Abstract
Surgical site events, including surgical site infections (SSI), represent a major problem in general surgery. SSI are responsible of nuisance for patients, and can lead to important complications and disability, often needing prolonged postoperative stay with specific treatment and recovery in Intensive Care Units. These justify the higher costs due to SSI. Despite the growing body of evidence concerning SSI in general surgery, literature dealing with SSI after colorectal surgery is scarce, reflecting in suboptimal perception of such a relevant issue by colorectal surgeons and health authorities in Italy, though colorectal surgery is associated with higher rates of SSI. The best strategy for reducing the impact of SSI on costs of care and patients quality of life would be the development of a preventive bundle, similar to that adopted in the US through the colorectal section of the National Surgery Quality Improvement Project of the American College of Surgeons (ACS-NSQIP). This policy has been showed to significantly reduce the rates of SSI. In this scenario, incisional negative pressure wound therapy (NPWT) is likely to play a pivotal role. We herein reviewed the literature to report on the current status of preventive NPWT on surgical wounds of patients undergoing colorectal procedures with primary wound closure, suggesting evidence-based measures to reduce the impact of SSI, and to contain the costs associated with conventional NPWT devices by means of newer available technologies. Some explicative real life cases are presented.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Surgical site infections in colorectal surgery
Surgical site infections (SSI) are relatively common postoperative complications, reaching 38 % in surgical unit [1]. SSI are responsible of significant complications and disabilities [1–4], potentially life-threatening [5]. They also increase the costs of care and need for repeated hospitalizations or referral to Intensive Care Units [4–9]. It has been estimated that SSI are responsible of over 1.5 billion dollar excess costs per year in the US [10]. Surgical site complications affect the quality of wound healing and the perceived health-related quality of life (HRQoL), which is a major concern in general surgery, especially in young patients [11], in whom SSI represent the commonest hospital-acquired complication. The highest incidence is observed in patients undergoing small bowel and colon surgery [1–4].
SSI are the commonest complication after colorectal surgery [12], affecting up to 16.3 and 12.5 % of patients after small bowel or colon surgery, respectively [1–4]. Besides being responsible for prolonged stay in the hospital, with the need of further treatments and higher costs, SSI can delay potential adjuvant treatments in oncologic patients [12, 13].
The rates of wound complications and SSI are influenced by complex interactions between patient- and procedure-related factors, which can be partially modified by preventive strategies. Patient-related factors are represented by prolonged or high-dose intake of steroid drugs, smoking habit, diabetes, high body mass index (BMI), stomas at the time of surgery, concomitant conditions (e.g. immunosuppression) or predisposing diseases [e.g. inflammatory bowel diseases (IBD)] [8, 9, 14–16]. A report from the Cleveland clinic suggested that age >55 years and thrombocytopenia are additional risk factors to be considered [13]. Procedure-related factors consist of prolonged length of surgery, intra-operative hypotension, bacterial contamination (especially from colon and rectum), pre-existent infections or contaminations (e.g. penetrating traumas) [16–18]. In detail, the National Nosocomial Infections Surveillance (NNIS) Risk Index, developed by the Centre for Disease Control and Prevention (CDC), quantifies in 3 h the threshold for increased risk of SSI in colorectal surgery [18]. Minimally invasive surgery has been associated with a reduced risk of SSI [19].
Aiming to prevent SSI, modifiable factors should be corrected (e.g. smoking discontinuation, weight loss), and preventive strategies applied when possible. Evidence level I prophylactic approaches in colorectal surgery consist of timely and appropriate antibiotic prophylaxis, which must be suspended within 24 h from surgery [20], correct hair removal from the surgical site, maintenance of normothermia during the entire procedure. The CDC developed guidelines to prevent SSI, focussing on specific surgical specialties [21]. Antibiotics should be administered within 1 h before the procedure (2 h for vancomycin and fluoroquinolones) [20], and it is important to choose the most appropriate agents in relation to the procedure performed. Potential pre-operative infections must be suspected, diagnosed, and treated. Adequate antiseptic skin agents must be used [21]. Enemas and oral cathartics are to be administered for the mechanical colon preparation, and oral non-absorbed antimicrobials should be given in two different administrations the day before surgery [21]. During surgery, the OR traffic should be kept at the minimum [21], and wounds should be covered with sterile gauzes to be removed after 24/48 h [21].
Little is known concerning the impact on SSI of prophylactic negative pressure wound therapy (NPWT) on closed incisions in patients undergoing colorectal surgery. Aims of the review were to assess the current risk of SSI in colorectal surgery and their potential classifications, and to seek for the role of preventive NPWT.
Materials and methods
Aims
Primary
To assess the effects of NPWT on surgical wound healing by primary closure after colorectal surgery compared with conventional dressings.
Secondary
(1) To report on the impact of SSI in colorectal surgery, (2) to report on suggested classifications and risk-reduction strategies in colorectal surgery, and (3) to report on the rationale underlying the use of preventive NPWT in colorectal surgery.
Inclusion criteria
Prospective or retrospective cohort studies comparing the effects of prophylactic NPWT after colorectal surgery with conventional dressings were evaluated for inclusion. Studies evaluating NPWT for abdominal complications or in open abdomen were not included for primary aim purposes. Studies mentioning other-than-colorectal procedures were included only if colorectal patients were clearly identifiable. Studies were evaluated for potential replication of data. Studies were only included if adequate information were provided. Only studies published as full-text article were included.
To allow for easier comparisons and reliable data, studies dealing with patients who underwent surgery without bowel opening (e.g. plastic surgery procedures, gynaecologic surgery) were removed, as the different microbial contaminations would have biased the results.
Data search
Available data of all studies published between January 2000 and December 2014 were evaluated for inclusion. The literature searches were carried out on PubMed, Scopus, and the Cochrane Database of Systematic Reviews. Google search engine was searched. Keywords and medical subjects headings (MeSH) used are reported in Table 1. Limits: publication date between 2000 and 2014. Cross-referencing and related articles were reviewed. The reference lists of all articles were searched for further eligible studies. Experimental articles were excluded. Article published in English, French, Spanish, Dutch, or Italian were included.
Outcome measures
SSI and wound dehiscence were defined and referred according to CDC criteria [18].
Data extraction
Selected publications were read and all information gathered. Data of interest: years and location of studies; year of publication; inclusion/exclusion criteria; perioperative care and postoperative follow-up duration/pathway; number of patients; patient- and disease-related characteristics; concomitant medications; type of surgery; SSI, wound complications; and all perioperative complications.
Results and discussion
Characteristics of included studies are reported in Table 2. All studies were used for the secondary aim, along with specific reports without clinical data or comparisons, which were not eligible for the primary aim. The flow chart for systematic study selection is reported in Fig. 1.
After careful screening of data, five studies were eligible and were included for the primary aim (see “Clinical trials on preventive NPWT in Colorectal surgery” section).
SSI risk stratification in colorectal surgery
Patients undergoing colorectal surgery are at increased risk of SSI and wound complications compared with other surgical specialties [12], and this could be explained in part with differences in terms of risk factors for SSI between baseline diseases [13].
Colorectal surgeons [22] and National Institutes of Health [18, 21, 23–25] are gradually becoming aware of the need of developing and put to use strategies aimed at timely identification, treatment, and prevention of SSI.
In the US, the Joint Commission recently introduced the routine adoption of SSI preventing measures as necessary criteria for Health Institutes to obtain accreditation [26], and the Institute for Healthcare Improvement established a campaign to improve patient-reported outcomes in the eventuality of SSI [23]. In addition, pay-for-performance health systems based on SSI have been introduced. Starting from October 2008, the Centre for Medicare and Medicaid Services [27] suspended refunds for hospital-acquired infections deemed preventable (rule CMS-1488-P), among which SSI are enlisted. The National Surgery Quality Improvement Project of the American College of Surgeons (ACS-NSQIP) was the first attempt of correlating the risk of SSI and the surgical procedure, aimed at simplifying comparisons between different Hospitals, which was established in 1994 in some Hospitals of Northern America Veteran’s Administration [24]. In the included Centres, a 50 and 30 % drop in morbidity and mortality was observed [25]. Under the light of such results, after 10 years, the ACS-NSQIP has been extended to include all interested health centres, allowing for the publication of a model to predict the risk of SSI based on a multivariate logistic regression including general and vascular surgery procedures [13, 28]. By only evaluating centres highly-weighted on colorectal surgery, the latter increased per se the risk of SSI, with a predicted-observed ratio of 1.27 [13].
Wick et al. [13] reported on 1646 patients operated on at the Cleveland Clinic in US, of whom 17 % received colorectal procedures. The authors proposed several predictors of SSI: (1) age ≥55 years (OR 1.69, 95 % CI 1.01–2.81; p = 0.045), (2) BMI ≥30 kg/m2 (OR 2.04, 95 % CI 1.20–3.44; p = 0.008), (3) platelet count ≤150 × 103/μL (OR 2.87, 95 % CI 1.17–7.03; p = 0.021), and (4) surgical procedure lasting ≥180 min (OR 1.73, 95 % CI 1.04–2.87; p = 0.034). Out of 280 patients receiving colic or rectal resections, 40 (14.3 %) developed SSI, compared with 155/1366 (9.4 %) undergoing other procedures [13]. The relatively limited number of patients did not allow for developing a predictive score or algorithm.
Aiming at preventing SSI, the CDC proposed a score, the NNIS risk index [18], obtained by analysing data of SSI in several Centres. The score is easily computed, with only three variables, hence ranging between 0 and 3 points. These consist of surgery being classified as “contaminated” or “dirty”, preoperative American Society of Anaesthesiologists (ASA) score >3, and surgery lasting over the 75th standard percentile according to the procedure being performed (e.g. 3 h for colorectal surgery). The risk of SSI increases with higher scores [18]. However, the ease of computation of this score is overwhelmed by several limitations. The restricted number of total scores limits the discriminatory capacities, the weight of each variable may not be equal to the others, and it is not possible to stratify the risk according to specific procedures [29–31].
These observations lead van Walraven et al. [29] to propose an alternative score, the Site Infection Risk Score (SSIRS), using data obtained from the ACS-NSQIP. By evaluating 363.040 surgical procedures performed in 2010, the authors identified the following predictors of SSI:
-
Patient-related factors: smoking, high BMI, peripheral vasculopathies, metastatic cancers, steroid medications, preoperative infections.
-
Procedure-related factors: patients operated on in inpatient or emergent settings, contaminated or dirty surgical field, ASA score ≥3, general rather than local anaesthesia, more than one procedure performed, prolonged surgical time.
Discriminatory power of SSIRS was confirmed with a validation group (c-statistics = 0.800) [29]. The authors also suggested a web-page where the risk score can be computed (http://www.ohri.ca/SSI_risk_index/Default.aspx) [29]. However, such a score is difficult to be applied in everyday practice.
The system proposed by Hedrick et al. [3] in 2013 is of easier application and more focussed on colorectal surgery. This consists of a nomogram designed from Colorectal ACS-NSQIP data, including 18.403 patients undergoing colorectal procedures, in whom 1447 (7.9 %) superficial and 278 (1.5 %) deep SSI were observed. Factors associated with higher risk of SSI were (1) age >75 years, (2) smoking habit, (3) alcohol intake >2 drinks/die in the week before surgery, (4) dependent functional status before the procedure, (5) ASA score ≥3, (6) intra-operative transfusions, (7) surgery for enterocutaneous stoma reversal, (8) open surgery, (9) high BMI, (10) high preoperative haematocrit [3]. Each factor is assigned a score, the sum of which represents the risk of developing superficial or deep SSI. However, the study lacks a prospective validation of the findings.
Adherence to SSI preventive pathways in colorectal surgery: results in everyday practice
After the development of guidelines for the prevention and management of SSI by ACS-NSQIP, several centres successfully applied strategies to reduce wound-related complications.
Concerning colorectal surgery, a study from the Mayo Clinic in Rochester [2] published in 2013 reported on the results obtained by applying an algorithm to prevent SSI after Colorectal procedures. Cima et al. [2] compared the rates of SSI between 2009 and 2010, included in ACS-NSQIP program, with those of 2011, after the introduction of specific rules to prevent SSI. The authors found a reduction of overall SSI from 9.8 to 4 % (p < 0.05), and a reduction of both superficial (4.9 vs 1.5 %, p < 0.05) and organ/space SSI (4 vs 2.6 %, p = 0.10) [2].
Even if the authors are not able to identify a single factor to justify these good outcomes, the study offers some interesting perspectives. It should be considered that a consistent number of patients in the study were suffering from IBD, which are an independent predictor of SSI [8, 9]. The algorithm that the authors propose [2] has some features in common with the CDC NNIS concerning pre-, intra-, and post-operative management of colorectal surgery patients. Under the light of the recent studies it should be underscored that preventive NPWT is not considered, at least in a subgroup of patients at high risk of developing SSI.
The role of NPWT in the prevention of SSI and wound complications in colorectal surgery: rationale
Over the last decades NPWT has been used for several procedures. It has been effectively advocated and is now recommended in patients with open bone fractures [32], in donor sites of skin patches [33], burns [34], diabetic and pressure ulcers [35–37], post-traumatic wounds [38], split-thickness skin graft [39], sterna incisions [40], and open abdominal wounds [41]. More recently, NPWT has been recommended in obese patients after both clean [42] and clean-contaminated surgery [7]. Benefits are observed also in Crohn’s disease (CD) [8, 9] as well as in elderly patients [7].
The excellent outcomes obtained lead investigators to apply NPWT as a preventive measure against SSI and wound complications in patients undergoing surgery with primary wound closure [7–9, 43, 44]. However, despite the good results, this indication is not well-known.
NPWT consists of a closed, sealed system generating negative pressure in proximity of the wound surface. Depending on the wound characteristics, either sterile gauzes or other products (e.g. foams), covered by sterile sheets, may be indicated to apply NPWT. Intermittent or continuous negative pressure is obtained by connecting such medications to vacuum pumps with canisters to collect fluids. Negative pressure values commonly used range between −125 and −50 mmHg [45, 46].
Wound healing is achieved through three consecutive and overlapping phases: inflammation, new tissue formation/deposition, and remodelling [47]. The specific mechanism underlying the effectiveness of NPWT in facilitating healing still remains unknown. Recent studies suggest that this may be due to NPWT (1) generating a moist environment, removing the excess fluids, (2) reducing tissue oedema, (3) contracting wound edges, (4) causing a mechanical stimulus for the wound bed, (5) stimulating neo-angiogenesis and deposition of granulation tissue [48]. In addition, by keeping the wound “sealed”, it confers protection against exogenous agents and contamination due to repeated dressing changes, particularly useful in patients with enterocutaneous stomas [49].
In Colorectal surgery patients needing abdominal incisions, NPWT use needs to be implemented by a specific formation of the caregivers, concerning the benefits and potential risk related with this technology. Experimental models suggested that excessively negative pressure values could cause ischemic damages to the bowel loops directly exposed to NPWT in open abdomen [50, 51]. The blood flow in the wall of the bowel loops decreases with decreasing pressures applied [51]. These observations advocate the need of utilising correct negative pressure values and specific interfaces to preserve bowel loops, should NPWT be applied after wound dehiscence or in wound healing by secondary intention. Anyway, NPWT is widely used safely in digestive surgery, when appropriate rules are followed, even in wounds with exposed hollow viscera and in paediatric patients [52–55]. NPWT should be carefully considered in patients with bad health status or with coagulation disturbances predisposing to haemorrhage, as it may determine bleeding, or protein and fluid/electrolyte loss [54, 56].
The commonest disadvantages of conventional NPWT consist of the high costs of the therapy (considering the vacuum devices, the disposable canisters to collect fluids, and the need of charging batteries), the reduced transportability of cumbersome devices, and their difficult management. These shortcomings justify the interest raised by a new disposable, pocket device for NPWT (PICO, Smith & Nephew, UK) [7], useful for the prevention of SSI in wound healing by primary intention. The device is small and works with two AA batteries. It does not need a canister to collect fluids (canister-free), which are absorbed by specifically designed gauze. The exudates are removed through moisture absorption and transpiration. The fluids enter the airlock stratus of the gauze, and are then rapidly absorbed by the hydrophilic stratus. This results in the formation of a gel that keeps fluids away from the wound, while the film on the superior part of the gauze allows moisture to evaporate. The balance between produced and evaporated fluid is dynamically maintained, avoiding that the gauze becomes too thick and heavy while in place. PICO generates a continuous, pre-set negative pressure of -80 mmHg, the ideal value for abdominal incision healing by primary closure [52–54]. Once activated, batteries work for 7 days, and there is no need of dressing change—unless the gauze becomes too wet. Pocket devices are well accepted and easily managed by patients [8], making these suitable for home therapy. A stoma does not contraindicate NPWT, even with PICO, but application could be more difficult [55]. It is important to place gauzes and apply NPWT at the end of the procedure, immediately after closing the skin incision and before opening/maturating the stoma [9, 43].
Clinical trials on preventive NPWT in Colorectal surgery
The first prospective, pilot trial on preventive NPWT in Colorectal surgery was published in 2014 [9], and included patients suffering from CD, an independent predictor of SSI. It is a prospective, controlled, nonrandomised trial. Controls received standard sterile wound dressings. Between January 2012 and October 2013 30 patients with stricturing CD undergoing abdominal surgery were enrolled. Patients were included if during the procedure small and/or large bowel were opened or resected. Patients undergoing laparoscopy with no need of conversion, those who did not require opening bowel segments, and those who underwent massive bowel resection were excluded from the study. Patients fit for inclusion who were able to manage the device (PICO) and were willing to receive it, were assigned to NPWT group, and had the device placed at end of the procedure, after closure by primary intention (n = 13 patients). The control group (n = 17 patients) received conventional sterile dressings.
In all patients, perioperative management was carried out according to CDC guidelines, implemented with NPWT in PICO group [21]. Gauzes were temporarily removed on postoperative day three also in the group receiving PICO, to allow wound assessment. The device was removed on postoperative day seven, and in selected patients an additional NPWT treatment was considered. In controls, dressing were usually changed sterilely after 48 h, and removed within 3 days from surgery. Follow-ups were scheduled on postoperative day seven, 15, and 30, then every 14 days during the subsequent 3 months. Five patients in the NPWT group were sent home with the device, removed within 4 days from discharge. Primary outcomes consisted of SSI and wound complications, according to CDC criteria [21] and Global ASEPSIS score [57, 58]. The cosmetic results were evaluated by means of Patient and Observer Scar Assessment Scale (POSAS) [59] and of a 10-cm Visual Analogue Scale (VAS, “0”, worse, “10” best outcome) 3 months after surgery, as secondary aim.
NPWT with PICO significantly reduced overall SSI rates compared with conventional dressings. Only one patient receiving NPWT (7.7 %) had SSI, classified as superficial, compared with eight (47 %) controls (p = 0.042). No other differences were observed concerning both minor and major postoperative complications [9]. Cosmetic results were similar between groups [9]. Groups were homogeneous in terms of demographical and surgical data, whereas length of stay was significantly shorter with NPWT (7 ± 1.8 vs 10 ± 1.6 days, p = 0.0007) [9].
Under the light of the excellent results obtained, enrolment was extended to include up to 25 patients per group [8], and results confirmed the benefits obtained with preventive NPWT. Wound complications were reduced by NPWT (seroma 2 vs 11 patients, p = 0.008, Global ASEPSIS score 14 ± 7 vs 28 ± 5, p = 0.001, NPWT vs controls), as well as SSI (2 vs 12, p = 0.004), and length of stay (7 ± 2 vs 12 ± 2 days, p = 0.0001).
In addition, a logistic regression including all enrolled patients showed that perioperative corticosteroid drugs (which are often needed in IBD patients candidates to surgery) were independent predictors of surgical site events (OR 1.95, CI 95 % 1.12–4.33, p = 0.02), which were significantly reduced with NPWT (OR 0.21, CI 95 % 0.15–0.5, p = 0.001) [8]. A subgroup analysis including patients receiving >20 mg/day or steroids at surgery was hence performed, and showed that the effects of NPWT were even more apparent (SSI: 1/13 vs 9/12, p = 0.001) [8].
These results are confirmed by a retrospective study including patients undergoing colorectal surgery with primary wound closure [43], who received NPWT by means of conventional devices. Similarly, corticosteroid medications were significantly associated with SSI [43].
Another retrospective study from Canada [60] evaluated the impact of preventive NPWT on SSI after rectal cancer surgery with abdominoperineal excision. NPWT was applied on the perineal wounds of 27 patients, and these were compared with 32 receiving conventional dressings. The latter had higher incidence of SSI, causing significant morbidity [60]. However, the last two cited studies have two major limitations due to their retrospective nature and the use of expensive, cumbersome NPWT devices [43, 60]. This makes it difficult to lead readers to accept their use preventively, which means before complications occur.
Portable devices could be useful in increasing patient adherence to NPWT, and device-related comfort [8, 9]. It has been shown that most problems due to device activation are easily dealt with by patients themselves, even in home settings, with no need of unscheduled office visits [8, 9].
Some facets of the above reported studies need further comments. IBD may affect people at any age [61–64]. Patients suffering from CD often need prolonged perioperative antibiotic treatment, which is a risk factor for SSI. It is interesting to consider that samples from surgical specimens of these patients may show pathogens in lymph nodes and gut serosa even without macroscopic evidence of contamination [65]. This may be responsible for postoperative sepsis, a dreaded complication [65, 66]. In common practice, perioperative antibiotic treatment is often different from other colorectal patients.
IBD pathogenesis relies on a complex interaction between environmental and autoimmune mechanisms, which still remain largely unknown [67–70]. As a result, IBD patents have impaired responses to host factors, affecting reaction to environmental agents, and increasing the risk of malignancies through inflammatory pathways, different from those observed in sporadic carcinogenesis [70–72]. Approximately 80 % of CD patients undergo surgery during life, often at young age, and more than half may need repeated surgery in the following 20 years [73–75]. It is important to obtain a rapid wound healing, allowing for early and safe discharge, and to achieve optimal wound healing, especially relevant in young patients candidates to further surgery.
In addition, these patients frequently need surgery in emergent settings due to abrupt onset, often needed fashioning of stomas [76, 77], and sometimes receive immunomodulators at surgery. These medications modulate local and systemic inflammatory response, and may increase the rate of wound dehiscence, septic complications, and impaired synthesis of collagen, ultimately resulting in delayed wound healing [78]. Prolonged or high-dose corticosteroid intake increases the likeliness of SSI. An analysis of more than 7000 patients developing SSI after general or vascular surgery showed that patients receiving steroids had 1.387 OR of SSI (p < 0.0001) [79]. A more recent paper suggested that the rate of SSI can increase from 2.9 to 5 % with perioperative steroids (OR 1.724) [80]. These observations are confirmed in colorectal surgery [8, 9, 43]. The role of biologic medications, which are suggested to increase the risk of septic complications after surgery for IBD, is controversial concerning wound healing, and further data are needed to assess their effect [81].
The most recent clinical study dealing with preventive NPWT in general and colorectal surgery included 50 patients undergoing surgery for breast diseases and 50 patients undergoing colorectal surgery. Each group included 25 patients treated with postoperative prophylactic NPWT and 25 receiving conventional wound dressings. In addition, ten patients per sub-group were older than 65 years of age at surgery [7]. The primary outcome of the study was to assess safety and effectiveness of NPWT with PICO in preventing wound complication compared with standard wound management in patients undergoing both breast and colorectal procedures. The secondary aims were to assess (1) effectiveness and safety of NPWT in geriatric patients, and (2) to seek for potential differences in results between the two types of surgery (breast vs colorectal) [7]. Intuitively, all patients in the breast surgery group were female (50 vs 28 patients, p < 0.0001) and had shorter duration of surgical procedures (p < 0.0001) and wound length (p = 0.003) than colorectal patients. Sub-groups were homogeneous. Length of stay was similar in NPWT and controls after breast surgery, whereas in Colorectal patients receiving NPWT it was shortened by half (7 ± 2.1 vs 12 ± 3.5 days, p = 0.001). The rate of seroma was significantly reduced with NPWT in Colorectal patients (8 vs 40 %, p = 0.02), but no differences were observed in breast patients. All NPWT patients had lower incidence of SSI (breast: 8 vs 36 %, p = 0.04; Colorectal: 8 vs 44 %, p = 0.08) and global ASEPSIS score (breast: 12 ± 3.2 vs 18.2 ± 5.1, p = 0.03; colorectal: 14.6 ± 4.7 vs 25.3 ± 3.3, p = 0.01). Sub-group analyses on elderly patients (10 per sub-group) suggested that results were even more apparent considering surgical site complications (breast: 0 vs 50 %, p = 0.003; colorectal: 10 vs 60 %, p = 0.003), with no differences between breast and Colorectal diseases (breast vs colorectal surgery: p > 0.99) [7]. No undesired events or complications were observed with NPWT devices.
The latter paper offers other causes for reflection. Patients diagnosed with malignancies often need multimodal treatment, including radiotherapy and chemotherapy, or combined radiochemotherapy, which can be administered preoperatively [82–89]. These cause therapeutic immunodepression, but might increase the risk of surgical site events. This is showed by the very recent attention to patients undergoing surgery for Colorectal malignancies, with studies aiming at reducing the risk of SSI in patients undergoing rectal surgery after chemoradiation showing the usefulness of gentamicin implants in the pelvis [82]. Preventive NPWT could be considered complementary to other intraoperative measures to obtain a synergistic effect, increasing protection against SSI, acting against all sources of infection (endogenous and exogenous) [83, 90]. Besides reducing the costs of care due to SSI and increasing patients’ HRQoL, preventive NPWT could enhance wound healing allowing for early initiation of adjuvant therapy after surgery. In our opinion, this is a very important point. Finally, due to advances in multimodal management of patients with recurrent pelvic malignancies [84–86], the risk of further surgery is to be considered.
At present, no clear or widely accepted algorithms are available to predict likeliness of SSI, and select patients who would benefit from prophylactic NPWT, and the development of easily reproducible predicting scores (similar to those of other conditions [91–93]) would be desirable. In our practice, we currently consider and propose prophylactic placement of NPWT to patients with at least two accepted predictors of SSI [3, 17, 21, 28, 29], or when there is a break in perioperative prophylactic measures [21]. Patients receiving steroids, frail (in the elderly with comorbidities—who are not excluded from major procedures in current practice [7, 93–95] ) or immunodepressed patients, and young patients suffering from IBD [7–9] are the ideal candidates to preventive NPWT after Colorectal surgery. It can be predicted that the use of preventive NPWT is cost-effective and well accepted in these patients. In times of spending review and difficult patient-caregiver relationships [96], the role of pocket disposable devices for NPWT is crucial, due to their limited costs and patient satisfaction. The use of these technologies should be encouraged, because as long as Colorectal surgeons use these they are going to become conscious of their utility, and, most importantly, of their shortcomings, fostering further researches and implementations. Waiting for randomised controlled trials, this is likely to optimise the outcomes of colorectal surgery, hopefully reflecting in optimal HRQoL of patients.
References
Nichols RL (2001) Preventing surgical site infections: a surgeon’s perspective. Emerg Infect Dis 7:220–224
Cima R, Dankbar E, Lovely J et al (2013) Colorectal surgery surgical site infection reduction program: a national surgical quality improvement program—driven multidisciplinary single-institution experience. J Am Coll Surg 216:23–33
Hedrick TL, Sawyer RG, Friel CM, Stukenborg GJ (2013) A method for estimating the risk of surgical site infection in patients with abdominal colorectal procedures. Dis Colon Rectum 56:627–637
De Werra C, Schiavone D, Di Micco R, Triassi M (2009) Surgical site infections in Italy. Infez Med 17:205–218
Emmerson AM, Enston JE, Griffin M, Kelsey MC, Smith ET (1996) The Second National prevalence Survey of infections in hospitals-overview of the results. J Hosp Infect 32:175–190
Reilly JS, Baird D, Hill R (2001) The importance of definitions and methods in surgical wound infection audit. J Hosp Infect 47:64–66
Pellino G, Sciaudone G, Candilio G et al (2014) Preventive NPWT over closed incisions in general surgery: does age matter? Int J Surg 12(Suppl 2):S64–S68. doi:10.1016/j.ijsu.2014.08.378
Selvaggi F, Pellino G, Sciaudone G et al (2014) New advances in negative pressure wound therapy (NPWT) for surgical wounds of patients affected with Crohn’s disease. Surg Technol Int 24:83–89
Pellino G, Sciaudone G, Candilio G et al (2014) Effects of a new pocket device for negative pressure wound therapy on surgical wounds of patients affected with Crohn’s disease: a pilot trial. Surg Innov 21:204–212
de Lissovoy G, Fraeman K, Hutchins V, Murphy D, Song D, Vaughn BB (2009) Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control 37:387–397
Fraccalvieri M, Sarno A, Gasperini S et al (2013) Can single use negative pressure wound therapy be an alternative method to manage keloid scarring? A preliminary report of a clinical and ultrasound/colour-power-doppler study. Int Wound J 10:340–344
Dimick JB, Chen SL, Taheri PA et al (2004) Hospital costs associated with surgical complications: a report from the private-sector National Surgical Quality Improvement Program. J Am Coll Surg 199:531–537
Wick EC, Vogel JD, Church JM, Remzi F, Fazio VW (2009) Surgical site infections in a “high outlier” institution: are colorectal surgeons to blame? Dis Colon Rectum 52:374–379
Konishi T, Watanabe T, Kishimoto J, Nagawa H (2006) Elective colon and rectal surgery differ in risk factors for wound infection: results of prospective surveillance. Ann Surg 244:758–763
Dellinger EP, Hausmann SM, Bratzler DW et al (2005) Hospitals collaborate to decrease surgical site infections. Am J Surg 190:9–15
Smith RL, Bohl JK, McElearney ST et al (2004) Wound infection after elective colorectal resection. Ann Surg 239:599–605
Wick EC, Gibbs L, Indorf LA, Varma MG, Garcia-Aguilar J (2008) Implementation of quality measures to reduce surgical site infection in colorectal patients. Dis Colon Rectum 51:1004–1009
System NNIS (2004) National Nosocomial Infections Surveillance (NNIS) System Report, data summary from January 1992 to June 2004, issued October 2004. Am J Infect Control 32:470–485
Romy S, Eisenring MC, Bettschart V, Petignat C, Francioli P, Troillet N (2008) Laparoscope use and surgical site infections in digestive surgery. Ann Surg 247:627–632
Fry DE (2008) Surgical site infections and the surgical care improvement project (SCIP): evolution of national quality measures. Surg Infect 9:579–584
Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR (1999) Guideline for prevention of surgical site infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control 27:97–132
Burke JP, O’Connell PR (2014) Surgical site infection and colorectal surgery: a research priority. Dis Colon Rectum 57:e390
Patient safety: Surgical site infections. Institute for Healthcare Improvement. http://www.ihi.org. Accessed 29 Sep 2014
Khuri SF, Daley J, Henderson W et al (1995) The National Veterans Administration Surgical Risk Study: risk adjustment for the comparative assessment of the quality of surgical care. J Am Coll Surg 180:519–531
Khuri SF, Daley J, Henderson W et al (1998) The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 228:491–507
Accreditation program for hospitals: National patient safety goals. The Joint Commission. http://www.jointcommission.org/. Accessed 29 Sep 2014
Barie PS (2007) No pay for no performance. Surg Infect (Larchmt) 8:421–433
Campbell D, Neumayer L, Khuri S, Birkmeyer J, Hall B, Bass B, American College of Surgeons NSQIP Staff ACS NSQIP Semiannual Report 2006. ACS NSQIP Advisory Committee. https://acsnsqip.org. Accessed 29 Sep 2014
van Walraven C, Musselman R (2013) The Surgical Site Infection Risk Score (SSIRS): a model to predict the risk of surgical site infections. PLoS One 8:e67167 Print 2013
Gaynes RP (2000) Surgical-site infections and the NNIS SSI Risk Index: room for improvement. Infect Control Hosp Epidemiol 21:184–185
Ercole FF, Starling CE, Chianca TC, Carneiro M (2007) Applicability of the national nosocomial infections surveillance system risk index for the prediction of surgical site infections: a review. Braz J Infect Dis 11:134–141
Stannard JP, Volgas DA, Stewart R, McGwin G Jr, Alonso JE (2009) Negative pressure wound therapy after severe open fractures: a prospective randomized study. J Orthop Trauma 23:552–557
Chio EG, Agrawal A (2010) A randomized, prospective, controlled study of forearm donor site healing when using a vacuum dressing. Otolaryngol Head Neck Surg 142:174–178
Molnar JA, Simpson JL, Voignier DM, Morykwas MJ, Argenta LC (2005) Management of an acute thermal injury with subatmospheric pressure. J Burns Wounds 4:e5
Padovano Sorrentino V, Della Corte A, Campitiello F, Freda F, Petronella P, Canonico S (2010) Wound bed preparation with NPWT in diabetic foot ulcers: case report. BMC Geriatrics 10:A69. doi:10.1186/1471-2318-10-S1-A69
Eneroth M, van Houtum WH (2008) The value of debridement and vacuum-assisted closure (V.A.C.) therapy in diabetic foot ulcers. Diabetes Metab Res Rev 24(Suppl 1):S76–S80
Mandal A (2007) Role of topical negative pressure in pressure ulcer management. J Wound Care 16:33–35
Kanakaris NK, Thanasas C, Keramaris N, Kontakis G, Granick MS, Giannoudis PV (2007) The efficacy of negative pressure wound therapy in the management of lower extremity trauma: review of clinical evidence. Injury 38(Suppl 5):S9–S18
Blume PA, Key JJ, Thakor P, Thakor S, Sumpio B (2010) Retrospective evaluation of clinical outcomes in subjects with split-thickness skin graft: comparing V.A.C.® therapy and conventional therapy in foot and ankle reconstructive surgeries. Int Wound J 7:480–487
Sjogren J, Gustafsson R, Nilsson J, Lindstedt S, Nozohoor S, Ingemansson R (2011) Negative-pressure wound therapy following cardiac surgery: bleeding complications and 30-days mortality in 176 patients with deep sternal wound infection. Interact Cardiovasc Thor Surg 12:117–120
Stevens P (2009) Vacuum-assisted closure of laparotomy wounds: a critical review of the literature. Int Wound J 6:259–266
Dragu A, Schnürer S, Unglaub F, Wolf MB, Beier JP, Kneser U, Horch RE (2011) Wide topical negative pressure wound dressing treatment for patients undergoing abdominal dermolipectomy following massive weight loss. Obes Surg 21:1781–1786
Bonds AM, Novick TK, Dietert JB, Araghizadeh FY, Olson CH (2013) Incisional negative pressure wound therapy significantly reduces surgical site infection in open colorectal surgery. Dis Colon Rectum 56:1403–1408
Stannard JP, Atkins BZ, O’Malley D et al (2009) Use of negative pressure therapy on closed surgical incisions: a case series. Ostomy Wound Manag 55:58–66
Ubbink DT, Westerbos SJ, Nelson EA, Vermeulen H (2008) A systematic review of topical negative pressure therapy for acute and chronic wounds. Br J Surg 95:985–992
Vikatmaa P, Juutilainen V, Kuukasjarvi P, Malmivaara A (2008) Negative pressure wound therapy: a systematic review on effectiveness and safety. Eur J Vasc Endovasc Surg 36:438–448
Gurtner GC, Werner S, Barrandon Y, Longaker MT (2008) Wound repair and regeneration. Nature 453:314–321
Malmsjö M, Borgquist O (2010) NPWT settings and dressing choices made easy. Wounds Int 1:1
DeCarbo WT, Hyer CF (2010) Negative-pressure wound therapy applied to high-risk surgical incisions. J Foot Ankle Surg 49:299–300
Lindstedt S, Malmsjö M, Hansson J, Hlebowicz J, Ingemansson R (2012) Microvascular blood flow changes in the small intestinal wall during conventional negative pressure wound therapy and negative pressure wound therapy using a protective disc over the intestines in laparostomy. Ann Surg 255:171–175
Hlebowicz J, Hansson J, Lindstedt S (2012) Microvascular blood flow response in the intestinal wall and the omentum during negative wound pressure therapy of the open abdomen. Int J Colorectal Dis 27:397–403
Gutierrez IM, Gollin G (2012) Negative pressure wound therapy for children with an open abdomen. Langenbecks Arch Surg 397:1353–1357
Perez D, Wildi S, Demartines N, Bramkamp M, Koehler C, Clavien PA (2007) Prospective evaluation of vacuum-assisted closure in abdominal compartment syndrome and severe abdominal sepsis. J Am Coll Surg 205:586–592
Stevens P (2009) Vacuum-assisted closure of laparostomy wounds: a critical review of the literature. Int Wound J 6:259–266
Bryan S, Dukes S (2009) Case study: negative pressure wound therapy in an abdominal wound. Br J Nurs 18:S15–S21
Hourigan LA, Linfoot JA, Chung KK et al (2010) Loss of protein, immunoglobulins, and electrolytes in exudates from negative pressure wound therapy. Nutr Clin Pract 25:510–516
Wilson AP, Treasure T, Sturridge MF, Gruneberg RN (1986) A scoring method (ASEPSIS) for postoperative wound infections for use in clinical trials of antibiotic prophylaxis. Lancet 1:311–313
Wilson AP, Gibbons C, Reeves BC et al (2004) Surgical wound infection as a performance indicator: agreement of common definitions of wound infection in 4773 patients. BMJ 329:720
van de Kar AL, Corion LU, Smeulders MJ, Draaijers LJ, van der Horst CM, van Zuijlen PP (2004) Reliable and feasible evaluation of linear scars by the Patient and Observer Scar Assessment Scale. Plast Reconstr Surg 116:514–522
Chadi SA, Kidane B, Britto K, Brackstone M, Ott MC (2014) Incisional negative pressure wound therapy decreases the frequency of postoperative perineal surgical site infections: a cohort study. Dis Colon Rectum 57:999–1006
Pellino G, Sciaudone G, Miele E et al (2014) Functional outcomes and quality of life after restorative proctocolectomy in paediatric patients: a case-control study. Gastroenterol Res Pract 2014:340341. doi:10.1155/2014/340341
Sciaudone G, Pellino G, Riegler G, Selvaggi F (2011) Infliximab in drug-naïve patients with failed ileorectal anastomosis for Crohn’s disease: a new chance for sparing the rectum? Eur Surg Res 46:163–168
Selvaggi F, Pellino G, Canonico S, Sciaudone G (2014) Systematic review of cuff and pouch cancer in patients with ileal pelvic pouch for ulcerative colitis. Inflamm Bowel Dis 20:1296–1308
Pellino G, Sciaudone G, Candilio G et al (2013) Complications and functional outcomes of restorative proctocolectomy for ulcerative colitis in the elderly. BMC Surg 13(Suppl 2):S9
Ambrose NS, Johnson M, Burdon DW, Keighley MR (1984) Incidence of pathogenic bacteria from mesenteric lymph nodes and ileal serosa during Crohn’s disease surgery. Br J Surg 71:623–625
Selvaggi F, Sciaudone G, Limongelli P et al (2010) The effect of pelvic septic complications on function and quality of life after ileal pouch-anal anastomosis: a single center experience. Am Surg 76:428–435
Lawrance IC, Rogler G, Bamias G, Breynaert C et al (2014) Cellular and molecular mediators of intestinal fibrosis. J Crohns Colitis. doi:10.1016/j.crohns.2014.09.008 Epub ahead of print
Latella G, Rogler G, Bamias G et al (2014) Results of the 4th scientific workshop of the ECCO (I): pathophysiology of intestinal fibrosis in IBD. J Crohns Colitis 8:1147–1165
Canani RB, Cirillo P, Mallardo G et al (2003) Effects of HIV-1 Tat protein on ion secretion and on cell proliferation in human intestinal epithelial cells. Gastroenterology 124:368–376
Romano M, Cuomo A, Tuccillo C et al (2007) Vascular endothelial growth factor and cyclooxygenase-2 are overexpressed in ileal pouch-anal anastomosis. Dis Colon Rectum 50:650–659
Egan L, D’Inca R, Jess T et al (2014) Non-colorectal intestinal tract carcinomas in inflammatory bowel disease: results of the 3rd ECCO Pathogenesis Scientific Workshop (II). J Crohns Colitis 8:19–30
Pellino G, Sciaudone G, Patturelli M et al (2014) Relatives of Crohn’s disease patients and breast cancer: an overlooked condition. Int J Surg 12(Suppl 1):S156–S158
Terdiman JP (2008) Prevention of postoperative recurrence in Crohn’s disease. Clin Gastroenterol Hepatol 6:616–620
Peyrin-Biroulet L, Loftus EV Jr, Colombel JF, Sandborn WJ (2010) The natural history of adult Crohn’s disease in population-based cohorts. Am J Gastoenterol 105:289–297
Pellino G, Sciaudone G, Selvaggi F, Riegler G (2015) Delayed diagnosis is influenced by the clinical pattern of Crohn’s disease and affects treatment outcomes and quality of life in the long term: a cross-sectional study of 361 patients in Southern Italy. Eur J Gastroenterol Hepatol 27:175–181
Selvaggi F, Pellino G, Canonico S, Sciaudone G (2012) Is omitting pouchography before ileostomy takedown safe after negative clinical examination in asymptomatic patients with pelvic ileal pouch? An observational study. Tech Coloproctol 16:415–420
Pellino G, Sciaudone G, Canonico S, Selvaggi F (2012) Role of ileostomy in restorative proctocolectomy. World J Gastroenterol 18:1703–1707
Busti AJ, Hooper JS, Amaya CJ, Kazi S (2005) Effects of perioperative antiinflammatory and immunomodulating therapy on surgical wound healing. Pharmacotherapy 25:1566–1591
Neumayer L, Hosokawa P, Itani K et al (2007) Multivariable predictors of postoperative surgical site infection after general and vascular surgery: results from the Patient Safety in Surgery Study. J Am Coll Surg 204:1178–1187
Ismael H, Horst M, Farooq M et al (2011) Adverse effects of preoperative steroid use on surgical outcomes. Am J Surg 201:305–309
Selvaggi F, Pellino G, Canonico S, Sciaudone G (2015) Effect of preoperative biologic drugs on complications and function after restorative proctocolectomy with primary ileal pouch formation: systematic review and meta-analysis. Inflamm Bowel Dis 21:79–92
Rutkowski A, Zając L, Pietrzak L et al (2014) Surgical site infections following short-term radiotherapy and total mesorectal excision: results of a randomized study examining the role of gentamicin collagen implant in rectal cancer surgery. Tech Coloproctol 18:921–928
Pellino G, Selvaggi F (2014) Surgical site events in colorectal surgery: prevention is better than cure. Tech Coloproctol 18:1187–1188
Selvaggi F, Cuocolo A, Sciaudone G, Maurea S, Giuliani A, Mainolfi C (2003) FGD-PET in the follow-up of recurrent colorectal cancer. Colorectal Dis 5:496–500
Saladino E, Fleres F, Irato S, Famulari C, Macrì A (2014) The role of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the treatment of ovarian cancer relapse. Updates Surg 66:109–113
Selvaggi F, Fucini C, Pellino G, Sciaudone G, Maretto I, Mondi I, Bartolini N, Caminati F, Pucciarelli S (2014) Outcome and prognostic factors of local recurrent rectal cancer: a pooled analysis of 150 patients. Tech Coloproctol. doi:10.1007/s10151-014-1241-x Epub ahead of print
Selvaggi F, Pellino G, Pellegrino T et al (2014) Role of scintigraphy in differential diagnosis of adrenal lesions. Minerva Chir 69(Suppl 1):105–109
Melotti G, De Antoni E, Habr-Gama A, Minicozzi A (2013) Management of distal rectal cancer: results from a national survey. Updates Surg 65:43–52
Accardo G, Esposito D, Barbato F et al (2014) Pheochromocytoma: from the bench to the surgery. Minerva Chir 69(Suppl 1):97–103
Benevento R, Santoriello A, Pellino G et al (2014) The effects of low-thrombin fibrin sealant on wound serous drainage, seroma formation and length of postoperative stay in patients undergoing axillary node dissection for breast cancer. A randomized controlled trial. Int J Surg 12:1210–1215
Cerullo G, Cassini D, Baldazzi G (2012) Application of Petersen Index score for Dukes’B colorectal cancer in a population of 103 consecutive resected patients. Updates Surg 64:95–99
Selvaggi F, Pellino G, Sciaudone G et al (2014) Development and validation of a practical score to predict pain after excisional hemorrhoidectomy. Int J Colorectal Dis 29:1401–1410
Speicher PJ, Ligh C, Scarborough JE et al (2014) A simple scoring system for risk-stratifying rectal cancer patients prior to radical resection. Tech Coloproctol 18:459–465
Canonico S, Pellino G, Pameggiani D et al (2014) Thyroid surgery in the elderly: a comparative experience of 400 patients from an italian university hospital. Int Surg 99:523–527
Pellino G, Sciaudone G, Candilio G et al (2013) Early postoperative administration of probiotics versus placebo in elderly patients undergoing elective colorectal surgery: a double-blind randomized controlled trial. BMC Surg 13(Suppl 2):S57
Santoro E (2014) Clinical-judicial syndrome: how a doctor becomes a patient through general indifference. Updates Surg 66:173–175
Conflict of interest
The authors have no conflicts of interests to disclose.
Ethical Standard
All procedures in our paper were in accordance with the ethical standards of the institutional as well as National research committee and with the 1964 Helsinki declaration and its later amendments.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained and anonymity maintained.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendix: Preventing SSI with NPWT in colorectal surgery: real life experience
Appendix: Preventing SSI with NPWT in colorectal surgery: real life experience
We below describe some exemplifying cases to show the effects of preventive NPWT in patients undergoing colorectal procedures.
Case 1
-
1.
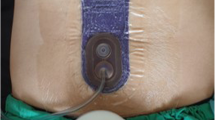
Figure 2. Male, >50-year-old patient undergoing laparoscopic colonic resection for a benign stricture of the transverse colon due to CD. Additional risk factors for SSI: corticosteroid drugs, obesity, smoking habit. A mini-laparotomy is performed in left hypochondrium to allow for digital examination of the small bowel, specimen extraction, and extra-corporeal anastomosis [8].
-
2.
Figure 3. Follow-up: appearance of the gauze after 5 days from surgery. NPWT is working due to absorbent capacity of the gauze [8].
-
3.
Figure 4. Follow-up: wound appearance at 30-days follow-up [8].
Case 2
-
1.
Figure 5. Female, 31-year-old patient undergoing ileal resection and multiple strictureplasties for CD. Additional risk factor for SSI: enterocutaneous stoma, smoking habit [9].
-
2.
Figure 6. Follow-up: gauze partially removed on postoperative day 3 to allow wound assessment as per protocol [9].
Case 3
Figure 7. Male, 28-year-old patient undergoing ileocaecal resection for CD. Additional risk factors: smoking habit. The patient refused NPWT application. The picture shows 14-days follow-up, when suboptimal healing is observed. The patient had superficial SSI.
Rights and permissions
About this article
Cite this article
Pellino, G., Sciaudone, G., Selvaggi, F. et al. Prophylactic negative pressure wound therapy in colorectal surgery. Effects on surgical site events: current status and call to action. Updates Surg 67, 235–245 (2015). https://doi.org/10.1007/s13304-015-0298-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13304-015-0298-z