Abstract
In the higher vertebrate brain, the delicate balance between structural stabilization and remodeling of synaptic networks changes over the life span. The juvenile brain is characterized by high structural plasticity. A critical step in brain maturation is the occurrence of the extracellular matrix (ECM) that structurally stabilizes neuronal tissue restricting the potential for neuronal remodeling and regeneration. Current research has only begun to understand how this putative limitation of adult neuronal plasticity might impact on learning-related plasticity, lifelong memory reformation and higher cognitive functions. In this review, we summarize recent evidence that recognizes the ECM and its activity-dependent modulation as a key regulator of learning-related plasticity in the adult brain. Experimental modulation of the ECM in local neuronal circuits further opens short-term windows of activity-dependent reorganization, promoting complex forms of cognitive flexible adaptation of valuable behavioral options. This further bears implications for guided neuroplasticity with regenerative and therapeutic potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Both neurons and glial cells in the brains of higher vertebrates produce the extracellular matrix (ECM). Pioneers of brain cell biology, including Camillo Golgi and Santiago Ramon y Cajal have discovered the mesh-like structure surrounding most neurons and synapses in the adult brain. Initially, the ECM has been seen as a key component for guaranteeing the structural stability of neuronal tissue. Later studies recognized that the ECM undergoes significant changes during brain maturation. During late embryonic phases the ECM is composed of proteoglycans and glycoproteins like neurocan and tenascin-C. These components are down-regulated in the adult brain. The ECM is then mainly made of the glycoprotein tenascin-R and chondroitin sulfate proteoglycans (CSPGs) such as brevican and aggrecan. The structural backbone of the adult ECM is formed by the unbranched polysaccharide hyaluronic acid (HA). Interestingly, it has been shown that the occurrence of the ECM closely matches the closure of critical periods in the respective brain regions. This led to the hypothesis that the brain’s ECM converts juvenile into adult plasticity by structural tenacity restricting the potential for neuronal reorganization [1]. The adult brain has thereby established an effective way to structurally stabilize neuronal networks shaped during experience-dependent learning, which is fundamental for an effective long-term memory storage and recall. However, current research now evidences that dynamic adaptation of the ECM can change forms of synaptic plasticity and further impact flexible learning and memory organization in the adult brain.
Structural and functional features of the ECM in the brain
The linear HA backbone binds and coordinates proteoglycans, especially of the lectican family that are cross-linked by glycoproteins to form the ECM meshwork. HA-based ECM is rich in the glycosaminoglycan chondroitin sulfate (CS) attached to CSPGs of the lectican family, among them brevican and aggrecan. Brevican and aggrecan both bind to the ECM glycoprotein tenascin-R (Fig. 1a). Cartilage link proteins further contribute to the ECM stability by forming a complex with lecticans and HA. A large variety of other components, including reelin, laminins, thrombospondins, heparin-sulfate proteoglycans, guidance molecules, and even transcription factors are incorporated in the complex ECM structure (Fig. 1a). The meshwork of ECM loosely enwraps cell bodies and dendritic and synaptic structures of most neurons. In the mature brain, a highly elaborate and specialized ECM structure around synapses and somata of a small proportion of neurons is called the perineuronal nets (PNN), especially rich in aggrecan and CS. PNNs are most abundant on GABAergic interneurons expressing the calcium buffer protein parvalbumin (PV; Fig. 1b). However, recent studies evidenced that PNNs are highly heterogeneous and can be found on various types of neurons throughout the CNS.
Cell-type specific forms of the hyaluronan-based ECM. a Schematic view of the PNN (left) and loose ECM (right) surrounding synapses. Left, PNNs are densely packed, rich in CS and aggrecan. Small molecules such as semaphorin 3a or Oxt2 are bound to CS within the PNN and mediate signaling functions or regulate gene expression. Axons form synapses within the holes of the PNN. Right, the loose ECM around excitatory, spiny synapses is rich in brevican and contains only little aggrecan and is thus less rich in CS. ECM proteins (e.g., reelin) signal through their receptors (e.g., integrins) and regulate several cellular processes, including trafficking of glutamate receptors (see c). ECM function is modulated by proteases (scissors) that may free synapses by removing ECM or unmask signaling molecules. b Left, parvalbumine positive interneuron (PV, red) with typical PNN stained for aggrecan (AGG, green). Middle, dendritic spines and synapses of PV cells (red) are also surrounded by brevican (BC, green), although it is less specific for PNNs. Right, dissociated cortical neuron stained with the dendritic marker Map2 (blue) and Homer (red) to stain excitatory synapses and hyaluronic acid binding protein (HABP, green) to label extracellular matrix. Note the loose appearance around dendrites, spines, and synapses. c Experimental enzymatic removal of the ECM by glycosidases (e.g., hyaluronidase) facilitates structural plasticity such as formation of new synapses and increases lateral diffusion and synaptic exchange of AMPA receptors (blue arrows, left)
Functionally, the ECM is not restricted to provide mechanical stability of neuronal networks. In the juvenile brain, the ECM regulates neuro- and gliogenesis, cell migration, axonal pathfinding and synaptogenesis. In the adult brain, the ECM is in the service of multiple functions, including regulation of synaptogenesis and synaptic plasticity, compartmentalization of the neuronal surface, neuroprotection, regulation of ion homeostasis and neuron–glia-interactions. In this review, we will summarize several ECM-based mechanisms for regulating synaptic plasticity and their effects on brain development and adult learning behavior.
Switch from developmental to adult forms of synaptic plasticity
In the developing brain, phases of high structural plasticity allow profound shaping of brain circuits by experience. In a number of experiments it has been shown that such critical periods are limited by the ECM implementing adult brain plasticity modes. As an example mutant mice for the cartilage link protein 1 (Crtl1/HPLN1 −/−) do not develop normal PNN structures in the visual cortex. This leads to juvenile forms of ocular dominance (OD) plasticity and sensitivity of the visual system to deprivation throughout the life span. Further, dark rearing delays not only the critical periods of developmental plasticity in visual cortex of rodents but also the formation of PNNs. The key study that elucidated the ECM as the regulatory switch between juvenile and adult plasticity came from Pizzorusso and colleagues [2]. The authors injected the ECM-cleaving enzyme chondroitinase ABC (chABC) into visual cortex of adult rats combined with monocular deprivation. Local weakening of the ECM was “re-juvenating” visual cortex and restored the critical period form of OD plasticity. In a follow-up study they restored visual acuity in grown-up adult animals with long-term monocular deprivation by the same manipulation. Similarly, application of the serine protease tissue-type plasminogen activator (tPA) into the visual cortex can prolong critical periods of OD plasticity in visual cortex.
Later studies supported the role of the ECM as a regulatory switch in other forms of developmental plasticity during brain maturation. For instance, in the zebra finch birdsong learning occurs during a sensitive period that is similar to the language development of humans. It has been shown that the emergence of PNNs around parvalbumin-positive neurons in brain areas that are dedicated to singing correlate with this sensory critical period. In another set of experiments, Gogolla et al. [3] have shown that ECM networks in the amygdala are essential for making fear memories erasure-resistant in adult animals. A conditioned fear memory trace can be erased permanently by extinction, that is, the presentation of the conditioned stimulus without the aversive stimulus. However, such permanent loss of the fear memory is only found in rats not older than 3–4 weeks. After this period the ECM in the amygdala forms and preserves fear memory. Extinction only attenuates the fear response, but this reinstates instantaneously if the aversive stimulus is presented again. Attenuation of the ECM in the amygdala by chABC injections in adult rats led to a complete erasure of the fear response after an extinction phase even if the aversive stimulus is presented again.
The reviewed studies have shown that the different modes of neuronal plasticity during brain development are mediated by a change of the juvenile form of the ECM to a more rigid adult ECM. Such restriction of adult reorganizational and regenerative plasticity by an elaborate ECM has only evolved in higher vertebrates. The evolutionary benefit may be to preserve the costly acquired hardwired connections during early life experience, which are fundamental for rapid experience-based behavioral adaptations of higher vertebrates [1]. However, the adult, healthy brain retains a remarkable capability of plastic reorganization essential for adapting to our ever-changing environment.
The extracellular space affects adult synaptic plasticity
The functional mechanisms by which the HA-based ECM implements adult brain plasticity are still elusive. Mainly, effects on classical (Hebbian) and homeostatic plasticity have been investigated in this respect (for an overview see [4]). First insights into the impact of the ECM on adult synaptic plasticity came from knockout models lacking particular components of the ECM. For instance, mice deficient in tenascin-R, brevican, or neurocan all showed impaired hippocampal long-term potentiation (LTP). However, compensatory mechanisms during brain development of k.o. models might limit the significance of the findings. Therefore, other studies used acute enzymatic weakening of the ECM based on different matrix-degrading enzymes. Hippocampal slices treated with chABC also showed impaired LTP, which may be due to the increased excitability of GABAergic perisomatic interneurons [4]. Injection of the hyaluronidase (HYase) from Streptomyces hyalurolyticus, which is in contrast to other hyaluronidases highly specific to HA and does not digest CS, impairs expression of LTP. This phenotype could be rescued by perfusion with HA. It has been suggested that this is due to the fact that HA directly regulates L-type voltage-gated calcium channels (L-VDCC; Cav1.2) of CA1 neurons and thus postsynaptic Ca2+ entry and hippocampal-dependent forms of learning [4]. In addition, we found that acute ECM removal was also altering short-term dependent plasticity [5]. We measured paired pulse ratios in dissociated hippocampal cultures and found robust paired pulse depression (PPD) under control conditions. Interestingly, digestion of the ECM prevented cells from expressing PPD. We found increased lateral diffusion of extrasynaptic AMPA receptors after ECM digestion as key correlate of this effect. This results in a higher exchange between synaptic desensitized receptors for extrasynaptic naïve ones ([5]; Fig. 1c). In such conditions, synapses are able to follow higher firing frequencies. Thus, the perisynaptic ECM forms surface compartments that act as diffusion barrier for membrane proteins such as AMPA receptors. Interestingly, lack of the major hyaluronan synthetase HAS3 in the hippocampus did not lead to a striking morphological change in the ECM. However, the extracellular space was reduced, which resulted in a more dense packing of cells and lower diffusion of soluble molecules in the CA1 stratum pyramidale. Further, these animals were prone to epileptic seizures which underlines the importance of the regulatory function of the HA-based ECM that is required, for instance, for volume transmission and ion homeostasis. Taken together, these results indicate that remodeling of the ECM results in altered synaptic plasticity due to a changed extracellular micromillieu.
Activity-dependent synaptic plasticity by proteolysis of the ECM
Endogenous ECM-modulating enzymes have a major impact on synaptic function in the juvenile and adult brain. Such enzymes may exert their function by altering extracellular milieu by digesting the ECM or by generating proteolytic fragments that may act as signaling molecules. An important group of such enzymes are the metalloproteases of the ADAMTS-family (A disintegrin and metalloproteinase with thrombospondin motifs). Especially ADAMTS-4/-5 have long been known for their ability to digest aggrecan and brevican—therefore previously termed aggrecanase-1/-2. In the meantime, it has been shown that these enzymes are able to digest all members of the lectican family. Interestingly, their activity increases after epileptic seizures and during homeostatic plasticity [6]. However, their impact on synaptic plasticity remains elusive and is subject of current research. The best studied extracellular protease is the matrix metalloprotease 9 (MMP9). The activity-dependent expression of MMP9 influences synaptic plasticity by regulating spine enlargement and synaptic potentiation. Similarly, the brain-specific serine protease neurotrypsin is regulated in an activity-dependent manner and requires concomitant activation of the postsynaptic neuron [1]. Proteolytic cleavage of agrin by neurotrypsin unmasks a signaling molecule harboring a single laminin G3 domain. This 22 kDa molecule can further regulate spine morphology and de novo synapse generation. In humans, neurotrypsin has been identified as essential component for cognitive functions. Deficits in the neurotrypsin genotype have been correlated with severe mental retardation. Together, this suggests that proteolysis of components of the ECM by exoenzymes not only modifies the structural rigidity but also activates instructive signal molecules that locally modulate synaptic functions [7]. This may temporally restore local divisions of “juvenile” environments as a major constituent of the balance between plasticity and tenacity in the mature brain.
Impact of the ECM on adult learning behavior and cognitive flexibility
Experimental weakening of the ECM by local injection of matrix-digesting enzymes can promote functional neurorehabilitation in the injured brain. This has been related mostly to injuries on the level of the peripheral nervous system and spinal cord [1]. Experience-driven plasticity does, however, not only lead sensory development or neuronal rehabilitation but is also indispensable during learning, memory formation and reconsolidation throughout life. The question now arises how forms of ECM-dependent plasticity in the adult brain might govern learning-related plasticity, lifelong memory reformation and the organization of cognitively flexible behavior. In this respect, only few studies have investigated the involvement of ECM functions in memory storage in adult animals. This has been characterized best for long-term plasticity in the hippocampus and fear memory in the amygdala. However, available evidence is controversial about how ECM functions may impact learning and memory processes.
For instance, it has been reported that tenascin-R knockout mice show normal hippocampus-dependent spatial memory acquisition in a water maze. In subsequent reversal learning though animals showed more vulnerable spatial long-term memory yielding enhanced relearning performance due to less conflicting past and actual learning contingencies. Another study found an already impaired acquisition of hippocampus-dependent contextual memory in same knockout mice [8].
In addition to deficits in matrix components, studies also found effects of deficits in exoproteases modeling the ECM. Loss of MMP9 activity has been associated with impaired hippocampal-dependent learning and amygdala-dependent learning. This is in line with findings of wildtype mice trained in an inhibitory avoidance (IA) learning paradigm. Hippocampal LTP has been related to increased levels of MMP3 and MMP9. Both proteases were upregulated for at least ~ 48 h promoting local plastic synaptic environments underlying the learning performance. Intra-hippocampal injections of MMP9 blockers completely abrogated memory for the IA response when tested days later. Comparably, hippocampal MMP3 and MMP9 were found to be increased during water maze acquisition learning in a NMDA-dependent manner. Hippocampal injection of the broad-spectrum MMP9 inhibitor FN-439 also prevented elevated MMP9 levels, altered hippocampal LTP and prevented spatial acquisition learning. Hence, hippocampal-dependent learning induced a period of focal MMP-mediated proteolysis driving long-lasting synaptic modifications underlying memory consolidation. Effects of changes in the ECM on initial learning are, however, still unclear [7, 8].
More recently, insights into the impact of the ECM onto behavior came from studies using experimental, enzymatic and local weakening of the ECM. The study of Gogolla et al. [3] manipulating adult fear extinction suggested that memory acquisition differs in juvenile and adult brains due to changes of the mature ECM functions. The authors further argued that intra-amygdala injections of chABC in adult rats had no effect on acquisition learning of fear, but only on extinction, reinstatement, and renewal of the fear memory [3]. A further study showed that intra-hippocampal and prefrontal injection of chABC and HYase in mice impair long-term trace contextual fear conditioning. This finding has been related to the impairment in the L-VDCC-dependent component of hippocampal LTP by cleaved extracellular HA.
In addition to spatial memory, another set of studies examined the function of the ECM in memory consolidation of drug seeking. Intracerebral injection of FN-439 has been shown to impair the acquisition of a cocaine-induced conditioned place preference (CPP) of rats. FN-439 injection 30 min prior to cocaine memory reactivation further attenuated the reinstatement of CPP in extinguished animals. A recent study showed that intra-amygdala injections of chABC during active extinction of cocaine-induced CPP prevented its subsequent priming-induced reinstatement. ChABC injections alone had no effect on the retention, retrieval, or relearning of CPP [8].
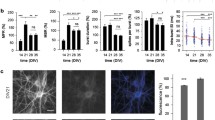
With respect to cognitively flexible adaptation of behavior, we have recently shown that weakening of the ECM in auditory cortex promotes complex forms of cortex-dependent relearning in the Mongolian gerbil [9]. In our experiments, we trained animals on frequency modulated tone discrimination based on the rising or falling modulation direction in a go/nogo-task. Such auditory learning is known to depend on learning-induced plastic reorganization of neuronal circuits in the auditory cortex. After acquiring robust discrimination of the stimulus contingencies, the animals were trained to reverse their choice. We found that ECM weakening by local HYase injection in bilateral auditory cortex accelerated the demanding relearning performance. Specifically, animals had to inhibit the obsolete initial behavioral strategy and then establish its successful reversal (Fig. 2). Importantly, attenuation of the ECM did neither affect general sensory learning nor erased already established, learned memory traces. That means attenuation of the ECM in sensory cortex of these animals promoted the flexible adaptation of the effectively appropriate strategy during cortex-dependent learning behavior that bases on previously acquired auditory memories. The ECM reconstitutes after several days to weeks, limiting again the promoting effects onto cognitive flexibility (Fig. 2a). A comparable finding investigated log-term object recognition memory in knockout mice of the link protein Crtl1/Hapln1— a key molecule for stabilization of PNNs. The Ctrl1/Hapln1 knockout mice have attenuated PNNs in the perirhinal cortex. Long-term object recognition memory, a task depending on perirhinal cortex, was enhanced in these mice. Local injection of chABC in wild-type mice had the same memory-prolonging effect in the object recognition task but also attenuated over time [10]. In this study, the attenuation of the PNNs was accompanied by enhanced perirhinal LTD, which is thought to be the major synaptic mechanism underlying object recognition memory.
Local enzymatic weakening of the ECM in auditory cortex of Mongolian gerbils enhanced the cognitive flexibility in a relearning paradigm. a Top, quantification of the ECM density after local injection of HYase in auditory cortex (right) based on a Wisteria floribunda (WFA) fluorescein staining against sugar chains of the CSPGs. Injection of 0.9 % sodium chloride solution (NaCl) served as control (left). Bottom, HYase injection significantly reduced ECM levels for about 1 week, after which it took 2 weeks after injection to reconstitute the ECM fully. b Gerbils show successful acquisition of the discrimination between two frequency-modulated sounds (modulation direction indicated by rising and falling arrows) depending on their contingency as a go-stimulus (red) or Nogo-stimulus (green). After 7 training days the contingency was reversed. Henceforth, the conditioned response rate is strongly reduced in both experimental groups indicating the active inhibition of the previously established discrimination strategy. HYase-treated animals are significantly better in correcting the behavioral strategy and successfully relearning the task. Modified from [9]
Both the mentioned studies therefore promote the view that the perineuronal ECM in the adult brain actively organizes the balance between memory stability and flexibility. Cortical attenuation of the ECM in the mature brain might hence promote the cognitive flexibility that can build on learned behaviors and allows for an enhanced activity-dependent memory reorganization. And regeneration of the ECM gradually restores normal, restrictive adult plasticity levels. Generally, all studies summarized in this review emphasized that the increased experience-based plasticity by acute, enzymatic PNN diminution is activity-dependent and the rather inconspicuous effect of mere ECM attenuation in general.
With respect to age, the importance of providing profound tenacity to conserve experience-based memories might increase over the life span. Indeed, hippocampal ECM levels have been suggested to show an age-dependent increase conquering age-related cognitive decline. In this line, the Alzheimer’s disease (AD) mouse model APP/PS1 showed a significant upregulation of several matrix components correlating with impairments in hippocampal LTP and contextual memory. Intra-hippocampal injections of chABC restored both, suggesting an important, but yet elusive role for the ECM in early memory impairment in AD, as findings about ECM alterations in dementia are highly controversial and are far from conclusive [8].
Outlook
We have summarized recent evidence showing that experimental modulation of the ECM promotes “windows of opportunities” with an increase in learning-related plasticity yielding cognitively flexible adaptation of learned behaviors and the underlying memories. How the ECM, in addition, impacts several mental disorders that generally develop after the closure of major critical periods for higher brain functions, as for instance affective disorders or schizophrenia, are exciting new research directions. We are envisaging future challenges in developing new tools for guided neuroplasticity with therapeutic potential for memory disorders, stroke or neuroprosthetic applications based on ECM manipulations.
References
Gundelfinger ED, Frischknecht R, Choquet D, Heine M (2010) Converting juvenile into adult plasticity: a role for the brain’s extracellular matrix. Eur J Neurosci 31:2156–2165
Pizzorusso T, Medini P, Berardi N, Chierzi S, Fawcett JW, Maffei L (2002) Reactivation of ocular dominance plasticity in the adult visual cortex. Science 298:1248–1251
Gogolla N, Caroni P, Lüthi A, Herry C (2009) Perineuronal nets protect fear memories from erasure. Science 325:1258–1261
Dityatev A, Schachner M, Sonderegger P (2010) The dual role of the extracellular matrix in synaptic plasticity and homeostasis. Nat Rev Neurosci 11:735–746
Frischknecht R, Heine M, Perrais D, Seidenbecher CI, Choquet D, Gundelfinger ED (2009) Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat Neurosci 12:897–904
Valenzuela JC, Heise C, Franken G, Singh J, Schweitzer B, Seidenbecher CI, Frischknecht R (2014) Hyaluronan-based extracellular matrix under conditions of homeostatic plasticity. Philos Trans R Soc Lond B Biol Sci 369(1654):20130606
Huntley GW (2012) Synaptic circuit remodelling by matrix metalloproteinases in health and disease. Nat Rev Neurosci 13(11):743–757
Dityatev A, Wehrle-Haller B, Pitkänen A (2014) Brain extracellular matrix in health and disease. Prog Brain Res 214:xiii–xvii
Happel MFK, Niekisch H, Castiblanco Rivera LL, Ohl FW, Deliano M, Frischknecht R (2014) Enhanced cognitive flexibility in reversal learning induced by removal of the extracellular matrix in auditory cortex. Proc Natl Acad Sci USA 111:2800–2805
Romberg C, Yang S, Melani R, Andrews MR, Horner AE, Spillantini MG, Bussey TJ, Fawcett JW, Pizzorusso T, Saksida LM (2013) Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J Neurosci 33:7057–7065
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Frischknecht, R., Happel, M.F.K. Impact of the extracellular matrix on plasticity in juvenile and adult brains. e-Neuroforum 7, 1–6 (2016). https://doi.org/10.1007/s13295-015-0021-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13295-015-0021-z