Abstract
Proliferating cell nuclear antigen (PCNA) has been suggested as a potential diagnostic biomarker for early hepatocellular carcinoma (HCC). However, its prognostic significance in HCC remains unclear. In the present study, we investigated the expression and significance of PCNA in HCC and then analyzed the role of PCNA in clinical outcomes. Our findings show that the expression intensity of PCNA is much higher in HCC tissues than that in paracarcinoma tissues and associated with AFP, albumin, tumor number, clinical grade, vascular invasion, and tumor-node-metastasis (TNM) stage (all p < 0.000). Kaplan-Meier analysis indicated that high PCNA expression was associated with poor disease-free survival (DFS) (p < 0.000) and overall survival (OS) (p < 0.000) in a training cohort of 76 HCC patients. Multiple Cox regression analysis indicated PCNA acts as an independent predictor for DFS (p = 0.002) and OS (p = 0.004) in HCC patients. Along with pathological results, our systematic review also identified the expression of PCNA was closely associated with DFS and OS (both p < 0.000). In conclusion, this study suggested that PCNA is increased in HCC patients and is indeed a novel unfavorable biomarker for prognostic prediction for patients with this deadly disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hepatocellular carcinoma is the second leading cause of cancer-related death worldwide [1]. Long-term clinical results demonstrated by our team suggest that liver resection is still considered as the first choice and the most effective “curative” treatment for hepatocellular carcinoma (HCC), especially for solitary large HCC [2]. However, the overall prognosis of HCC is still far from satisfactory due to high incidences of tumor recurrence and metastasis [2, 3], with a 5-year recurrence rate of approximately 60 % after hepatic resection in our and other centers [2, 4]. Therefore, it is important to further study the molecular mechanisms underlying metastasis of HCC and to research novel prognostic biomarkers for HCC.
Proliferating cell nuclear antigen, an essential regulator of the cell cycle, is a 36-kDa molecule, which is highly conserved among species. Proliferating cell nuclear antigen (PCNA) is an evolutionally well-conserved protein and found in all eukaryotic species from yeast to humans [5, 6]. Its functions are related to vital cellular processes such as DNA replication, chromatin remodeling, DNA repair, sister chromatid cohesion, and cell cycle control [7]. The role and interaction of PCNA are modulated by posttranslational regulation, whose exact mechanisms are controversial and not completely understood [8, 9]. Many reports showed posttranslational regulation of PCNA modifications, including phosphorylation, acetylation, and methyl esterification. In addition, PCNA is important to determine its role in proliferative activity in different tumors including HCC. However, the diagnostic role of PCNA in clinical outcome of HCC patients remains controversial. Thereby, it is essential to conduct a complete in vitro analysis and reviews.
In this study, the expression of PCNA in HCC was examined. The relationship between PCNA expression and clinicopathological features was investigated. The role of PCNA in HCC prognosis was assessed. Our results reveal that PCNA is noticeably upregulated in HCC and significantly correlated with unfavorable prognosis.
Material and methods
Patients, specimens, and follow-up
This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute and complied with the Declaration of Helsinki. All of the patients provided written informed consent. Patient tissue samples, 76 paired primary HCC tumor and non-tumorous tissue samples, were collected immediately after surgery resection at Shandong Cancer Hospital and Institute between December 2010 and September 2011. The enrollment criteria were as follows: (a) definitive HCC diagnosis by pathology based on WHO criteria, (b) no preoperative trans-hepatic arterial chemoembolization or chemotherapy or radiotherapy before surgery, (c) surgical resection, defined as the complete resection of all tumor nodules with the cut surface being free of cancer by histologic examination, and (d) complete clinicopathologic and follow-up data. In this study, non-tumoral liver tissues were defined as 2.0 cm from the tumor margin, which had been described previously. Hepatitis B history was defined as history with positive serum hepatitis B surface antigen (HBsAg). Tumor encapsulation was defined as the presence of a clear fibrous sheath around the tumor at gross inspection. Tumor differentiation was based on the Edmondson and Steiner classification. HCC metastasis was defined as the presence of vascular invasion in the portal vein or the presence of satellite nodules surrounding a larger main tumor. Tumor staging was determined according to the 7th edition tumor-node-metastasis (TNM) classification of the American Joint Committee on Cancer.
Western blot
Total proteins were extracted and separated by 10 % SEMS-PAGE and then transferred onto polyvinylidene difluoride (PVDF) membrane (Millipore, Bedford, MA) as described previously [10]. Equal amounts of protein (30 μg) were resolved by SDS-PAGE and then electrophoretically transferred onto PVDF membranes. After being blocked in 5 % non-fat milk 1 h at room temperature, the membranes were incubated with appropriately diluted primary antibodies overnight at 4 °C. After being washed thrice with TBST, the blotted membranes were incubated with anti-PCNA (1:1000, Sigma, Cambridge, England). The membranes were incubated with HRP-conjugated secondary antibody at 1:20,000 dilutions for 1 h at room temperature. The membranes were visualized by the enhanced Phototope TM-HRP Detection Kit and exposed to Kodak medical X-ray processor (Carestream Health, USA). Anti-GAPDH (1:1000, Santa Cruz, CA, USA) was used as a loading control.
Literature search
A literature search was carried out using MEDLINE, PubMed, WANFANG, and CNKI databases up to August 2015. There was no restriction of origin and languages. Search terms included the following: “PCNA,” “HCC [MeSH],” “prognosis or prognostic or outcome,” “survival,” etc. All references in retrieved articles were scanned to identify other potentially applicable reports. All searched studies were retrieved and the bibliographies were reviewed for other relevant publications. Review articles and bibliographies of other relevant studies identified were searched manually to identify additional eligible studies. We tried to identify potential relevant studies from the whole reference lists by orderly reviewing title, abstract, and full text.
Selection criteria and data extraction
Two reviewers independently selected eligible studies. Disagreement between the two reviewers was settled by discussion with the third reviewer. Inclusion criteria were as follows: (1) the patients were confirmed the diagnosis of HCC, who underwent surgical resection; (2) the main outcome of interest focuses on disease-free survival (DFS), overall survival (OS), and other clinicopathological indicators; (3) PCNA expression status was detected by immunohistochemistry (IHC), RT-PCR, and Western blot; (4) the value of hazard ratio (HR), OR, and 95 % confidence interval (CI) between PCNA expression and the survival status could be obtained from the literature directly or recalculated based on the figure and table in articles; (5) for duplicate articles, only the most complete and/or recently published one was included.
Statistical analysis
Statistical analyses were performed using the SPSS 16.0 software (SPSS, Chicago, IL). Wilcoxon matched pairs test was used to determine the significant difference. Student t test was performed to analyze the correlation between PCNA expression and clinicopathological parameters. Kaplan-Meier analysis (log-rank test) was conducted for survival analysis and univariate analysis. Independent analyses were performed according to the selected population: overall population and different morphological and pathological subgroups. Cox proportional hazards regression model was used to identify the independent prognostic factors. Statistical significance was set at p < 0.05.
As for systematic review, the HR with 95 % CI was estimated for each study. A chi-square-based Q statistic test was performed to assess heterogeneity across the studies. p < 0.10 indicated obvious between-study heterogeneity.
Results
Clinicopathologic characteristics of HCC patients
As shown in Table 1, the study included 63 men and 13 women with an average age of 52.0 years (range, 19–78). The adjacent non-tumor liver had liver cirrhosis in 63 (82.9 %) cases (Table 1). Solitary tumor was detected in 53 (69.7 %) patients, and multiple tumors in 23 (30.2 %) patients. Thirty-four (44.7 %) patients had HCC with vascular invasion. The size of the majority of tumor (56.6 %) was less than 5 cm. Forty-three HCC patients (56.6 %) were diagnosed with TNM II–III stage. Overall, most of the HCC patients had positive common markers, including AFP, TBil, and albumin.
Expression of PCNA in HCC samples
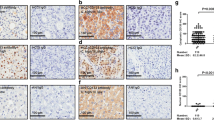
To determine the expression of PCNA in HCC samples, 76 pairs of tumor samples and the corresponding adjacent non-tumorous samples were collected and subjected to Western blot assay. Results of Western blotting showed that the expression intensity of PCNA protein in HCC carcinoma samples was markedly increased when compared with the paracarcinoma samples (Fig. 1a). The mean intensity of PCNA in HCC carcinoma tissues was 0.756 ± 0.112, while that in non-tumorous samples was 0.221 ± 0.083 (Fig. 1b). The difference between carcinoma samples and non-tumorous samples was significant (p < 0.001).
PCNA expression is increased in HCC samples by Western blot. a Human HCC carcinoma and normal tissues were detected using Western blot. Protein levels of PCNA in carcinoma samples (C) and corresponding adjacent non-tumorous samples (N) were examined in nine pairs of HCC fresh tissues by Western blot. b PCNA protein expression normalized to GAPDH was calculated as semi-quantity analysis. The measurements were performed in triplicate, and data are presented as means ± SD. *p < 0.001 vs. control; using Student t test
Association of PCNA expression and clinical outcome in HCC
To disclose the clinical value of PCNA in HCC patients, the relationship between PCNA expression and the clinical variables of HCC patients was analyzed. As shown in Table 1, no significant associations were depicted between PCNA expression and the clinicopathological parameters, including age, gender, HBsAg, TBil, tumor size, and capsular formation (all p > 0.05). On the other hand, significant associations were found between PCNA expression and AFP (p < 0.000), albumin (p < 0.000), tumor number (p < 0.000), clinical grade (p < 0.000), vascular invasion (p < 0.000), and TNM stage (p < 0.000), indicating PCNA expression has some significances in the development of HCC. Therefore, the prognostic implication of PCNA in HCC was next determined.
Based on the mean intensity of PCNA in normal samples, patients were divided into two groups defined as low PCNA expression (less than the mean intensity of PCNA in normal samples) and high PCNA expression (more than the mean intensity of PCNA in normal samples). Randomly, 76 patients with HCC were separated into two cohorts. The DFS and OS rates for 76 HCC patients were 26.3 and 34.2 % at 5 years. The 5-year DFS rate of the high PCNA expression group was significantly lower than that of the low PCNA expression group (16.0 vs. 46.2 %) (p < 0.000) (Fig. 2a). The mean DFS was 24.2 months for the high PCNA expression group and 39.5 months for the low PCNA expression group. The 5-year OS rate of the high PCNA expression group was significantly lower than that of the low PCNA expression group (24.0 vs. 53.8 %) (p < 0.000) (Fig. 2b). The mean OS was 24.9 months for the high PCNA expression group and 43.3 months for the low PCNA expression group.
High PCNA expression is correlated with unfavorable prognosis in HCC patients. Kaplan-Meier analysis disclosed the significant differences in disease-free survival (a) and overall survival (b) between postoperative patients with high and low PCNA expression in overall (n = 76) cohort. *p < 0.000 vs. control; using log-rank test
Prediction factors of disease-free survival in HCC patients
As shown in Table 2, in univariate analysis, in addition to identified PCNA association with DFS, DFS also showed significant correlations with tumor number (p = 0.002), clinical grade (p = 0.031), vascular invasion (p = 0.008), TNM stage (p = 0.002), capsular formation (p = 0.035), and satellite nodules (p = 0.028). To seek independent factors, we conducted the multivariate analysis and found that tumor number (p = 0.008), clinical grade (p = 0.033), vascular invasion (p = 0.005), TNM stage (p = 0.003), and satellite nodules (p = 0.048) were independent predictors of DFS. Importantly, we validated that high PCNA expression also acts as an independent predictors of DFS (p = 0.002).
Prediction factors of overall survival in HCC patients
According to univariate analysis, tumor number (p = 0.006), clinical grade (p = 0.029), vascular invasion (p = 0.022), tumor size (p = 0.042), TNM stage (p = 0.001), capsular formation (p = 0.031), and satellite nodules (p = 0.019) showed unfavorable effects on OS (Table 3). In multivariate analysis, tumor number (p = 0.009), clinical grade (p = 0.009), vascular invasion (p = 0.006), TNM stage (p = 0.002), capsular formation (p = 0.018), and satellite nodules (p = 0.009) were independent predictors of shorter OS (Table 3). Also, high PCNA expression was an independent predictor of shorter OS (p = 0.004), indicating patients with high PCNA expression were more likely to suffer from death than those with low PCNA expression.
Systematic review about PCNA expression
In previous studies, we found that the significance of PCNA in DFS or OS of HCC patients was controversial. In the present study, to determine the role of PCNA expression in pathological diagnosis, we investigated the association of PCNA expression with prognosis. Table 4 shows the eight included studies on PCNA and DFS/OS. We used systematic review to assess the HR and 95 % CI of DFS and OS. Our results revealed that high PCNA expression was associated with DFS (HR = 2.779, p < 0.000) and OS (HR = 3.266, p < 0.000) in HCC patients (Table 5).
Discussion
Despite the combined efforts of surgeons and scientists worldwide, there is a constant increase in the incidence of hepatocellular carcinoma during the last two decades [19]. Successful HCC treatment requires an adequate therapeutic index reflecting the specific treatment effects on target cells. Cellular proliferation is one of the important indexes for the biologic aggressiveness of a malignant lesion. The dysregulated proliferation may be a significant change to determine the potential prognosis of various malignant tumors. PCNA is a 36-kDa protein involved in several cellular mechanisms, including DNA synthesis and repair, cell cycle regulation, and apoptosis [5, 7]. An alteration in PCNA structure might contribute to DNA damage accumulation in cancer cells [10]. The present study was aimed at evaluating the PCNA expression pattern in HCC patients.
In the present study, results of Western blotting showed that the expression intensity of PCNA protein in HCC carcinoma samples was markedly increased when compared with the paracarcinoma samples. Similar to our study, the highly valuable tumor molecular markers PCNA had higher expression levels in NSCLC samples, which may be important for the early diagnosis of lung cancer and individualized therapy, having also an important role in predicting tumor prognosis [20]. Our result was also important for the early diagnosis of HCC. Besides, we observed significant associations were found between PCNA expression and AFP, albumin, tumor number, clinical grade, vascular invasion, and TNM stage, indicating PCNA expression has some significance in the development of HCC.
As reported, PCNA index can be used to assess the proliferation and aggressiveness in dysplasia and different grades oral squamous cell carcinoma [21]. Lan et al. assessed the intensity of immunoreactivity of PCNA and correlated it with tumor differentiation, nuclear atypia, and the patterns of invasive margins in the underlying connective tissue. Increased expression of PCNA was indicative of poor differentiation, higher nuclear atypia, and more invasive growth of tumor cells [22]. Using systematic review, Lv et al. suggested PCNA expression is significantly associated with poor 5-year survival, advanced stage, or higher grade, which might be suggested as a useful prognostic and diagnostic biomarker or an effective therapy target in cervical cancer, gliomas, or even more cancers [23]. Thus, we further analyzed the role of PCNA in the prognosis of HCC and found that high PCNA expression also acts as an independent predictor of DFS and OS.
However, recent reports showed different results about PCNA and HCC, which have some conflicts, so we conducted the systematic review to determine the significance of PCNA in HCC. Our results revealed that high PCNA expression was associated with DFS and OS in HCC patients. Our findings determined that PCNA expression predicted a poor prognosis in HCC patients. Mechanically, PCNA was originally recognized as an antigen characteristic of proliferating cells that is expressed in cell nuclei during S phase of the cell cycle. PCNA is a widely recognized cell proliferation marker serving also as a prognostic indicator for a variety of tumors [24, 25].
In conclusion, our findings demonstrated that PCNA is increased in HCC patients and is a novel unfavorable biomarker for prognostic prediction for patients with this deadly disease. It can be concluded that PCNA index along with clinical features can be used for predicting aggressiveness and recurrence rate in more cancers.
References
Allemani C, Weir HK, Carreira H, Harewood R, Spika D, Wang XS, et al. Global surveillance of cancer survival 1995-2009: analysis of individual data for 25,676,887 patients from 279 population-based registries in 67 countries (CONCORD-2). Lancet. 2015;385(9972):977–1010.
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
Li XP, Cao GW, Sun Q, Yang C, Yan B, Zhang MY, et al. Cancer incidence and patient survival rates among the residents in the Pudong New Area of Shanghai between 2002 and 2006. Chin J Cancer. 2013;32(9):512–9.
Altekruse SF, Henley SJ, Cucinelli JE, McGlynn KA. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109(4):542–53.
Stoimenov I, Helleday T. PCNA on the crossroad of cancer. Biochem Soc Trans. 2009;37(Pt 3):605–13.
Wang SC, Nakajima Y, Yu YL, Xia W, Chen CT, Yang CC, et al. Tyrosine phosphorylation controls PCNA function through protein stability. Nat Cell Biol. 2006;8(12):1359–68.
Li Y, Dowbenko D, Lasky LA. AKT/PKB phosphorylation of p21Cip/WAF1 enhances protein stability of p21Cip/WAF1 and promotes cell survival. J Biol Chem. 2002;277(13):11352–61.
Naryzhny SN, Lee H. The post-translational modifications of proliferating cell nuclear antigen: acetylation, not phosphorylation, plays an important role in the regulation of its function. J Biol Chem. 2004;279(19):20194–9.
Hoelz DJ, Arnold RJ, Dobrolecki LE, Abdel-Aziz W, Loehrer AP, Novotny MV, et al. The discovery of labile methyl esters on proliferating cell nuclear antigen by MS/MS. Proteomics. 2006;6(17):4808–16.
Pan Y, Zhang Y, Chen L, Liu Y, Feng Y, Yan J. The critical role of Rab31 in cell proliferation and apoptosis in cancer progression. Mol Neurobiol. 2015. [Epub ahead of print] PMID: 26245486
Ng IO, Lai EC, Fan ST, Ng M, Chan AS, So MK. Prognostic significance of proliferating cell nuclear antigen expression in hepatocellular carcinoma. Cancer. 1994;73(9):2268–74.
Cao J, Hu X, Xie M, Sun J, Cheng H. Expression of proliferating cell nuclear antigen and alpha-fetoprotein in hepatocellular carcinoma and its clinical significance. Pract J Cancer. 1998;13(2):105–7.
Zhang Z, Wu Q, Hua Z, Zhou Z, Jia Z, Wang H. The expression of PCNA CD44v6 in human hepatic carcinoma and its clinical significance. Hainan Med J. 2000;11(6):8–11.
Claudio PP, Russo G, Kumar CA, Minimo C, Farina A, Tutton S, et al. pRb2/p130, vascular endothelial growth factor, p27(KIP1), and proliferating cell nuclear antigen expression in hepatocellular carcinoma: their clinical significance. Clin Cancer Res. 2004;10(10):3509–17.
Qin HX, Nan KJ, Yang G, Jing Z, Ruan ZP, Li CL, et al. Expression and clinical significance of TAp73alpha, p53, PCNA and apoptosis in hepatocellular carcinoma. World J Gastroenterol. 2005;11(18):2709–13.
Zhao Z, Zhan Y, Ni Y, Zhang M, Wang C. Prognosis and the expression of survivin protein and proliferating cell nuclear antigen (PCNA) in hepatocellular carcinoma. Shenzhen J Int Trad Chin West Med. 2007;17(6):355–7.
Hu TH, Wang CC, Huang CC, Chen CL, Hung CH, Chen CH, et al. Down-regulation of tumor suppressor gene PTEN, overexpression of p53, plus high proliferating cell nuclear antigen index predict poor patient outcome of hepatocellular carcinoma after resection. Oncol Rep. 2007;18(6):1417–26.
Gao F, Yang J, Liu B, Ma F, Geng Y, Li J. Expressions of PCNA and Hpa and their correlations with prognosis of patients with hepatocellular carcinoma. Hebei Med J. 2013;35(16):2410–2.
Qiu W, Wang G, Sun X, Ye J, Wei F, Shi X, et al. The involvement of cell surface nucleolin in the initiation of CCR6 signaling in human hepatocellular carcinoma. Med Oncol. 2015;32(3):75.
Li X, Liu X, Cui D, Wu X, Qian R. Clinical significance of nucleostemin and proliferating cell nuclear antigen protein expression in non-small cell lung cancer. J BUON. 2015;20(4):1088–93.
Poosarla C, Ramesh M, Ramesh K, Gudiseva S, Bala S, Sundar M. Proliferating cell nuclear antigen in premalignancy and oral squamous cell carcinoma. J Clin Diagn Res. 2015;9(6):ZC39–41.
Lan HA, Zain RB, Saitoh M, Muramatsu Y, Shrestha P, Mori M. Proliferating cell nuclear antigen and p53 in epithelial dysplasia and squamous cell carcinoma of oral mucosa—a marker for poor tumour differentiation, increasing nuclear atypia and invasiveness. Anticancer Res. 1996;16:3059–65.
Lv Q, Zhang J, Yi Y, Huang Y, Wang Y, Wang Y, et al. Proliferating cell nuclear antigen has an association with prognosis and risks factors of cancer patients: a systematic review. Mol Neurobiol. 2015. [Epub ahead of print] PMID: 26558632
Li J, Li H, Liu J, Feng B, Feng M, Lv B, et al. The clinical implications of human telomerase reverse transcriptase expression in grade and prognosis of gliomas: a systematic review and meta-analysis. Mol Neurobiol. 2015. [Epub ahead of print].
Bowman GD, Goedken ER, Kazmirski SL, O’Donnell M, Kuriyan J. DNA polymerase clamp loaders and DNA recognition. FEBS Lett. 2005;579:863–7.
Acknowledgments
We greatly thank other members of our lab for valuable suggestions and writing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This study was approved by the Ethics Committee of Shandong Cancer Hospital and Institute and complied with the Declaration of Helsinki. All of the patients provided written informed consent.
Rights and permissions
About this article
Cite this article
Ma, S., Yang, J., Li, J. et al. The clinical utility of the proliferating cell nuclear antigen expression in patients with hepatocellular carcinoma. Tumor Biol. 37, 7405–7412 (2016). https://doi.org/10.1007/s13277-015-4582-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4582-9