Abstract
ST6GalNAc I is the major Sialyl-Tn antigen (STn) synthase that is highly correlated with tumor invasion and metastasis. However, the roles and molecular mechanisms by which ST6GalNAc I mediates the malignant phenotypes of hepatocarcinoma cells still remain poorly unknown. In this study, we investigated the expression of STn and ST6GalNAc I in mouse hepatocarcinoma cell lines Hca-F, Hca-P, and Hepa1-6, which have different metastatic potential, as compared with normal mouse liver cell line IAR-20. The results showed that the expression of ST6GalNAc I and STn in Hca-F and Hca-P cells was much higher than that in Hepa1-6 and IAR20 cells. Knockdown of ST6GalNAc I by shRNA in Hca-F cells significantly decreased the expression of STn and inhibited the growth of tumor cells in vitro and in vivo. This reduction of ST6GalNAc I expression also led to the decreased migration and invasion of Hca-F cells. Furthermore, we found that ST6GalNAc I knockdown inhibited the expression levels of PI3k, p-Akt473, p-Akt308, NF-κB, and their downstream molecules. Together, our results suggest a role of ST6GalNAc I in promoting the growth and invasion of hepatocarcinoma cells through regulating PI3K/AKT signaling, and ST6GalNAc I might be a promising marker for the prognosis and therapy of hepatocarcinoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocarcinoma remains one of the leading causes of cancer death worldwide [1]. Hepatocellular carcinoma is one of the most common histological forms of primary liver cancer [2]. Most hepatocarcinoma patients die from cancer invasion or distant metastasis to other organs, such as lungs, regional lymph nodes, and bone. The mechanism of cancer invasion and metastasis is a complicated multistep process involving multiple genetic alterations [3, 4]. Therefore, exploring the molecular mechanisms underlying the metastasis of hepatocarcinoma may reveal new therapeutic targets for the prognosis and treatment of hepatocarcinoma.
It has been reported that alternation of glycosylation is a common phenotypic change observed in cancer cells and sialylated antigens overexpress at the surface of cancer cells [5]. Sialyl-Tn antigen (STn) is a short O-glycan containing a sialic acid residue in α2,6-linkage to GalNAc-O-Ser/Thr, usually absent in healthy tissues, but aberrantly expressed in most cancers, such as gastric, ovarian, breast, and bladder [6–10]. STn-positive cancer cells showed higher intraperitoneal metastatic ability in comparison with STn-negative control, resulting in shortened survival time of the mice [11]. STn has been reported to be correlated with cancer progression, poor prognosis, and immunosuppressive microenvironment [12, 13].
The transfer of sialic acid to N-acetylgalactosamin (GalNAc) from CMP-sialic acid is catalyzed by a family of sialytransferases, GalNAc α2,6-sialyltransferases (ST6GalNAc-family) [14]. ST6GalNAc family includes ST6GalNAc I∼VI; some members are involved in the malignant phenotypes of cancer cells [15, 16]. It has been reported that high expression of ST6GalNAc II was associated with breast cancer metastasis [17]. ST6GalNAc I is the major STn synthase and localizes in the Golgi apparatus, and competes with other glycosyltransferases for the Tn antigen. Marcos et al. reported that ST6GalNAc I was highly expressed in colon adenocarcinoma, transitional tissue, and gastrointestinal tissues [18]. The expression of the STn antigen in various cancer cells is due to ST6GalNAc I activity [19]. However, the pathological roles of ST6GalNAc I in hepatocarcinoma and molecular mechanisms underlying tumor migration and invasion remain poorly understood.
In this study, we checked the expression levels of ST6GalNAc I in mouse hepatocarcinoma cell lines (Hca-F, Hca-P, and Hepa1-6), as compared with normal mouse liver IAR-20 cells, and then analyzed the effects of ST6GalNAc I knockdown on cell proliferation, migration, and invasion. We found that the expression levels of ST6GalNAc I were higher in hepatocarcinoma cells than those in normal liver cells and correlated with malignant potential of hepatocarcinoma cells. Knockdown of ST6GalNAc I significantly decreased the expression of STn and inhibited the growth, migration, and invasion ability of Hca-F cells. In addition, ST6GalNAc I silencing inhibited the expression levels of PI3k, p-Akt473, p-Akt308, and NF-κB. Together, our results suggest that silencing of ST6GalNAc I could attenuate the proliferation, migration, and invasion capability of hepatocarcinoma cells by modulating the PI3K/AKT signaling pathway.
Materials and methods
Cell culture and mice
Mouse hepatocarcinoma cell lines Hca-F and Hca-P with high and low lymph node metastatic rate, respectively, were created and maintained by Department of Pathology of Dalian Medical University [20]. The cells were grown in RPMI 1640 (Gibco) supplemented with 10 % fetal bovine serum (Gibco) and 1× penicillin/streptomycin (Hyclone). Mouse hepatocyte cell line IAR-20 and hepatoma cell line Hepa1-6 were purchased from Cell Center of Peking Union Medical University in Beijing. IAR-20 cells were cultured in 90 % modified Eagle’s medium (Gibco) containing 10 % fetal bovine serum and 1× penicillin/streptomycin. Hepa1-6 cells were maintained in Dulbecco’s modified Eagle’s medium (Gibco) containing 1 g/L Hepes (hyclone), 1× penicillin/streptomycin, and 10 % fetal bovine serum. All cells were incubated in a humidified incubator at 37 °C containing 5 % CO2. A total of 615 mice (Hca-F and Hca-P cancer host) were obtained from the Animal Facility of Dalian Medical University. Inbred 615 mice, weighing 20 to 23 g, were used for this study.
Immunofluorescence analysis
Cells seeded on counting chamber slides were fixed with 4 % paraformaldehyde for 15 min and blocked with 5 % bovine serum albumin (BSA) for 1 h. Slides were then incubated with the FITC-conjugated STn antibody (STn219; Abcam) for 30 min on ice. Immunofluorescent images were acquired by fluorescence microscopy (Biozero BZ-8000, Keyence) [21].
Flow cytometry analysis
Cells (1 × 106) were blocked with PBS containing 1 % bovine serum albumin (BSA) and then incubated with the FITC-conjugated STn antibody in 1× phosphate-buffered saline (PBS) for 30 min on ice. After washing thrice with PBS containing 1 % BSA, cells were analyzed using a FACscan instrument (BD Biosciences).
Real-time PCR analysis
Real-time PCR was performed with ABI PRISM 7900 detection system (Applied Biosystems) using SYBR Premix DimerEraser Kit (TaKaRa). Primers for the experiment were as follows: ST6GalNAc I, forward 5′-AAGAACAGGTTTCTGAGGTCAA-3′; reverse 5′-AGATCAACCTTTTCCACGATGT-3′. Total RNA was isolated using TRIzol reagent (Invitrogen), and cDNA synthesis was performed using PrimeScript RT reagent Kit (TaKaRa) according to the manufacturer’s instructions. Specific primers for ST6GalNAc I and GAPDH were purchased (GenePharma). Relative changes in mRNA expression were normalized with GAPDH and calculated using 2−ΔΔCT method [22].
Western blot analysis
Protein concentration was measured with BCA assay kit (Pierce). Equal amounts of denatured proteins were subjected to 10 % SDS–PAGE and blotted onto nitrocellulose membranes (Pall Corporation). Antibodies against ST6GalNAc I, PI3k, p-Akt473, Akt, p-Akt308, NF-κB, MMP-2, MMP-7, MMP-9, VEGF, and GAPDH (Abcam) were used as the primary antibodies. The detection was performed using ECL kit (Amersham Biosciences) according to the manufacturer’s instructions. Use LabWorks software to determine the relative amount of protein.
Construction of vectors and transfection
Construction of expression vector, including ST6GalNAc I-specific small hairpin RNA (shRNA) sequences, had been described previously [23]. The oligonucleotides targeting ST6GalNAc I or the negative control were annealed and ligated into pGPU6/neo vector (GenePharma), respectively. Hca-F cells were transfected with the mixture of plasmids and Lipofectamine2000™ (Invitrogen) according to the manufacturer’s recommendation.
Cell viability assay
Cells (5 × 103 cells/well) were plated in 96-well plates overnight at 37 °C in 5 % CO2. After 24 h, 10 μl of CCK8 solution (Cell counting kit-8, Dojindo) was added to each well following the manufacturer’s instructions. The wavelength for measuring the absorbance is 450 nm, for the supernatant of each well using the plate reader Multiskan EX. The numbers of Hca-F, Hca-F/shNC, and Hca-F/shST6GalNAc I cells were counted over a period of 3 days. Growth rate = (cell counts at day x − cell counts at day 1) / cell counts at day 1 × 100 %. All experiments were repeated in triplicate.
Colony formation assay in soft agar
In brief, a bottom layer (0.6 % low-melt agarose, Sigma) was prepared with culture media. A top layer (0.3 % agar, Sigma) was prepared with culture media containing 5000 indicated cells. Plates were incubated at 37 °C in 5 % CO2 in a humidified incubator for about 2 weeks. The cell colonies were stained for 20 min with a solution containing 0.5 % crystal violet and 25 % methanol. The plates were then scanned and photographed, and the number of colonies was quantified by Quantity one v.4.0.3 software (Bio-Rad).
Subcutaneous tumorigenicity test
All experiments involving animals were performed in accordance with the Guide for the Care and Use of Laboratory Animals. A total of 615 mice (Hca-F and Hca-P tumor host) were obtained from the Animal Facility of Dalian Medical University. Inbred 615 mice, weighing 20 to 23 g, were used for this study. The 615 mice were injected subcutaneously in the right flank with 1.0 × 106 Hca-F cells, Hca-F/shST6GalNAc I cells, and Hca-F/NC cells. Mice weight and tumor diameter were measured every other day. After 2 weeks, tumors were isolated from mice, photographed and weighed.
Migration and invasion assay
A Transwell chamber (Corning Corporation) with an 8-μm pore size coated with ECMatrix gel (Chemicon) was used for migration and invasion assay. Cell migration and invasion assay were carried out in a transwell filter on membrane filters. Cells (3 × 105) were harvested in serum-free medium containing 0.1 % BSA and added into the upper chamber. The lower chambers were filled with MEM containing 10 % FBS. After 24 h of incubation, the cells on the upper surface of the filter were completely removed by wiping with a cotton swab. Then, the filters were fixed in methanol and stained with Wright–Giemsa. Cells that migrated through the membrane and reached the lower surface of the filter were counted using a light microscope. Triplicate samples were acquired and the data were expressed as the average cell number of five fields.
Immunohistochemistry
Mouse subcutaneously nodules were selected, fixed, embedded with paraffin, and cut into sections. Slides were deparaffinized in xylene, followed by treatment with a graded series of alcohol washes, rehydration in PBS (pH 7.5). After blocking endogenous peroxidase activity, slides were incubated with the primary antibody against STn (1:50 dilution; Abcam) at 4 °C overnight. After washing with PBS, biotinylated antibody (1:50 dilution; Vectastain Universal Elite ABC Kit, Vector Laboratories) was added to tissue sections. The negative control (blank) sections were also prepared by replacing the primary antibody with preimmune sera. STn was visualized with 3,3-diaminobenzidine (DAB), the chromogenic substrate for peroxidase. Hematoxylin was used to counter stain the nuclei.
Statistical analysis
All data were expressed as the mean ± SD. Statistical analysis was performed with SPSS 13.0 software. One-way ANOVA with post hoc Tukey’s test was performed for experiments that involved more than two groups, and Student’s t test was performed for comparisons between two groups. P < 0.05 was considered to be statistically significant.
Results
Expression of STn and ST6GalNAc I in hepatocarcinoma cell lines
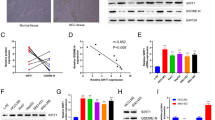
To explore the roles of ST6GalNAc I in hepatocarcinoma, we evaluated the expression of STn and ST6GalNAc I in Hca-F, Hca-P, and Hepa1-6 hepatocarcinoma cell lines with high, low, and no metastatic potential, as compared with normal mouse liver cell line IAR-20. Immunofluorescence staining and flow cytometry assays showed that the STn expression was higher in Hca-F and Hca-P cells, and almost negative in Hepa1-6 and IAR20 cells (Fig. 1a, b). A relatively high expression of ST6GalNAc I mRNA was detected in Hca-F and Hca-P cells, and a low expression was observed in Hepa1-6 and IAR20 cells (Fig. 1c, *P < 0.05). As determined by Western blotting, ST6GalNAc I expression in Hepa1-6 and IAR20 cells was substantially reduced as compared with the expression in Hca-F and Hca-P cells (Fig. 1d, *P < 0.05). Thus, these results indicate that ST6GalNAc I expression might be correlated with the malignant phenotypes of mouse hepatocarcinoma cells.
Expression of STn and ST6GalNAc I in mouse hepatocarcinoma cell lines and normal liver cells. a Immunofluorescence staining analysis of the expression of STn in Hca-F, Hca-P, Hepa1-6, and IAR20 cells. b Flow cytometry assay for analyzing the expression of STn in different cells. c The mRNA level of ST6GalNAc I was measured using real-time PCR with RNA extracted from different cells (*P < 0.05). d Western blot analysis of the expression of ST6GalNAc I in different cells. Relative signal intensities of ST6GalNAc I protein levels as compared with GAPDH were analyzed by LabWorks (TM ver4.6, UVP, BioImaging systems), respectively (*P < 0.05)
Silencing of the ST6GalNAc I gene by shRNA results in the inhibition of STn expression in Hca-F cells
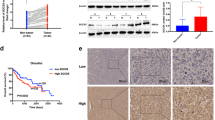
To further evaluate the functional roles of ST6GalNAc I in hepatocarcinoma, ST6GalNAc I was knocked down with ST6GalNAc I-specific shRNA in Hca-F cells that express high levels of ST6GalNAc I. The efficiency of this knockdown was demonstrated using RT-PCR and Western blot assays. The expression levels of ST6GalNAc I mRNA and protein in Hca-F cells transfected with ST6GalNAc I-shRNA (Hca-F/shST6GalNAc I) decreased when compared to the Hca-F and Hca-F/shNC cells (Fig. 2a–b, *P < 0.05). The expression of STn was also significantly inhibited in Hca-F/shST6GalNAc I cells compared to control cells (Fig. 2c–d). Thus, ST6GalNAc I-shRNA transfection could effectively inhibit the ST6GalNAc I mRNA and protein expression, and suppress STn levels in Hca-F cells.
Silencing of the ST6GalNAc I gene by shRNA results in the inhibition of STn expression in Hca-F cells. a, b Knockdown efficiency of ST6GalNAc I in Hca-F cells was analyzed by RT-PCR and Western blot assays. shNC negative control, shST6GalNAc I ST6GalNAcI-specific short hairpin RNA. Relative signal intensities of ST6GalNAc I protein levels as compared with GAPDH were analyzed by LabWorks (TM ver4.6, UVP, BioImaging systems), respectively (*P < 0.05). c Immunofluorescence staining analysis of the expression of STn in Hca-F, shNC, and shST6GalNAc I cells. d FACS analysis for the expression of STn in Hca-F, shNC, and shST6GalNAc I cells. The data were obtained from three independent experiments (*P < 0.05)
ST6GalNAc I knockdown inhibits the growth of Hca-F cells in vitro and in vivo
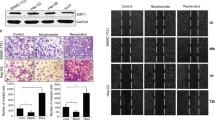
To investigate the effect of ST6GalNAc I knockdown on the growth of Hca-F cells, CCK8 and colony formation assays were conducted. As shown in Fig. 3a, the growth rates of the Hca-F/shST6GalNAc I cells were lower than those of Hca-F and Hca-F/shNC cells over a period of 3 days (P < 0.05). Colony numbers of Hca-F cells transfected with ST6GalNAc I-shRNA were markedly lower than those of the non-transfected cells and negative control cells (Fig. 3b, *P < 0.05). In vivo assay showed that the weight of tumor was obviously decreased in Hca-F/shST6GalNAc I cells (Fig. 3c, *P < 0.05). To provide evidence that the inhibition of tumor growth by shST6GalNAc I was due to its ability to downregulate the expression of STn in vivo, a separate study was accomplished to analyze STn expression levels in tumor tissues by IHC analysis. As shown in Fig. 3d, e, STn expression levels in Hca-F/shST6GalNAc I cells were less than those in control cells. These results suggest that ST6GalNAc I knockdown significantly inhibits the proliferation capability of Hca-F cells in vitro and in vivo.
ST6GalNAc I silencing inhibits the proliferation and clonogenicity of Hca-F cells. a Cell proliferation was determined by the CCK8 assay. The numbers of Hca-F, shNC, and shST6GalNAc I cells were counted over a period of 3 days. The results represent mean values from three independent experiments ± SD. P < 0.05. b Representative field photograph by colony-forming assay of Hca-F, shNC, and shST6GalNAc I cells (×100). Each value is the mean ± standard deviation (SD) of ten different fields (*P < 0.05). c Weight of tumor was compared between Hca-F/shST6GalNAc I and control cells. Each value is the mean ± SD (*P < 0.05). d, e HE staining for tumor tissues and STn expression in tumor tissue were assessed by immunohistochemistry. The data were obtained from three independent experiments
ST6GalNAc I knockdown inhibits the migration and invasion ability of Hca-F cells
To analyze the roles of ST6GalNAc I in cell migration and invasion, transwell chamber with 8-mm polyester membrane filter coated without or with ECMatrixgel was used for migration and invasion assay, respectively. The number of Hca-F/shST6GalNAc I cells migrating through the filter was markedly lower than the number of Hca-F and control cells (Fig. 4a, b, *P < 0.05).In addition, we found that less Hca-F/shST6GalNAc I cells penetrated the Matrigel-coated membrane when compared with the number of invading Hca-F and Hca-F/shNC cells (Fig. 4c, d, *P < 0.05). Collectively, these results indicate that ST6GalNAc I knockdown can inhibit the migration and invasion ability of Hca-F cells.
ST6GalNAc I knockdown inhibits the migration and invasion ability of Hca-F cells. a The migrated ability of cells was assessed using 24-well Transwell units with 8-mm pore size polycarbonate inserts. b The number of migrated cells is displayed on a bar diagram. Results are represented as means ± SD from three independent experiments (*P < 0.05). c The invasive ability of cells was assessed using 24-well Transwell units with 8-mm pore size polycarbonate inserts and Matrigel as a basement membrane. d The number of invaded cells is displayed on a bar diagram. Results are represented as means ± SD from three independent experiments (*P < 0.05)
ST6GalNAc I silencing inhibits the PI3K/AKT signaling in Hca-F cells
To explore the potential mechanisms underlying the poor migration and invasion of Hca-F/shST6GalNAc I cells, we analyzed the relative levels of PI3K/AKT molecules and metastatic regulators in Hca-F, Hca-F/shNC, and Hca-F/shST6GalNAc I cells by Western blot assay. The results showed that the relative levels of PI3k, p-Akt473, p-Akt308, and NF-κB expression were significantly lower in Hca-F/shST6GalNAc I cells than those in Hca-F and Hca-F/shNC cells, whereas the levels of total Akt remained unchanged (Fig. 5a, b, *P < 0.05). Furthermore, significantly reduced levels of MMP-2, MMP-7, MMP-9, and VEGF were also detected in Hca-F/shST6GalNAc I cells compared to Hca-F and Hca-F/shNC cells (Fig. 5c, d, *P < 0.05). Totally, these results indicate that ST6GalNAc I knockdown inhibits the malignant phenotypes of Hca-F cells, which might be attributed to the regulation of PI3K/AKT signaling pathway.
Effect of ST6GalNAc I knockdown on the expression of PI3K/AKT signaling pathway. a, c The relative levels of PI3K/AKT protein expression in Hca-F, Hca-F/shNC, and Hca-F/shST6GalNAc I cells were estimated by Western blot analysis. b, d Relative signal intensities of proteins as compared with GAPDH were analyzed by LabWorks (TM ver4.6, UVP, BioImaging systems), respectively. Data shown is representative of three independent experiments (*P < 0.05)
Discussion
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related mortality, and the poor prognosis of patients with HCC largely results from the distant metastasis after curative resection [24]. However, the molecular mechanisms underlying metastasis of hepatocarcinoma are still poorly understood. Currently, there has been little focus on the role of sialyltransferases in the tumorigenesis and development of hepatocarcinoma. This reason raises our interest in the identification of ST6GalNAc I and STn changes in hepatocarcinoma cells with different metastatic properties, and the analysis of their roles in the malignant phenotypes of carcinoma cells.
The sialyltransferases are enzymes that transfer sialic acid from the activated CMP-NeuAc to terminal positions on sialylated glycolipids (gangliosides) or to the N- or O-linked glycan chains of glycoproteins [25]. Sialyltransferases are subdivided into four families: ST3Gal, ST6Gal, ST6GalNAc, and ST8Sia. ST6GalNAc I (GalNAcα2,6-sialyltransferase) is an enzyme that sialylates O-linked glycoproteins. In this study, we found that the expression of ST6GalNAc I was at high levels in mouse hepatocarcinoma Hca-F, Hca-P, and Hepa1-6 cells compared with normal liver IAR20 cells. In addition, Hca-F and Hca-P cells have higher expression of ST6GalNAc I than Hepa1-6 cells. According to this finding, we speculate that ST6GalNAc I might play a key role in promoting malignant progression of hepatocarcinoma cells.
The overall increased sialylation on cell surface plays a role in cancer metastasis through the increased expression of the associated glycosyltransferases [26, 27]. ST6GalNAc I as the major enzyme controls the expression of carcinoma-associated STn in gastrointestinal tissues, gastric carcinoma [19, 21], and prostate cancer cells [13]. In this study, we demonstrated that transfection with ST6GalNAc I sh RNA in Hca-F cells significantly inhibited the expression of STn. It was reported that STn may play a role in initiating colorectal carcinogenesis and assist in evaluating malignant status of colorectal lesions [28]. Our data suggests that downregulated expression of STn inhibited the capability of proliferation, migration, and invasion of Hca-F cells. Thus, the expression of the cancer-associated STn mediates malignant phenotypes of cancer cells, and the synthesis of STn depends on the expression of ST6GalNAc I. However, it still deserves further study that the target of ST6GalNAc I as the single sialyltransferase primarily associated with generation of STn.
Overexpression of ST6GalNAc I was associated with the growth, invasion, and migratory capacity of gastric carcinoma cells [21]. Lo CY et al. found that overexpression of glycoprotein-specific ST6GalNAc-transferases altered cell adhesion properties [29]. We found that ST6GalNAc I knockdown inhibited the growth and proliferation of Hca-F cells in vivo and in vitro. Our study also demonstrated that ST6GalNAc I silencing significantly inhibited the migration and invasion ability of Hca-F cells. Thus, these results provide a basis that regulation of ST6GalNAc I expression has a direct impact on cancer development of hepatocarcinoma cells including proliferation, migration, and invasion ability.
Previous studies have reported that an increased level of PI3K/AKT is correlated with proliferation, progression, and metastasis of hepatocarcinoma [30, 31]. STn and its precursor Tn were found to be marker of poor prognosis in bladder carcinoma, and the activation of the PI3K/Akt/mTOR pathway is cancer-associated events [32]. The activation of PI3K/AKT signaling pathway can induce the expression of MMP-2, MMP-7, MMP-9 via regulating the transcriptional activation of NF-κB in many cell lines [33, 34]. The present study showed that ST6GalNAc I silencing inhibited the expression levels of PI3k/Akt signaling and their downstream molecules in hepatocarcinoma cells. However, further researches are needed to investigate the detailed molecular mechanisms by which ST6GalNAc I regulates the PI3K/AKT signaling in hepatocarcinoma cells.
In conclusion, ST6GalNAc I was involved in proliferation, migration, and invasion of hepatocarcinoma cells in vitro and in vivo through the regulation of the PI3K/AKT signaling and MMPs expressions. These findings suggest a potential targeting role of ST6GalNAc I in predicting the invasive property of hepatocarcinoma.
Change history
02 September 2021
Publisher’s Note: An erratum to this article is available online at https://doi.org/10.3233/TUB-219003
References
Fitzmaurice C, Dicker D, Pain A, Hamavid H, Moradi-Lakeh M, MacIntyre MF, et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015;1:505–27.
Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907–17.
Zhang J, Sun MZ, Li RK, Liu SQ, Mao J, Huang YH, et al. Ech1 is a potent suppressor of lymphatic metastasis in hepatocarcinoma. Biomed Pharmacother. 2013;67:557–60.
Zhang J, Song MY, Wang JW, Sun MZ, Li RK, Huang YH, et al. Enoyl coenzyme A hydratase 1 is an important factor in the lymphatic metastasis of tumors. Biomed Pharmacother. 2011;65:157–62.
Schneider F, Kemmner W, Haensch W, Franke G, Gretschel S, Karsten U, et al. Overexpression of sialyltransferase CMP-sialic acid: Galbeta1, 3GalNAc-R alpha6-Sialyltransferase is related to poor patient survival in human colorectal carcinomas. Cancer Res. 2001;61:4605–11.
Campos D, Freitas D, Gomes J, Magalhães A, Steentoft C, Gomes C, et al. Probing the O-glycoproteome of gastric cancer cell lines for biomarker discovery. Mol Cell Proteomics. 2015;14(6):1616–29.
Ricardo S, Marcos-Silva L, Pereira D, Pinto R, Almeida R, Söderberg O, et al. Detection of glyco-mucin profiles improves specificity of MUC16 and MUC1 biomarkers in ovarian serous tumours. Mol Oncol. 2015;9(2):503–12.
Leivonen M, Nordling S, Lundin J, von Boguslawski K, Haglund C. STn and prognosis in breast cancer. Oncology. 2001;61:299–305.
Ferreira JA, Videira PA, Lima L, Pereira S, Silva M, Carrascal M, et al. Overexpression of tumour-associated carbohydrate antigen sialyl-Tn in advanced bladder tumours. Mol Oncol. 2013;7:719–31.
Carrascal MA, Severino PF, Guadalupe Cabral M, Silva M, Ferreira JA, Calais F, et al. Sialyl Tn-expressing bladder cancer cells induce a tolerogenic phenotype in innate and adaptive immune cells. Mol Oncol. 2014;8:753–65.
Ozaki H, Matsuzaki H, Ando H, Kaji H, Nakanishi H, Ikehara Y, et al. Enhancement of metastatic ability by ectopic expression of ST6GalNAcI on a gastric cancer cell line in a mouse model. Clin Exp Metastasis. 2012;29:229–38.
Loureiro LR, Carrascal MA, Barbas A, Ramalho JS, Novo C, Delannoy P, et al. Challenges in antibody development against Tn and Sialyl-Tn antigens. Biomolecules. 2015;5:1783–809.
Munkley J, Oltean S, Vodák D, Wilson BT, Livermore KE, Zhou Y, et al. The androgen receptor controls expression of the cancer-associated sTn antigen and cell adhesion through induction of ST6GalNAc1 in prostate cancer. Oncotarget. 2015;6:34358–74.
Kurosawa N, Takashima S, Kono M, Ikehara Y, Inoue M, Tachida Y, et al. Molecular cloning and genomic analysis of mouse GalNAc alpha2, 6-sialyltransferase (ST6GalNAc I). J Biochem. 2000;127:845–54.
Vandermeersch S, Vanbeselaere J, Delannoy CP, Drolez A, Mysiorek C, Guérardel Y, et al. Accumulation of GD1α ganglioside in MDA-MB-231 breast cancer cells expressing ST6GalNAc V. Molecules. 2015;20:6913–24.
Senda M, Ito A, Tsuchida A, Hagiwara T, Kaneda T, Nakamura Y, et al. Identification and expression of a sialyltransferase responsible for the synthesis of disialylgalactosylgloboside in normal and malignant kidney cells: downregulation of ST6GalNAc VI in renal cancers. Biochem J. 2007;402:459–70.
Ren D, Jia L, Li Y, Gong Y, Liu C, Zhang X, et al. ST6GalNAcII mediates the invasive properties of breast carcinoma through PI3K/Akt/NF-kB signaling pathway. IUBMB Life. 2014;66:300–8.
Marcos NT, Bennett EP, Gomes J, Magalhaes A, Gomes C, David L, et al. ST6GalNAc-I controls expression of sialyl-Tn antigen in gastrointestinal tissues. Front Biosci (Elite Ed). 2011;3:1443–55.
Marcos NT, Pinho S, Grandela C, Cruz A, Samyn-Petit B, Harduin-Lepers A, et al. Role of the human ST6GalNAc-I and ST6GalNAc-II in the synthesis of the cancer-associated sialy-Tnantigen. Cancer Res. 2004;64:7050–7.
Chu H, Zhou H, Liu Y, Liu X, Hu Y, Zhang J. Functional expression of CXC chemokine recepter-4 mediates the secretion of matrix metalloproteinases from mouse hepatocarcinoma cell lines with different lymphatic metastasis ability. Int J Biochem Cell Biol. 2007;39:197–205.
Tamura F, Sato Y, Hirakawa M, Yoshida M, Ono M, Osuga T, et al. RNAi-mediated gene silencing of ST6GalNAc I suppresses the metastatic potential ingastric cancer cells. Gastric Cancer. 2016;19:85–97.
Yu S, Zhang L, Li N, Fan J, Liu L, Zhang J, et al. Caveolin-1 up-regulates ST6Gal-I to promote the adhesive capability of mouse hepatocarcinoma cells to fibronectin via FAK-mediated adhesion signaling. Biochem Biophys Res Commun. 2012;427:506–12.
Wang S, Chen X, Wei A, Yu X, Niang J. α2, 6-linked sialic acids on N-glycansnodulate the adhesion of hepatocarcinoma cells to lymph no des. Tumor Biol. 2015;36:885–92.
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–86.
Li Y, Chen X. Sialic acid metabolism and sialyltransferases: natural functions and applications. Appl Microbiol Biotechnol. 2012;94:887–905.
Julien S, Adriaenssens E, Ottenberg K, Furlan A, Courtand G, Vercoutter-Edouart AS, et al. ST6GalNAc I expression in MDA-MB-231 breast cancer cells greatly modifies their O-glycosylation pattern and enhances their tumourigenicity. Glycobiology. 2006;16:54–64.
Dimitroff CJ. Galectin-binding O-glycosylations as regulators of malignancy. Cancer Res. 2015;75:3195–202.
Xu F, Fan C, Fan S, Liu F, Wen T, An G, et al. Expression profile of mucin-associated sialyl-Tn antigen in Chinese patients with different colorectal lesions (adenomas, carcinomas). Int J Clin Exp Pathol. 2015;8:11549–54.
Lo CY, Antonopoulos A, Gupta R, Qu J, Dell A, Haslam SM, et al. Competition between core-2 GlcNAc-transferase and ST6GalNAc-transferase regulates the synthesis of the leukocyte selectin ligand on human P-selectin glycoprotein ligand-1. J Biol Chem. 2013;288:13974–87.
Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951–8.
Saxena NK, Sharma D, Ding X, Lin S, Marra F, Merlin D. Anania FA concomitant activation of the JAK/STAT, PI3K/AKT, and ERK signaling is involved in leptin-mediated promotion of invasion and migration of hepatocellular carcinoma cells. Cancer Res. 2007;67:2497–507.
Costa C, Pereira S, Lima L, Peixoto A, Fernandes E, Neves D, et al. Abnormal protein glycosylation and activated PI3K/Akt/mTOR pathway: role in bladder cancer prognosis and targeted therapeutics. PLoS One. 2015;16(10):e0141253.
Jin J, Shen X, Chen L, Bao LW, Zhu LM. TMPRSS4 promotes invasiveness of human gastric cancer cells through activation of NF-kB/MMP-9 signaling. Biomed Pharmacother. 2016;77:30–6.
Li C, Li F, Zhao K, Yao J, Cheng Y, Zhao L, et al. LFG-500 inhibits the invasion of cancer cells via down-regulation of PI3K/AKT/NF-kB signaling pathway. PLoS One. 2014;9(3):e91332.
Acknowledgments
This work was supported by grants from the Natural Science Foundation of China (31470799) and the Natural Science Foundation of Liaoning Province (2014023032).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None.
Additional information
Xiao Yu and Qiang Wu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Yu, X., Wu, Q., Wang, L. et al. Silencing of ST6GalNAc I suppresses the proliferation, migration and invasion of hepatocarcinoma cells through PI3K/AKT/NF-κB pathway. Tumor Biol. 37, 12213–12221 (2016). https://doi.org/10.1007/s13277-016-5086-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-016-5086-y