Abstract
Introduction
The recently published 7th edition of the American Joint Committee on Cancer (AJCC) TNM staging criteria for gastric adenocarcinoma contains important revisions to T and N classifications, as well as overall stage grouping. Our goal was to validate the new staging system using a cancer registry.
Methods
Retrospective review of gastric cancer patients from Surveillance, Epidemiology, and End Results (SEER) registry data (2004–2007). Patients were staged according to both 6th and 7th edition criteria, and 3-year disease-specific survival was compared.
Results
Thirteen thousand five hundred forty-seven patients with gastric adenocarcinoma were identified with complete staging information. When using 7th edition criteria, there was an increase in the number of patients classified as stage III (23% vs. 13%), and a decrease in patients classified as stage IV (47% vs. 53%). Statistically significant differences in 3-year disease-specific survival were observed for all T and N categories and re-staging the same population according to the 7th edition criteria improved survival discrimination. Multivariate analysis revealed statistically significant differences in survival and linear progression of hazard ratios for each stage grouping.
Conclusions
The 7th edition AJCC staging criteria for gastric adenocarcinoma demonstrate better survival discrimination and risk stratification than previous criteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer is the fourth most common malignant tumor, with an estimated one million cases diagnosed worldwide each year1 and remains the second leading cause of cancer deaths.2 Surgical resection with regional lymphadenectomy is the cornerstone of potentially curative multi-modality therapy and also allows for precise staging. Accurate staging is important for directing therapeutic decisions as well as prognostication. The 6th edition American Joint Committee on Cancer (AJCC) TNM staging criteria for gastric adenocarcinoma2 has been used extensively in the USA and worldwide since 2002. Numerous modifications have since been proposed, and the recently updated 7th edition (2010) contains important revisions. These include a re-classification of tumor depth (T) and lymph node number (N) groupings. Five new T categories have been defined, which more closely resemble T categories used for staging other gastrointestinal cancers; T1, T2, T3, T4a, and T4b. In addition, the N categories have also been redefined to include five groups, N0, N1, N2, N3a, and N3b. The major revisions reflect differences in 5-year cumulative survival rates according to 7th edition T and N categories and the corresponding 6th edition categories from USA, Korean, and Japanese databases.3
As well as revisions to both T and N classification, the updated staging criteria also include a re-classification of overall stage assignment; in particular, the 7th edition criteria mark the introduction of new stages IIA, IIB, and IIIC as well as re-classification of stage IV as patients having non-curable disease with distant metastases only.
Few studies have attempted to validate the new staging criteria, and until now have comprised only retrospective single-center Asian population studies.3–6 So far, these studies appear to support the revisions reflected in the latest AJCC staging criteria. However, the pattern of presentation and pathophysiology in Western populations is different from that of Asian populations. The incidence of gastric cancer is higher among Asian populations with a greater proportion of early gastric cancer, primarily because of national screening programs.7 In the USA, there is an increasing incidence of diffuse subtype vs. intestinal subtype and a shift towards more proximal lesions at the time of diagnosis. In this study, we aim to validate the new AJCC TNM staging criteria for gastric adenocarcinoma using a national population registry and compare it with the previous 6th edition staging criteria in terms of survival rates and survival discrimination. To our knowledge, this is the largest sample size studied and the only national multi-center study conducted in a Western population.
Materials and Methods
SEER Database
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) tumor registry was used for this study. SEER contains over three million cases from 17 geographic sites, covering approximately 26% of the US population. The database is designed to reflect the overall characteristics of the US population and is regarded as a model population-based cancer database. Quality control is an important component of the SEER program; the current standard for accuracy of data is an error rate of less than 5%.8 Registries that are part of SEER routinely collect data on patient demographics (e.g., age and sex), primary tumor characteristics (e.g., size, grade, and location), primary operation performed (partial vs. total gastrectomy), lymph node staging (number of lymph nodes removed, number of positive lymph nodes), vital status, and survival. Although information on radiation therapy is recorded, no information on hormone or chemotherapy is reported. The 2009 update was used for this study, providing information from 1973 to 2007.9 As a population-based study with no patient identifiers involved, our study was exempt from Institutional Research Board review.
Patient Selection
A retrospective review of all gastric cancer patients from SEER registry data from 2004 to 2007 was conducted. Eligibility criteria included patients entered into the SEER database with gastric adenocarcinoma confirmed by histopathology. Patients were excluded from survival analyses if they had incomplete or missing data regarding staging or survival in order to perform a valid analysis. We collected data for patient demographics, tumor characteristics, surgical characteristics, the utilization of adjuvant therapy, TNM stage, and 3-year disease-specific survival (DSS). The same patient dataset was used to stage patients according to both 6th and 7th edition criteria using information available for depth of invasion, number of positive nodes and distant metastases (Tables 1 and 2).
Statistical Analyses
Continuous variables were analyzed using independent sample t-tests and categorical variables using chi-squared analyses. Survival analysis was performed using the Kaplan–Meier method and the log-rank test was used to determine significance; multivariate analysis for survival was performed using the Cox proportional hazards model. We compared 3-year DSS from the time of diagnosis. All tests were two-tailed; significance levels were set at p < 0.05, and confidence intervals at 95%. Statistical analysis was performed using SPSS 16.0 statistical software (SPSS Inc., Chicago IL).
Results
A total of 19,167 patients with gastric adenocarcinoma were identified. After exclusions, 13,547 patients constituted the study population; of these, 8,193 patients (60%) underwent a cancer-directed operation (i.e., partial/total gastrectomy and excluding local excision), and 3,486 (26%) received radiation therapy. The median patient age was 68 years and 8,497 patients (63%) were male. Clinicopathologic characteristics are outlined in Table 3.
Effect of T and N Re-classification and Stage Designation
Tables 1 and 2 illustrate the effects of both T (tumor depth) and N (number of involved lymph node) re-classification on stage migration, as well as changes in allocation of overall stage grouping. Revision of T categories include the division of T1 tumors (mucosal) into T1a (tumors invading the lamina propria or muscularis mucosa), and T1b (tumors invading the submucosa); this sub-classification, however, did not feature in the overall stage groupings (which are all simply designated T1). A striking difference regarding the new T categories is the distinction between tumors invading the muscularis propria and subserosa, previously T2a and T2b, respectively; T2 now denotes patients with muscularis propria invasion of their tumor only, and T3 denotes those with subserosal invasion. Consequently, changes in T-classification resulted in 3,136 fewer T2 tumors; the number of T3 lesions increased to 3,136, 973 more than previously characterized by 6th edition criteria. As T4 lesions now include tumors that both penetrate the serosa (T4a) and invade surrounding tissues (T4b; replacing the previous T3 and T4 groups, respectively), the number of T4 lesions increased significantly from 2,042 to 4,205 (Table 4). There was hence an overall shift towards upstaging tumor depth.
Regarding N categories, differences include a subdivision of previously categorized N1 nodal count (one to six nodes) into a modified N1 (one to two nodes), and N2 (three to six nodes); this resulted in 1,503 fewer N1 patients. The other major revision to the N categories involved the combination of previous N2 and N3 groups into N3 (with N3a and N3b divisions, respectively); this resulted in 1,447 more N3 patients, and an overall minor increase in N2 patients (56) (Table 5). Again, there was a clear shift toward higher N grouping.
Changes in overall stage grouping are due to the re-designation of stage groupings and to stage migration as a result of changes to T and N classification. There was a slight shift in the proportion of patients classified as stage II (13% to 14%) and a marked increase in the number of patients classified as stage III when using the 7th edition criteria (13% to 23%). There was also a decrease in the number of patients classified as stage IV (53% to 47%), which now includes only patients with distant metastases (Table 6).
Survival Discrimination by T Stage
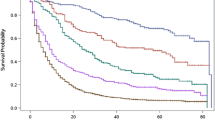
Three-year DSS based on both 6th and 7th T classification was calculated. Median survival (months) for T stage I–IV as determined by 6th edition TNM criteria was 32.1, 23.6, 15.7 and 8.7, respectively. The corresponding median survivals according to 7th edition staging were 33.0, 35.7, 21.7, and 10.8, respectively. There was a significant difference in survival between each T-classification according to both the 6th and 7th edition criteria (p < 0.001). Figure 1a shows Kaplan–Meier survival curves for the patient cohort based purely on T classification for each edition. Cumulative survival for T2 patients (after excluding tumors invading the subserosa) has improved (green line), such that it is comparable to survival for T1 tumors.
Survival Discrimination by N Stage
There was a significant difference in 3-year DSS survival between each N classification according to 7th edition criteria (p < 0.001). Median survival (months) for N stage (0–3) as determined by 6th edition TNM criteria was 36.0, 22.3, 15.7, and 11.7, respectively. The corresponding median survivals according to 7th edition staging were 36.0, 24.6, 20.6, and 14.4, respectively. Figure 1b shows Kaplan–Meier survival curves for the patient cohort based purely on N classification according to each criteria edition. Survival for both N1 (one to two nodes only) and N2 groups (three to six nodes, replacing seven to 15 nodes as in the 6th edition criteria) has improved.
Survival by Stage Grouping
The overall 3-year DSS was 41.7%. Re-staging the same patient population according to the 7th edition criteria improved survival discrimination from the 6th edition staging (Fig. 1c). Hazard ratios by stage (compared with stage IA) showed linear progression and a statistically significant survival difference from the prior stage (p < 0.001) (Fig. 2). Other independent variables predicting worse survival on Cox regression multivariate analysis included high tumor grade (HR, 1.17; CI, 1.10–1.26; p < 0.001), distal location of primary tumor vs. proximal location (HR, 1.78; CI, 1.64–1.93; p < 0.001), and no adjuvant radiation vs. radiation (HR, 1.85; CI, 1.72–1.99; p < 0.001).
Discussion
Accurate cancer staging provides the means for appropriate treatment selection and defining prognosis. It also serves as the standard for reporting cancer incidence and outcomes. The TNM staging criteria for gastric adenocarcinoma have seen numerous revisions, the most recent of which are reflected in the 7th edition AJCC TNM cancer staging manual.10 Each update reflects changes based on accumulated clinical experience with the disease and is meant to improve prognostication. In this study we use the SEER database, a national US-based cancer registry, to show improved survival discrimination from the 6th to the 7th stage for gastric adenocarcinoma.
Previous 6th edition T categories T2a and T2b (denoting muscularis propria and subserosal invasion, respectively) have been reclassified into T2 (muscularis propria) and T3 (subserosal), respectively; this change reflects a significantly longer disease-specific 5-year survival rate with tumors invading the muscularis propria compared with those invading the subserosa.11–13 Upstaging from T2 to T3 places each tumor in a higher stage grouping for all stages. The previously categorized T3 category (serosal invasion) has now been changed to T4a, with the classification of adjacent structure involvement changed from T4 to T4b. No longer do T4b tumors denote stage IV disease by default; M1 disease is now characterized only by the presence of distant metastases. This is important as en bloc surgical resection for T4 tumors is considered a viable surgical strategy for potentially curative therapy. We show a trend towards upstaging with respect to tumor depth; we were also able to show statistically significant survival discrimination between all T categories, including between newly categorized T2 and T3 groups. This is consistent with reports that show that prognosis is worse for tumors invading the subserosa compared with tumors invading the muscularis propria. We demonstrated improved survival for T2 tumors, similar to that of T1 tumors; this may require incorporation of T1 tumors with best prognosis (i.e., T1a tumors invading the lamina propria vs. submucosa) into overall stage grouping, to further reflect improved survival with T1a tumors vs. T1b tumors.
There is some debate about how the extent of nodal metastases should best be characterized for inclusion into staging systems. The fifth edition of the TNM staging criteria (1997) was the first to introduce a numerical staging system for nodal staging, based upon number of positive regional lymph nodes containing metastatic disease independent of anatomic location. Since this time, there have been several reports validating the use of a numerical system.14–17 The other major approach proposed is the metastatic lymph node ratio, which involves taking the ratio of metastatic to examined lymph nodes to determine the N classification. The lymph node ratio has been shown by some to be a more precise and effective indicator of metastatic disease than the current numerical grouping.18 The most recent modifications adopted into the 7th edition nodal grouping criteria include the subdivision of the previous group N1 (one to six involved regional nodes) into N1 (one to two nodes) and N2 (three to six nodes); N3a now denotes the presence of seven to 15 involved LNs (replacing N2), and N3b denotes ≥16 involved LN (replacing N3). The current N3 category has therefore been expanded to include the involvement of ≥7 nodes. In a recent retrospective study of 295 patients undergoing curative gastrectomy with D2 lymphadenectomy, the 7th edition TNM N classification was found on multivariate analysis to be an independent prognostic factor rather than the 6th edition N classification.4 The value of new designations N3a and N3b in not yet clear, as previous validation studies have failed to show that survival is equal for both groups. In our study we also show that survival for N2/N3a and N3/N3b patients was significantly different (p < 0.001).
Although the distinction between N3a and N3b is clear, the subgroups are not incorporated into final stage grouping. Rather, they are grouped together as N3. It appears that >15LNs (N3b) indicates worse prognosis within this group, and suggests that revisions may be required to further re-define nodal groupings (e.g., the addition of a possible N4 category) to more accurately reflect prognosis. In addition, we demonstrated a large survival difference between N0 and N1 groups; as N1 denotes one to two involved nodes, this may suggest that prognosis is different for patients with a single metastatic node compared with two nodes. It also suggests that there may be subsets of N0 patients with poor prognostic features not captured by current staging criteria. Current recommendations are for removal of ≥15 lymph nodes for accurate nodal staging. Although in this study the proportion of patients having ≥15 lymph nodes removed was only 26% (3,465/13,547), survival for each stage according to 7th edition criteria showed similar improved discrimination to that obtained when studying all patients regardless of extent of lymphadenectomy (statistically significant, data not shown). This was also true when a stage-specific survival analysis was done between patients with <15 and ≥15 lymph nodes removed.
In our study of the SEER population, an important change is the revision of advanced locoregional cancer, with downstaging from stage IV to stage III, despite overall trends towards upstaging for both T and N groupings. Stage IV patients include those with distant metastases only and hence incurable disease (i.e., “true” stage IV), similar to staging criteria of other gastrointestinal carcinomas. More patients are now defined as potentially operable, e.g., T4b tumors invading adjacent structures. Similarly, N3 patients are also down-staged from stage IV. With recent studies suggesting an improvement in survival following peri-operative chemotherapy19 (and the potential for better systemic therapy in the pipeline) prior nihilism for this group of patients can no longer be justified, and is appropriately reflected by the new staging criteria. We also show superior survival discrimination when patients were staged according to the 7th edition criteria. Furthermore, a linear progression in hazard ratios is seen with advancing stage and a relatively homogenous distribution of risk within each stage, as reflected by the 95% confidence intervals.
The few published validation studies also conclude that the 7th edition TNM criteria provides a more detailed classification of prognosis and improved survival discrimination.3,5,6 However, validation studies to date have involved single-center retrospective reviews of Asian gastric cancer patients. In the USA, as in other Western countries, there is a trend towards increasing incidence of proximal lesions (despite an overall decreasing incidence of gastric cancer).10,20 In our study most patients were diagnosed with proximal lesions (31%) compared with body (22%) and distal lesions (25%). Furthermore, we identified distal location of tumor as an independent significant predictor of poor survival, which appears inconsistent with other reported data and nomograms that incorporate proximal location as a poor prognostic indicator.20–22 In addition, high tumor grade was also identified as a significant independent predictor of poor survival. Anatomic location does not feature in the current staging system for gastric cancer as it does with esophageal cancer,10 neither does grade or tumor histology; separate stage groupings have been introduced for squamous cell carcinoma and adenocarcinoma in the 7th edition staging criteria for esophageal carcinoma. With this in mind, additional features including tumor grade, location, and histology may need to be incorporated into future TNM staging criteria.
Another problem that exists is the distinction between tumors in close proximity to either the gastric cardia or esophagogastric (EG) junction, where the anatomic distinction between gastric and esophageal carcinoma is more ambiguous. It is important to make such a distinction accurately and consistently, as the staging criteria for each are quite different. In order to clarify this, the 7th edition TNM criteria have stated that tumors arising in the EG junction or those ≤5 cm from the EG junction that cross the EG junction should be classified as esophageal carcinomas. As well as providing more consistency, another goal of the updated criteria was to harmonize T groupings for gastric cancers with those assigned to esophageal and small/large bowel cancers.23 With esophageal carcinoma, for example, T2 denotes tumors invading the muscularis propria only, with T3 lesions invading the adventitia. The SEER registry currently does not provide enough data to be able to make this distinction. Other limitations include the retrospective nature of the data, the possibility of coding errors and a lack of granularity in a population-based dataset.
Conclusions
In conclusion, the AJCC 7th edition staging criteria demonstrate better survival discrimination than the 6th edition criteria. A smaller proportion of patients were classified as stage IV using the updated criteria, with 13% of stage IV patients down-staged to IIIC. The revisions reflect better prognostic accuracy and allow for more appropriate selection of therapeutic options, of particular importance in the modern era of multi-modality therapy. Our study using SEER registry data validates the recent revisions made in the 7th edition AJCC staging criteria for gastric adenocarcinoma. Further validation studies are required to more accurately evaluate current nodal groupings and how they impact prognosis.
References
Garcia M, Jemal A, Ward EM, et al. Global cancer facts and figures 2007. Atlanta: American Cancer Society, 2007.
Greene, FL, Page, DL, Fleming, ID, et al (Eds.). AJCC Cancer staging manual, 6th edition. New York: Springer, 2002.
Ahn HS, Lee HJ, Hahn S, Kim WH, Lee KU, Sano T, Edge SB, Yang HK. “Evaluation of the 7th American Joint Committee on Cancer/International Union Against Cancer Classification of gastric adenocarcinoma in comparison with the 6th classification.” Cancer 116, no. 24 (2010): 5592–8.
Chae S, Lee A, Lee JH. “The effectiveness of the new (7th) UICC N classification in the prognosis evaluation of gastric cancer patients: a comparative study between the 5th/6th and 7th UICC N classification.” Gastric Cancer, 2011: (in press).
Jung H, Lee HH, Song KY, Jeon HM, Park CH. “Validation of the 7th edition of the American joint committee on cancer TNM staging system for gastric cancer.” Cancer, 2011: (in press).
Wang W, Sun XW, Li CF, Lv L, Li YF, Chen YB, Xu DZ, Kesari R, Huang CY, Li W, Zhan YQ, Zhou ZW. “Comparison of the 6th and 7th Editions of the UICC TNM Staging System for Gastric Cancer: Results of a Chinese Single-Institution Study of 1,503 Patients.” Ann Surg Oncol, 2011: (in press).
Leung WK, Wu MS, Kakugawa Y, Kim JJ, Yeoh KG, Goh KL, Wu KC, Wu DC, Sollano J, Kachintorn U, Gotoda T, Lin JT, You WC, Ng EK, Sung JJ. “Screening for gastric cancer in Asia: current evidence and practice.” Lancet Oncol 9, no. 3 (2008): 279–87.
Havener, L. “Standards for cancer registries volume III: standards for completeness, quality, analysis, and management of data. North American Association of Central Cancer Registries.” 2004.
National Cancer Institute. http://seer.cancer.gov/index.html . (accessed October 2010).
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A (Eds.). AJCC cancer staging manual, 7th edition. New York: Springer, 2010.
Lu Y, Liu C, Zhang R, Li H, Lu P, Jin F, Xu H, Wang S, Chen J. “Prognostic significance of subclassification of pT2 gastric cancer: a retrospective study of 847 patients.” Surg Oncol 17, no. 4 (2008): 317–22.
Nitti D, Marchet A, Mocellin S, Rossi GM, Ambrosi A, Mencarelli R. “Prognostic value of subclassification of T2 tumours in patients with gastric cancer.” Br J Surg 96, no. 4 (2009): 398–404.
Park Do J, Kong SH, Lee HJ, Kim WH, Yang HK, Lee KU, Choe KJ. “Subclassification of pT2 gastric adenocarcinoma according to depth of invasion (pT2a vs pT2b) and lymph node status (pN).” Surgery 141, no. 6 (2007): 757–63.
Aurello P, D’Angelo F, Rossi S, Bellagamba R, Cicchini C, Nigri G, Ercolani G, De Angelis R, Ramacciato G. “Classification of lymph node metastases from gastric cancer: comparison between N-site and N-number systems. Our experience and review of the literature.” Am Surg 73, no. 4 (2007): 359–66.
Ichikura T, Tomimatsu S, Uefuji K, Kimura M, Uchida T, Morita D, Mochizuki H. “Evaluation of the New American Joint Committee on Cancer/International Union against cancer classification of lymph node metastasis from gastric carcinoma in comparison with the Japanese classification.” Cancer 86, no. 4 (1999): 553–8.
Kodera Y, Yamamura Y, Shimizu Y, Torii A, Hirai T, Yasui K, Morimoto T, Kato T, Kito T. “Lymph node status assessment for gastric carcinoma: is the number of metastatic lymph nodes really practical as a parameter for N categories in the TNM classification? Tumor Node Metastasis.” J Surg Oncol 69, no. 1 (1998): 15–20.
Roder JD, Böttcher K, Busch R, Wittekind C, Hermanek P, Siewert JR. “Classification of regional lymph node metastasis from gastric carcinoma. German Gastric Cancer Study Group.” Cancer 82, no. 4 (1998): 621–31.
Asoglu O, Karanlik H, Parlak M, Kecer M, Muslumanoglu M, Igci A, Ozmen V, Gulluoglu M, Kapran Y. “Metastatic lymph node ratio is an independent prognostic factor in gastric cancer.” Hepatogastroenterology 56, no. 91–92 (2009): 908–13.
Chua YJ, Cunningham D. “The UK NCRI MAGIC trial of perioperative chemotherapy in resectable gastric cancer: implications for clinical practice.” Ann Surg Oncol 14, no. 10 (2007): 2687–90.
Strong VE, Song KY, Park CH, Jacks LM, Gonen M, Shah M, Coit DG, Brennan MF. “Comparison of gastric cancer survival following R0 resection in the United States and Korea using an internationally validated nomogram.” Ann Surg 251, no. 4 (2010): 640–6.
Hundahl SA, Phillips JL, Menck HR. “The National Cancer Data Base Report on poor survival of U.S. gastric carcinoma patients treated with gastrectomy: Fifth Edition American Joint Committee on Cancer staging, proximal disease, and the ”different disease“ hypothesis.” Cancer 88, no. 4 (2000): 921–32.
Kattan MW, Karpeh MS, Mazumdar M, Brennan MF. “Postoperative nomogram for disease-specific survival after an R0 resection for gastric carcinoma.” J Clin Oncol 21, no. 19 (2003): 3647–50.
Washington, K. “7th edition of the AJCC cancer staging manual: stomach.” Ann Surg Oncol 17, no. 12 (2010): 3077–9.
Acknowledgments
None.
Conflicts of interest
The authors declare that they have no conflicts of interest
Author information
Authors and Affiliations
Corresponding author
Additional information
Discussant
Dr. Han-Kwang Yang (Seoul, Korea): I am glad to hear that your conclusion is favorable with the new 7th staging system, in which our institute’s survival data were used as one of the principal reference data. You used 3DFS in this analysis. GASTRIC group presented that 3DFS can replace 5ys in gastric cancer at ASCO meeting 2009. Now, Japanese Gastric Cancer Association adopted this 7th edition, too.
But there are a few problems on the 7th edition as you mentioned.
1. Esophago Gastric junction tumor invading esophagus was classified to Esophageal cancer, which is not appropriate according to our analysis.
2. N3a vs. N3b should be further investigated.
For the next revision of TNM staging system, International Gastric Cancer Association just launched a TFT which will include database from all over the world.
When I look at the general characteristics table of your SEER database, I have a few questions on the general practice of surgery for gastric cancer in the USA.
Question#1. Average or median number LN examined per patient for each stage?
Question#2. In the type of surgery, what does it mean “local excision” (39%) in the era of 2004–2007?
I am concerned on this description of surgery. If I assume proximal cancers are more operated in the specialized centers, this might be related to the independently significantly poor prognosis of distal location compared with proximal location. We know that radiation can not compensate inadequate surgery. This could explain why the survival for each stage of SEER database is worse than those from Asia.
Question #3. Who dissects the LN of the specimen? If surgeon does specimen dissection, it will improve the pathologic evaluation of LN status.
Closing Discussant
Drs. Lee J. McGhan & Nabil Wasif: Thank you Dr. Yang for reviewing our manuscript, and for your insightful comments and questions. I would like to first address the point you raised about classification of proximal gastric tumors within 5 cm of the GE junction involving the GE junction as esophageal cancers in the AJCC classification. We are limited in our analysis of this dataset by being unable to classify tumors by location beyond being “proximal” in location. It is also possible that many of these tumors are treated as esophageal cancers and underwent neo-adjuvant chemoradiation with subsequent downstaging which may have resulted in the improved survival seen in our analysis.
In answer to your first question, the median lymph node counts for stage I–III patients were 10, 11, and 15, respectively. Although most patients in our series did not have >15 lymph nodes excised, we re-analyzed our data to look at patients who did have >15 lymph nodes examined and noticed the same improvement in survival discrimination with the 7th AJCC classification as compared with the 6th.
Secondly, the term “local excision” as used in our study encompasses all techniques used to remove the tumor that do not involve surgical resection. This includes techniques such as polypectomy and endoscopic mucosal resection or EMR.
Finally, lymph nodes are typically found in the specimen by the pathology assistant and then processed and examined by the pathologist, as is the case at our institution. The surgeons are usually not involved. Studies on the subject have shown an improvement in lymph node counts when a standardized processing protocol is applied in the pathology laboratory and we aim to achieve this at our institution; this may need to applied at the national level. The involvement of surgeons in labeling lymph node stations may indeed also be useful.
Rights and permissions
About this article
Cite this article
McGhan, L.J., Pockaj, B.A., Gray, R.J. et al. Validation of the Updated 7th Edition AJCC TNM Staging Criteria for Gastric Adenocarcinoma. J Gastrointest Surg 16, 53–61 (2012). https://doi.org/10.1007/s11605-011-1707-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11605-011-1707-3