM7 remained IIIC TNM 8. These changes resulted in increased number of IIIA patients (from 44 in TNM7 to 52 in TNM8) and IIIB patients (from 50 in TNM7 to 64 in TNM8) and decreased number in IIIC patients (from 54 in TNM7 to 32 in TNM8).
In summary, when shifting from gastric TNM7 to TNM8, stage migration was observed in 54 of 309 patients (17.5%), in particular 12 patients upstaged (3.9%) and 42 downstaged (13.6%) by just one tier. Overall, the agreement between gastric TNM7 and TNM8 was very good (weighted kappa 92.3%, IC 95% 90.3–94.1%), also because discrepancies between the two TNM editions consisted downward or upward shifts by just one tier.
Differences were much larger, when shifting from esophageal TNM7 to gastric TNM8. Indeed, stage migration was observed in 196 of 309 patients (63.4%), and all these patients downstaged. Stage migration involved a downshift of two stages in 27 patients (8.7%), and even three stages in 1 case, as shown in Table 2. As a consequence, the agreement between esophageal TNM7 and gastric TNM8 (weighted kappa 69.1%, 65.2–73.2%) was much lower than the agreement between gastric TNM7 and TNM8 classifications.
Univariable survival analysis
Entire study population
As shown in Fig. 1, prognosis progressively worsened with increasing stage, irrespective of the TNM edition used (test for trend: p < 0.001).
When patients were classified according to gastric TNM7 (Fig. 1a), overall survival at 3 years was 79% (63–88%) in stage I; 60% (38–77%) and 55% (36–71%), respectively in stage IIA and IIB; 43% (26–59%), 50% (34–65%), 26% (15–38%), respectively in stage IIIA, IIIB and IIIC; 6% (1–16%) in stage IV. When patients were regrouped according to gastric TNM8 (Fig. 1b), 3-year overall survival became 41% (25–56%) in stage IIIA, 47% (34–59%) in stage IIIB and 18% (7–35%) in stage IIIC. Of note, survival in the IIIB group was higher than in the preceding stage (IIIA) with both editions.
When adopting esophageal TNM7 (Fig. 1c), 3-year overall survival was 79% (62–89%) in stage I, 67% (45–81%) in stage II, 53% (35–68%) in stage IIIA, 35% (15–56%) in stage IIIB, 40% (30–49%) in stage IIIC, 6% (1–16%) in stage IV. The most striking finding was the rather favorable prognosis in stage IIIC; it should be reminded that this class was inflated by esophageal TNM7 and comprised nearly 40% of patients, including also patients staged as IIB or IIIA by gastric TNM8.
Only upfront surgery
When considering only patients who had undergone upfront surgery (Fig. 2a, b), prognosis progressively worsened with increasing stage (test for trend: p < 0.001), with about the same pattern recorded in the entire series. When using gastric TNM7, overall survival at 3 years was 81% (62–91%) in stage I; 60% (36–78%) and 54% (33–72%), respectively in stage IIA and IIB; 42% (24–59%), 63% (40–79%), 24% (12–38%), respectively in stage IIIA, IIIB and IIIC; 8% (1–22%) in stage IV. When patients were regrouped according to present TNM8, 3-year overall survival became 40.5% (23–57%) in stage IIIA, 51% (35–65%) in stage IIIB and 15% (4–33%) in stage IIIC. Of note, also in this case survival in IIIB group was higher than in the preceding stage (IIIA) with both editions.
When upfront surgery patients were coded according to esophageal TNM7, 3-year survival was 81% (60–92%) in stage I, 67% (44–82%) in stage II, 52% (31–69%) in stage IIIA, 28% (9–51%) in stage IIIB, 42% (31–53%) in stage IIIC, and 8% (1–22%) in stage IV) (p < 0.001, Fig. 2c).
Of note, when the study base was restricted to patients submitted to upfront surgery and adequately staged (> 16 retrieved lymph nodes), the unexpected improvements in survival with worsening stage, observed in gastric TNM7 and TNM8 from stage IIIA to stage IIIB and in esophageal TNM7 from stage IIIB to stage IIIC, persisted (data not shown).
Multivariable survival analysis
When controlling for sex, age, Lauren histotype, number of excised nodes by Cox regression model, and stratifying by centre, the risk of death from all causes markedly increased with increasing tier in all classifications (Fig. 3). Of note, it can be appreciated that both TNM7 and TNM8 did not allow differentiating the risk of death between IIA and IIB tiers, and between IIIA and IIIB tiers. However, while in TNM7 mortality risk slightly decreased from IIIA to IIIB, in TNM8 mortality risk slightly increased as expected. Moreover, while in gastric TNM7 and TNM8 the hazard of death in stage IIIC was intermediate between those of stages IIIB and IV, in esophageal TNM7 it was close to that of stage IIIB, so that a fourfold step in prognosis occurred between stages IIIC and IV.
Hazard ratio of death from all causes as a function of three different TNM classifications: gastric TNM7 (TNM7), gastric TNM8 (TNM8), esophageal TNM7 (TNM7_esophagus). HR (95% CI) were computed by a Cox model, controlling for sex, age, Lauren histotype, number of excised nodes, and stratifying by center
The model with stage coded according to gastric TNM7 had a lower AIC (1008.3) than the model with stage coded according to TNM8 (1014.2), while having a similar C-index (0.738 and 0.740, respectively). The model with esophageal TNM7 was the worst one, having the highest AIC (1028.6) and the lowest C-index (0.722).
Discussion
Siewert III is an orphan disease. Although representing around 40% of EGJ cancers and being the EGJ cancer with worst prognosis, it does not have a homogenous treatment and is often excluded from study protocols. There are no dedicated studies for Siewert III, and it is either explicitly excluded from trials on gastric or esophageal cancers or included in trials like MAGIC, which considered all esophagogastric cancers together [9]. It is now defined as a gastric cancer by TNM8, and this should solve most of the problems. Nonetheless, the lack of specific literature allows only for speculation.
In our trial, we considered the largest series to our knowledge of solo Siewert III patients from a single western country. We compared overall survival using esophageal TNM7, gastric TNM7 and the new gastric TNM8 for the entire study population and for patients treated with upfront surgery. Our main findings are the following:
-
1.
The agreement between gastric TNM7 and TNM8 was very good (k = 0.92), stage migration being restricted to stage III patients.
-
2.
Survival models with either gastric TNM7 or TNM8 had similar discrimination ability, as regards prognosis. However, the model with gastric TNM7 achieved a slightly higher goodness-of-fit.
-
3.
At variance, stage migration was substantial when shifting from esophageal TNM7 to gastric TNM8, and the agreement was lower (k = 0.69). Moreover, survival models with esophageal TNM7 presented the worst goodness-of-fit and the lowest discrimination ability, suggesting that a staging system including Siewert III among esophageal cancers is worse than staging systems placing Siewert III among gastric cancers.
-
4.
These findings were similar when considering either the entire study population or only upfront surgery patients.
TNM7 for gastric cancer was a “hybrid” between esophageal categories proposed by WECC [1, 10] and Japanese and Korean stage groups [11]. As a matter of fact, this staging system was made to harmonize gastric staging to the esophageal system. However, in esophageal system all patients with more than six nodes were defined as pN3, and this was done considering that in esophageal cancer having more than six nodes involved equaled to diffuse disease with very low prognosis. Actually this is not exactly true in gastric cancer, where survival progressively decreases with increasing number of involved nodes, but patients with a considerable number of involved nodes have substantial survival, and anyway patients with more than 15 nodes involved have statistically worst prognosis than patients with 7–15 positive nodes [12, 13]. So in gastric cancer dividing pN3 into pN3a and pN3b seemed important. An international multicenter study, carried on by International Gastric Cancer Association (IGCA) on more than 25,000 patients, demonstrated that discriminating between pN3a and pN3b patients into gastric TNM7 better stratified survival [11]. To note, all those patients came from data retrospectively collected by specialized centers, mainly from eastern countries (91%), without induction treatments. In detail, these Authors proposed a different stage grouping that introduced the above-mentioned partition on pN3 in determining final stage, and that caused a stage migration among stage III patients, with increased number of IIIA patients and decreased number of IIIC patients compared to gastric TNM7. This different distribution correlated to a better stratification of survival compared to TNM7. Authors’ conclusion was that, in the new TNM8, pN3 patients should have been further subdivided according to the number of involved nodes. Actually, the classification proposed by IGCA was translated into facts and TNM8 now considers pN3a and pN3b.
Although Siewert III is different from distal gastric cancer, and data must be compared with caution, it is now defined as a gastric cancer, and has to be staged as such.
Only the IGCA study considered also Siewert II and III patients and demonstrated that gastric TNM7 was better than esophageal TNM7 in determining prognosis, but worse than the IGCA proposal. When considering only Siewert III cases, the best staging system remained the IGCA proposal, although prognosis was globally worse than that of the other gastric cancer patients and Authors attributed the difference to different tumor biology or more difficult surgical approach. To note, although the number of Siewert III was 616, only 160 came from Western series. Our study represents the largest Western series by a single country, with 225 patients treated with surgery alone. Anyway, in our series, survival discrimination was similar considering only upfront surgery and the entire study population, which included multimodal treatment. Also, in our series, consistent with Sano et al. [11], esophageal TNM7 did not fit Siewert III patients, and TNM8 seemed to clinically better determine prognosis.
Different studies on gastric cancer tested the new classification comparing it to previous TNM version [6,7,8]. All these studies did not consider Siewert III patients nor induction-treated patients. The American and Chinese study [6, 8] reported an increased number of stages IIIA and IIIB and a reduction of IIIC, with around a 20% stage migration for the Chinese trial, which was consistent with the 17.5% of our study. A minimal migration (6%) was on the contrary reported by the Korean study [7], were upstaging was slightly more common. Only this last study reported a sure improved survival discrimination with TNM8, while the others reported a discrimination potential similar to TNM7. Indeed, although TNM8 was probably clinically more successful, TNM7 was statistically superior in both studies. Our results, although Siewert III is probably a different disease and is not considered in these trials, are in line with what reported in the abovementioned Chinese and American studies in terms of stage migration and comparison of discrimination potential between staging systems. Indeed, considering our entire study population, overall survival of stages IIA and IIB was very similar in both TNM7 and TNM8. In TNM7, stages IIIA and IIIB were inverted, on the contrary, in TNM8 they overlapped, with IIIB worsening and IIIA improving. Moreover, stage IIIC clearly worsened in TNM8. All these were indicative of better calibration of TNM 8. When only upfront surgery patients were considered, figures were similar.
Interestingly in the Chinese trial, TNM8 became statistically superior when more than 30 lymph nodes were removed. This can indicate that a correct lymphadenectomy correlates to improved staging: to define N stage in TNM8 at least 16 nodes must be removed. Interestingly, in the American trail the median number of removed nodes was 2, preventing from correctly using the new TNM8. Our results come from specialized centers, where the median number of removed nodes was 31, with only 8 patients with < 10 nodes removed (2.5%) and 29 with < 16 nodes removed (9%). Hence, this indicates that TNM8 was correctly applicable in 91% of our study population.
The issue of correct lymphadenectomy is and has always been relevant. Barbour et al. [14]. in 2007 demonstrated in Siewert types II and III adenocarcinoma that adequately staged patients (≥ 15 nodes examined) had more positive lymph nodes compared with inadequately staged patients (< 15 nodes examined); and this translated into important prognostic differences, with inadequately staged N0 patients demonstrating similar survival to adequately staged N1 patients.
Total number of resected nodes is a good marker of lymphadenectomy adequacy: more nodes harvested translated into more precise staging, reducing stage migration and giving more accurate survival information. However, extended lymphadenectomy would be justified only if it correlated to improved survival. Many trials investigated the topic, reporting an overall survival advantage and/or a reduced hazard of death in case of increased number of resected nodes [15,16,17,18] in esophageal and EGJ cancer. Similarly, on gastric cancer, two large-scale Chinese studies [19, 20] demonstrated improved survival with more than 21 and 30 nodes harvested, respectively. Considering only N0 gastric cancer, other studies [21,22,23] showed an improved survival with increasing number of lymph nodes removed: respectively with cut-offs of 18, 22 and 25 nodes harvested, respectively. This advantage was noted especially in advanced cancers. The reason why increasing number of resected nodes reflects on survival is not fully understood: if for N + patients it is probably related to stage migration with the possibility to identify other positive nodes that might be missed with less rigorous lymph node sampling, a possible explanation for N0 patients is the elimination of micrometastases. These are defined as metastases detectable only with immunostaining, in nodes considered negative with imaging and by routine histological examination. The presence of micrometastases in supposed node-negative patients could explain the improved survival after extended lymphadenectomy in pathological N0 patients [9].
An adequate number of harvested nodes is, therefore important not only to avoid stage migration, but also to ensure a correct pN0 allocation. TNM8 can be useful and in particular better than TNM7 only if used correctly, which means only if an adequate number of nodes is removed, allowing for correct staging.
Limitations
This study presents, however some limitations. First of all, although presenting the largest single country series of solo Siewert III patients, it is a retrospective study from a not prospectively collected database. Its multicenter nature accounts for the differences among centers in terms of surgical approaches and multimodal treatments. Moreover, in line with other studies cited, patients could have undergone adjuvant treatments after surgery, possibly altering some of the survival analyses. Again, all the centers were specialized in foregut malignancies, and this is shown by the high number of lymph nodes removed. Siewert III patients require difficult surgical operations and are then often centralized in specialized centers; nonetheless our results may not represent a fully descriptive picture of the reality in not specialized hospitals.
Often Siewert III patients undergo multimodal treatments and this may alter the results compared with surgery alone. In the present investigation, results considering patients with or without multimodal treatments were similar. However, other studies with largest series are necessary to confirm or confute these findings.
Conclusions
In conclusion, Siewert III is a gastric cancer, but it is also different. Its location with involvement of the esophagus makes it a unique type of cancer. Although gastric TNM7 seemed better from a statistical point of view, gastric TNM8 seemed to improve staging, better separating stage III patients also for Siewert III. Gastric TNM8 is, therefore, the best staging tool available so far, provided that a correct lymphadenectomy, with at least 16 nodes removed, is carried out. Nonetheless probably a specific staging system would better fit this unique type of cancer and a worldwide collaboration to collect data of this rising in incidence type of cancer would be of utmost importance.
References
Rice TW, Blackstone EH, Rusch VW (2010) 7th edition of the AJCC cancer staging manual: esophagus and esophagogastric junction. Ann Surg Oncol 17(7):1721–1724. https://doi.org/10.1245/s10434-010-1024-1
Lutz MP, Zalcberg JR, Ducreux M et al (2012) Highlights of the EORTC st. Gallen international expert consensus on the primary therapy of gastric, gastroesophageal and oesophageal cancer—differential treatment strategies for subtypes of early gastroesophageal cancer. Eur J Cancer 48(16):2941–2953. https://doi.org/10.1016/j.ejca.2012.07.029
Ajani JA, D’Amico TA, Almhanna K, et al (2015) Esophageal and esophagogastric junction cancers, version 1.2015. J Natl Compr Cancer Netw 13(2):195–227
Siewert JR, Stein HJ (1998) Classification of adenocarcinoma of the oesophagogastric junction. Br J Surg 85(11):1457–1459. https://doi.org/10.1046/j.1365-2168.1998.00940.x
Brierley JD, Gospodarowicz MKWC (2017) TNM classification of malignant tumours, 8th edn. Wiley-Blackwell, Oxfrod
In H, Solsky I, Palis B, Langdon-Embry M, Ajani J, Sano T (2009) Validation of the 8th edition of the AJCC TNM staging system for gastric cancer using the National Cancer Database. Ann Surg Oncol. https://doi.org/10.1245/s10434-017-6078-x
Kim SG, Seo HS, Lee HH, Song KY, Park CH (2017) Comparison of the differences in survival rates between the 7th and 8th editions of the AJCC TNM staging system for gastric adenocarcinoma: a single-institution study of 5,507 patients in korea. J Gastric Cancer 17(3):212–219. https://doi.org/10.5230/jgc.2017.17.e23
Lu J, Zheng C, Cao L et al (2017) The effectiveness of the 8th American Joint Committee on Cancer TNM classification in the prognosis evaluation of gastric cancer patients: A comparative study between the 7th and 8th editions. Eur J Surg Oncol. https://doi.org/10.1016/j.ejso.2017.09.001
Di Leo A, Zanoni A (2017) Siewert III adenocarcinoma: treatment update. Updates Surg 69(3):319–325. https://doi.org/10.1007/s13304-017-0429-9
Rice TW, Rusch VW, Apperson-Hansen C et al (2009) Worldwide esophageal cancer collaboration. Dis Esophagus 22(1):1–8. https://doi.org/10.1111/j.1442-2050.2008.00901.x
Sano T, Coit DG, Kim HH et al (2017) Proposal of a new stage grouping of gastric cancer for TNM classification: international Gastric Cancer Association staging project. Gastric Cancer 20(2):217–225. https://doi.org/10.1007/s10120-016-0601-9
Marrelli D, Morgagni P, de Manzoni G et al (2012) Prognostic value of the 7th AJCC/UICC TNM classification of noncardia gastric cancer. Ann Surg 255(3):486–491. https://doi.org/10.1097/SLA.0b013e3182389b1a
McGhan LJ, Pockaj BA, Gray RJ, Bagaria SP, Wasif N (2012) Validation of the updated 7th edition AJCC TNM staging criteria for gastric adenocarcinoma. J Gastrointest Surg 16(1):53–61. https://doi.org/10.1007/s11605-011-1707-3
Barbour AP, Rizk NP, Gonen M et al (2007) Lymphadenectomy for adenocarcinoma of the gastroesophageal junction (GEJ): impact of adequate staging on outcome. Ann Surg Oncol 14(2):306–316. https://doi.org/10.1245/s10434-006-9166-x
Zhang X, Watson DIJG (2007) Lymph node metastases of adenocarcinoma of the esophagus and esophagogastric junction. Chin Med J 120(24):2268–2270
Rizk NP, Ishwaran H, Rice TW et al (2010) Optimum lymphadenectomy for esophageal cancer. Ann Surg 251(1):46–50. https://doi.org/10.1097/SLA.0b013e3181b2f6ee
Altorki NK, Zhou XK, Stiles B et al (2008) Total number of resected lymph nodes predicts survival in esophageal cancer. Ann Surg 248(2):221–226. https://doi.org/10.1097/SLA.0b013e31817bbe59
Peyre CG, Hagen JA, DeMeester SR et al (2008) The number of lymph nodes removed predicts survival in esophageal cancer: an international study on the impact of extent of surgical resection. Ann Surg 248(4):549–556. https://doi.org/10.1097/sla.0b013e318188c474
Shen Z, Ye Y, Xie Q, Liang B, Jiang K, Wang S (2015) Effect of the number of lymph nodes harvested on the long-term survival of gastric cancer patients according to tumor stage and location: a 12-year study of 1,637 cases. Am J Surg 210(3):431–440. https://doi.org/10.1016/j.amjsurg.2015.01.029
Lu J, Wang W, Zheng C et al (2017) Influence of total lymph node count on staging and survival after gastrectomy for gastric cancer: an analysis from a two-institution database in China. Ann Surg Oncol 24(2):486–493. https://doi.org/10.1245/s10434-016-5494-7
Ji X, Bu Z-D, Li Z-Y et al (2017) Prognostic significance of the total number of harvested lymph nodes for lymph node-negative gastric cancer patients. BMC Cancer. 17(1):558. https://doi.org/10.1186/s12885-017-3544-6
He H, Shen Z, Wang X, Qin J, Sun Y, Qin X (2016) Survival benefit of greater number of lymph nodes dissection for advanced node-negative gastric cancer patients following radical gastrectomy. Jpn J Clin Oncol 46(1):63–70. https://doi.org/10.1093/jjco/hyv159
Baiocchi GL, Tiberio GA, Minicozzi AM et al (2010) A multicentric Western analysis of prognostic factors in advanced, node-negative gastric cancer patients. Ann Surg 252(1):70–73. https://doi.org/10.1097/sla.0b013e3181e4585e
Acknowledgements
We thank Giovanni Vittimberga (General Surgery, Morgagni-Pierantoni Hospital, Forlì, Italy), Fausto Rosa (Division of Digestive Surgery, Department of Surgical Sciences, Catholic University, Rome, Italy), Giovanni Sgroi (Department of General Surgery, Treviglio Hospital, ASST of Bergamo, Bergamo, Italy) and Francesco Ricci (Unit of General Surgery, Rovereto Hospital (APSS of Trento), Italy) for their precious participation in this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Human participants and/or animals statement
The research does not involve human participants and/or animals.
Informed consent
There was no need to get informed consent.
Additional information
The article is part of topical collection on Gastric Cancer Surgery.
Rights and permissions
About this article
Cite this article
Zanoni, A., Verlato, G., Baiocchi, G.L. et al. Siewert III esophagogastric junction adenocarcinoma: does TNM 8th save us?. Updates Surg 70, 241–249 (2018). https://doi.org/10.1007/s13304-018-0537-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-018-0537-1