Abstract
Previous studies proved that Vav3 gene was overexpressed in cancers. However, the molecular mechanism of Vav3 in apoptosis still keeps unclear; therefore, the relationship between Vav3 gene and apoptosis of gastric cancer (GC) was explored in the present study. Vav3-siRNA was transfected into MGC803 cells, and then cell activity and apoptosis rate were tested with MTT and FCM; apoptosis-related genes and proteins in MAPK signaling pathway were also tested. Results showed that Vav3 was overexpressed in GC than in adjacent normal tissues (all P < 0.05), and expression of Vav3 was related to degree of histological differentiation, cancer invasion depth, and lymphatic metastasis (Χ 2 = 7.185, P = 0.007; Χ 2 = 18.654, P < 0.001; Χ 2 = 5.058, P = 0.025). Vav3 silencing inhibited activity of MGC803 cells, and apoptosis rate of cells was affected. Vav3-siRNA transfection led to changes of apoptosis-related genes such as Survivin, xIAP, Bcl-2, caspase-3, and Bax (all P < 0.01). After transfection, ratio of phosphorylation of ERK significantly reduced. We concluded that Vav3 inhibition can suppress cell activity and promote apoptosis by regulating the apoptosis-related genes through the ERK pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastric cancer (GC) is currently the second leading cause of death from all cancers in the world. It is also the leading cause of death from malignant tumors in Eastern Asia including China [1]. Recent studies indicate that GC cells have strong resistance to apoptosis, which is an important factor responsible for poor prognosis; multiple apoptosis-related genes and pathways together with expression products exert control in the development of apoptosis resistance for GC cells [2–6]. And, there were some reports with certain results that GC cell inhibition could be achieved by promoting apoptosis [7–9]. However, there is still no breakthrough in molecular mechanism on apoptosis regulation in GC, so it is of significance to search new apoptosis-regulated genes targeting GC cells for molecular targeted GC therapy.
Members of oncogene Vav family, activator of small GTP kinase in Rho family, could activate RhoA, Racl, Cdc42, and so on. In Vav family, Vav3 plays an important role in the process of tumor development and metastasis [10]. It has been demonstrated that many researches focused on the effect of Vav3 in bladder cancer, lymphoma, and breast cancer, and these researches showed that expression of Vav3 was upregulated in these cancers [11–13]. So, it can be concluded that Vav3 is invovled in carcinogenesis.
Our previous study found that expression of Vav3 was upregulated in GC cells, and when expression of endogenous Vav3 was inhibited, the growth and migration of GC cells was also inhibited [14]. However, expression of Vav3 and its biological function in apoptosis remains halfway in GC, so we observed expression of Vav3 in GC tissues and cell lines in this study. Specific small interfering RNA (siRNA) was employed to knockdown the expression of endogenous Vav3, leading to decreased activity of GC cell line MGC803 and significantly increased apoptosis. The results suggest that overexpressed Vav3 in GC cells inhibits apoptosis of GC.
Materials and methods
Patients and tissue specimens
Fifty-five cases (38 males and 17 females, with a mean age of 61.8 ± 10.2 years) of GC that had been surgically removed and pathologically confirmed were obtained from the Fourth Affiliated Hospital of Hebei Medical University from January 2014 to August 2014. All these patients did not receive preoperative treatment including chemotherapy, radiotherapy, or biotherapy for GC. One piece of tissue (approximately 1.0 × 0.5 × 0.5 cm) from both cancerous and adjacent normal tissues (over 2 cm off the edge of cancerous tissues; it was confirmed pathologically after surgery that no cancer cell was found) was harvested. Each specimen was divided into two parts, from which one part was fixed with 10 % of neutral formalin, embedded with paraffin, and then cut serially with the thickness of 4 μm for immunohistochemistry (IHC); the other part was stored at −80 °C for real-time fluorescent quantitative PCR (qPCR) and Western blotting.
Cell lines and reagents
Gastric cancer cell lines AGS, OCUM-2MD3, SGC7901, BGC823, MGC803, MKN28, and MKN45 and gastric epithelial cell line GES-1 were purchased from Cell Resource Center of Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences (Shanghai, China); RPMI 1640 culture medium and trypsin were from Gibco Company; and Trizol reagent and Lipofectamine™ 2000 transfection reagents were purchased from Invitrogen. Reverse transcription kit and fluorescence quantitative RT-PCR (qPCR) reagents were purchased from Promega Corporation, USA; PCR primers and small interfering RNA were synthesized by the Shanghai Biological Engineering Co. (China); protein extraction kit was purchased from Bio-Rad company, USA; Vav3, Survivin, x-IAP, Livin, caspase-3, p53, Bcl-2, Bax, ERK1/2, JNK, and p38MAPK in mitogen-activated protein kinase (MAPK) signaling pathways and their phosphorylated antibodies, as well as the internal standard β-actin antibody were purchased from Santa Cruz, USA; and MTT was purchased from Sigma, USA. Flow cytometer (Epics-XL, typeII) was the product from Beckman Coulter, USA.
Detection of Vav3 protein with IHC assay
Expressions of Vav3 in GC tissues and adjacent cancer tissues was examined with IHC assay (two-step), which was operated strictly in accordance with the instructions in the kit. Vav3 protein was positive if brown granules appeared in the cytoplasm. Five visual fields were observed randomly under microscope at ×400, and 100 cells were counted in each field. Secondary scoring method was employed: First, cells were scored based on the staining intensity: 0 for colorless, 1 for pale yellow, 2 for brownish yellow, and 3 for tan; second, positive cells were scored by percentage: 0 for positive cells <25 %, 1 for those between 25 and 50 %, 2 for those between 51 and 75 %, and 3 for those >75 %. The sum of staining intensity and the percentage of positive cells was regarded as expression results, with 0 as negative (−), 1–2 as weakly positive (+), 3–4 as positive (++), and 5–6 as strongly positive (+++).
Cell culture and treatment
Human GC cell lines and gastric epithelial cell line GES-1 were cultured in RPMI 1640 (Invitrogen) containing 10 % fetal calf serum, 100 U/ml penicillin, 100 mg/ml streptomycin, and incubated at 37 °C supplemented with 5 % of CO2. Trypsin solution (0.25 %) containing 0.02 % of EDTA was applied for trysinze and split cells.
RNA extraction and real-time fluorescent qPCR
Total RNAs of tissues or cell lines were isolated using Trizol reagent, and 2-μg RNA was reverse transcribed to synthesize template cDNA. Two microliters of reverse transcription product was detected by PCR to get the expression levels of mRNA in target molecules, and GAPDH served as an internal reference gene. According to kit instructions, we established PCR reaction with a final volume of 20 μl, 2-μl reverse transcription product, 10-μl SYBR Green Mix (Applied Biosystems, Foster City, CA), and 0.5-μl of both upstream and downstream primers (10 μmol/l), deionized water (7 μl). PCR reaction started with 1 cycle of 95 °C for 5 min, followed by 45 cycles of 3 steps as 94 °C for denaturation for 30 s and 60 °C for annealing for 30 s. Primers were designed by using Primer 5.0 and detected for specificity in Blast comparison test for the experiments, and primers were synthesized with the sequences as follows:
-
Vav3: 5′-CAAATTCACCGAGATCCTGT-3′ (F) and 5′-TGCTGGAGTGCTGTACGAAA-3′ (R)
-
Survivin: 5′-GCCAGATTTGAATCGCGGGA -3′ (F) and 5′-GCAGTGGATGAAGCCAGCCT -3′ (R)
-
x-IAP: 5′-CCGTGCGGTGCTTTAGTTGT-3′ (F) and 5′-TTCCTCGGGTATATGGTGTCTGAT-3′ (R)
-
Livin: 5′-TCCACAGTGTGCAGGAGACT-3′ (F) and 5′-ACGGCACAAAGACGATGGAC-3′ (R)
-
caspase-3: 5′-AGAGCTGGACTGCGGTATTGAG-3′ (F) and 5′-GAACCATGACCCGTCCCTTG-3′ (R)
-
p53: 5′-GTACCGTATGAGCCACCTGAG-3′ (F) and 5′-CGTCCCAGAAGATTCCCAC-3′ (R)
-
Bcl-2: 5′-TGTGTGGAGAGCGTCAACC-3′ (F) and 5′-TGGATCCAGGTGTGCAGGT- 3′ (R)
-
Bax: 5′-TTTCTGACGGCAACTTCAAC-3′ (F) and 5′-AGTCCAATGTCCAGCCCAT- 3′ (R)
-
GAPDH: 5′-GACCCCTTCATTGACCTCAAC-3′ (F) and 5′-CGCTCCTGGAAGATGGTGAT-3′
-
RT-PCR results were detected by 1.5 % agarose gel electrophoresis, and quantitative PCR results were calculated utilizing the 2−ΔΔCt method.
Western blotting analysis
Total proteins from clinical tissue samples or cultured cell samples were extracted. After protein quantification, 60-μg protein samples in each group were electrically transferred to a PVDF membrane after gel electrophoresis with 12 % polyacrylamide. They were added with 5 % skim milk powder supplemented with Tris-buffered saline with Tween 20 (TBST), closed at room temperature for 1 h, incubated with diluted target molecule primary antibody or internal reference β-actin primary antibody overnight at 4 °C, and rinsed three times with TBST. Appropriate peroxidase-labeled secondary antibody was added for incubation at room temperature for 1 h; chemiluminescence was employed for coloration and bands underwent scanning for integral absorbance.
siRNA transfection
Vav3 specific small interfering RNA (Vav3-siRNA) sequence was synthesized by Shanghai Sangon, and the sequence were as follows:
-
siRNA#1: 5′-GACAGCAGCAGAATTTGATTCAGTA-3′
-
siRNA#2: 5′-GAGCAATGGAAAGATTGCAAGCAGA-3′
-
siRNA#3: 5′-CCCAGTTTCTCTGTTTGAAGAACAT-3′
-
Control siRNA (Non siRNA): 5′-CCCTTCTCTGTTTGTAAAGAGACAT-3′
Before transfection, the synthesized siRNA was dissolved in solution at a concentration of 20 μmol/l. MGC803 cells were seeded in 6-well plates with the density of 4 × 105/ml for 24 h. Prior to transfection, cells were rinsed with serum-free and antibiotics-free RPMI 1640. According to the reagent instructions or by comparing control siRNA and the corresponding proportion, Vav3-siRNA diluted with serum-free and antibiotics-free RPMI 1640 was transfected into Lipofectamine™ 2000. It was held and then transfected into MGC803 cells. Twenty-four hours after transfection, the transfection efficiency was tested for subsequent experiments.
Cell survival test of MTT assay
MGC803 cells were trypsinized using 0.25 % containing 0.02 % of EDTA and were seeded in 96-well plates with the density of 5 × 104 cells/mL. Cells at a confluency of 60 to 70 % or compared with control siRNA were transfected with Vav3-siRNA. Six replicate wells were made in each group. Four hours before the end of experiment, 20 μl of MTT with a concentration of 5 mg/mL was added to each well. After 4 h, the culture medium was discarded, and 150 μl of DMSO was added in each well and shaken at room temperature for 15 min. Absorbance value (OD) at a wavelength of 490 nm was measured by a microplate reader. The above experiment was repeated three times. Growth inhibition rate (%) = (1 − experimental group OD value / control group OD value) × 100 %.
FCM assay (Annexin V/PI technique)
Quantitative apoptotic cell was measured by using an Annexin V-FITC/PI detection kit (Jiamei, Beijing, China), according to the manufacturer’s instruction. Briefly, cells were harvested and resuspended in binding buffer (106 cells/mL). After addition of 5-μl Annexin V-FITC and 10 μl of propidium iodide (PI) with mixing, the tubes were incubated for 15 min at room temperature in the dark. Annexin V-FITC binding was detected by a FACSCalibur cytometer (Becton Dickinson, USA). The data was analyzed by the Cell Quest software.
Statistical analysis
Experimental results were expressed with expressed ±standard deviation (\( \overline{x}\pm s \)), and SPSS 13.0 was used for the ANOVA analysis and Dunnett test. A P value of <0.05 indicated a significant difference.
Results
Expression of Vav3 in GC (qPCR and IHC, Western blot) and the relationship between Vav3 protein (IHC) and clinicopathological characteristics
qPCR results demonstrated that, compared with adjacent cancer tissues, mRNA level of Vav3 was higher in GC tissues (P < 0.05), as shown in Fig. 1a. IHC and Western blot analysis showed that protein level of Vav3 in GC tissues was significantly higher than that in adjacent tissues, as shown in Fig. 1b, c. Results of IHC showed that Vav3 protein were mainly expressed in cytoplasm of cells, and generally diffused distribution was shown (Fig. 1b), and positive expression rate of Vav3 protein in GC group (IHC) was 70.91 % (39/55), which was significantly higher than that in the adjacent tissues (14.55 %, 8/55) (Χ 2 = 35.70, P < 0.001). Results showed that Vav3 expression (IHC) had no significant correlation to the patient’s gender and age (Χ 2 = 1.742, P = 0.187; Χ 2 = 1.408, P = 0.236), and it also had no correlation to nerve/vessel invasion (Χ 2 = 0.234, P = 0.629) nor TNM stages (Χ 2 = 1.661, P = 0.198). Vav3 expression was related to degree of histological differentiation, cancer invasion depth, and lymphatic metastasis: that was, Vav3 expression was increased with reduction in the degree of tumor differentiation (Χ 2 = 7.185, P = 0.007); Vav3 expression in patients with tumor invasion reached T1/T2 was significantly lower than that in patients with tumor invasion that was limited to the T3/T4 (Χ 2 = 18.654, P < 0.001); Vav3 positive expression rate in GC patients without lymph node metastasis was significantly lower than in patients with lymph node metastasis (Χ 2 = 5.058, P = 0.025) (Table 1).
Vav3 expression in gastric cancer tissues and adjacent cancer tissues. Clinical samples of gastric cancer tissues and adjacent cancer tissues were subjected to qPCR (a), IHC (b), and Western blot (c) assays to determine the expression levels of Vav3. The relative mRNA expression level was shown in a and protein levels in c. Vav3 expressions of IHC in gastric cancer tissues and adjacent cancer tissues were shown in b (×400), and in gastric cancer tissues, strong expression of Vav3 protein was shown as a, weak expression as b; in adjacent cancer tissues positive expression of Vav3 protein was shown as c, negative expression as d. Values were shown as mean ± SD, for tissue samples n = 55. *P < 0.05 versus adjacent cancer tissues group
Expression of Vav3 in gastric cell lines (qPCR and Western blot)
Detection of cell lines indicated that mRNA and protein levels of Vav3 are variable in different human GC cell lines, gastric epithelial cell line GES-1. Out data showed Vav3 had the highest mRNA and protein levels in MGC803, while gastric epithelial cell line GSE-1 had the lowest Vav3 expression (Fig. 2a, b). Then, MGC803 was selected for subsequent study.
Vav3 expression in gastric cell lines. Gastric cell lines GES-1, AGS, OCUM-2MD3, SGC7901, BGC823, MGC803, MKN28, and MKN45 cell lines were subjected to qPCR (a) and Western-blot (b) assays to determine the expression levels of Vav3. The relative mRNA expression levels were shown in a, and protein levels in b. Values were shown as mean ± SD, n = 4 in each group. *P < 0.05 versus control group (GES-1 group). Highest Vav3 levels (both mRNA and protein) were discovered in MGC803 cells
Vav3-siRNA inhibition to Vav3 in MGC803 cells
Three Vav3-siRNAs with different sequences were transfected into MGC803. Cells transfected with control siRNA (Non group) was utilized as a negative control. qPCR and Western blot analysis showed that expression level of Vav3 did not change after being transfected with control siRNA (Non group); after being transfected with three Vav3-siRNAs, mRNA and protein levels of Vav3 in MGC803 cells were all decreased, among which in MGC803 cells transfected with Vav3-siRNA-3, Vav3 decreased most significantly (Fig. 3a, b). Different doses of Vav3-siRNA-3 were utilized to transfect cells, and qPCR and Western-blot analysis showed that expression of Vav3 was reduced in a dose-dependent manner (Fig. 3c, d) (all P < 0.05). In subsequent sections, Vav3-siRNA-3 was described as Vav3-siRNA.
Vav3-siRNA downregulates Vav3 expression in MGC803 cells. Cells were transfected with different sequence of Vav3-siRNA (s1, s2, s3) or control NS-siRNA (Non group) (a, b), alternatively. Cells were transfected with different amount of the sequence of Vav3-siRNA-3 for 24 h (c, d), the expression of Vav3 were identified by qPCR and Western blot, and the expression levels were represented as results of c (mRNA) and d (proteins). *P < 0.05 versus Non group
Vav3-siRNA transfection to MGC803 cell activity (MTT assays)
After different doses of Vav3-siRNA, including 20, 40, and 80 nM, were used to transfect MGC803 cells, MTT detected changes in cell activity. Compared with the control siRNA (Non group) transfected with control siRNA, inhibition of cell proliferation in MGC803 transfected with Vav3-siRNA was increased in a dose-dependent manner (P < 0.05) (Fig. 4).
Effects of Vav3-siRNA on the activity of gastric cancer cell line MGC803 (MTT assay). Cells were transfected with Vav3-siRNA or control NS-siRNA, and then were subjected to MTT assay to show inhibition rates of Vav3-siRNA to MGC803 cells. The results showed that inhibition rate was reduced in a concentration-dependent manner
Influences of Vav3-siRNA to the MGC803 apoptosis (FCM result)
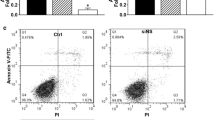
Twenty-four hours after 80 nM of Vav3-siRNA was transfected into MGC803 cells, changes in cell apoptosis ability were detected by flow cytometry (FCM). The results manifested that, compared with the control siRNA (Non group), apoptosis rate of GC cells transfected with Vav3-siRNA was significantly increased after transfection (Fig. 5) (P < 0.05).
Effects of Vav3-siRNA on the cell apoptosis of gastric cancer cell line MGC803 (FCM assay). Cells transfected with Vav3-siRNA or control NS-siRNA were also tested by FCM. Apoptosis rates were shown as a (histogram of apoptosis rates) and b (figure of apoptosis in each group). *P < 0.05 versus Non group
Influences of Vav3-siRNA to apoptosis related genes in MGC803 cells
We used qPCR and Western blot to detect expression of apoptosis-related genes. Eighty nanomolars of Vav3-siRNA or control siRNA was transfected into MGC803 cells. After 24 h, expressions of Survivin, xIAP, Livin, caspase-3, p53, Bcl-2, and Bax were detected. The results showed that mRNA and protein levels of apoptosis-related genes Survivin, xIAP, and Bcl-2 significantly decreased, while mRNA and protein levels of caspase-3 and Bax significantly increased; expressions of Livin and p53 had no obvious changes (P > 0.05) (Fig. 6).
Effects of inhibiting Vav3 on the apoptosis-related genes of gastric cancer cell line MGC803. Cells were transfected with Vav3-siRNA or control NS-siRNA (Non group), then were subjected to qPCR (a) or Western blot (b) assays to detect the mRNA or protein expression levels of Survivin, xIAP, Livin, caspase-3, p53, Bcl-2, and Bax. *P < 0.05 versus Non group
Influences of Vav3-siRNA to MAPK signaling pathways in MGC803 cells
Western blot was employed to detect changes of expression levels and total protein levels in phosphorylation of ERK1/2, JNK, and p38 of Vav3-siRNA-transfected MGC803 cells. The results showed that compared with Non group, the total protein level of signaling pathways mentioned above had no obvious changes in the Vav3-siRNA group, and phosphorylation levels of JNK and p38MAPK were not significantly different, whereas phosphorylation levels of extracellular signal regulated kinase (ERK) were significantly lowered (Fig. 7) (P < 0.05).
Inhibition of Vav3 expression affected MAPK pathway in MGC803 gastric cancer cells. MGC803 cells were transfected with Vav3-siRNA or control NS-siRNA, and then the expression and phosphorylation of MAPK pathway molecules were assayed by Western blot. Values were presented as mean ± SD. *P < 0.05 versus Non group
Discussion
GC is the most common digestive cancer in China. The mortality rate of GC is high among various malignant tumors and seriously threatens the life and health of people [15, 16]. The main mechanism of GC is the activation of oncogenes and inactivation of tumor suppressor genes. Study has proved Vav3 gene was upregulated in GC [14]. Our present study also found that high expression of Vav3 in GC tissues and cell lines and Vav3 expression was related to degree of histological differentiation, cancer invasion depth, and lymphatic metastasis, which suggested that Vav3 played a key role in promoting the development and progression of GC. After siRNA technology was applied to inhibition of expression of endogenous Vav3 in GC MGC803 cells, decreased cell growth capacity and increased apoptosis rate of GC cells were discovered, which confirmed Vav3 could promote cell growth and also induced apoptosis of GC cells, providing an experimental basis for exploring the mechanism of GC and targeted gene therapy.
Vav3 gene, located on chromosome 1, encodes a protein of 847 amino acid with a molecular mass of 97.8 kD. Vav3 is able to regulate various signaling pathways through modulating activities of members of the Rho family. According to report, the major downstream signaling pathways affected by Vavs are MAPK and PI3K-Akt [17, 18], which are essential for the development of the body. Results of animal studies have confirmed that Vav3 plays important roles in the pathologies of nervous system and cardiovascular system, and the defect of Vav3 expression could lead to abnormality of sympathetic nerve and cardiovascular system [19, 20]. Therefore, it is of importance in the field of cancer research to characterize and identify biological functions of Vav3 in the pathogenesis of malignant tumor.
RNA interference (RNAi) technique is able to maintain transcriptional silencing of specific genes by delivering specific double-stranded RNA into cells. siRNAs have commonly been employed as reverse genetic methods to study the biological functions of target genes in vitro and in vivo in recent years due to the specificity, efficiency, and persistence of the technology [21]. In present study we found that inhibition of high expression of Vav3 in GC cell line MGC803 by siRNA resulted in alterations on growth and apoptosis of MGC803 cells. It has been reported that Vav3 plays a role in prostate cancer and breast cancer proliferation [11, 13]. Other studies also show that Vav3 functions in apoptosis in bladder cancer [17, 22] and breast cancer [23]. Flow cytometry results of this study displayed that when Vav3-siRNA inhibited Vav3 in MGC803 cells. After transfection with Vav3-siRNA, apoptosis rate of GC cells was significantly increased, suggesting that Vav3 overexpression enhanced apoptosis resistance of GC cells.
Mechanism of tumor cell apoptosis is extremely complicated, with many genes involved in different manners. Bcl-2 family members in mitochondrial pathway play an important role in this process. Bcl-2 is able to enhance the apoptotic resistance of tumor cells in a variety of ways. Bcl-2 can be combined with Bax to form Bcl-2/Bax dimers, and changes in the ratio of Bcl-2/Bax make a difference in tumor cell apoptosis [24–26]. p53 is the most researched tumor gene, and wild-type p53 can promote tumor cell apoptosis, and mutant tumor p53, a key factor in carcinogenesis, has more effects on inhibition of apoptosis [27, 28]. Apoptosis inhibition protein family play key roles in inhibition of tumor cell apoptosis pathway, and Survivin, x-IAP, and Livin in the family have a strong suppression of apoptosis [29–34]. Caspase family is the core in apoptosis, which can directly induce apoptosis, and caspase-3 is one of the main members [35, 36]. The results of this study showed that after Vav3 expression was inhibited, expression of Survivin, x-IAP, and Bcl-2 were decreased, expressions of caspase-3 and Bax increased, and expressions of Livin and p53 had no obvious change, which indicated Vav3 gene was closely related to GC cell apoptosis. Its regulation mechanism may be caused by regulating genes in mitochondrial pathway and inhibition of apoptosis pathway.
Studies have shown that Vav3 regulatory mechanisms are in connection with MAPK [17, 37] signaling pathway, and the MAPK pathway is closely related to apoptosis of tumor cells [38]. MAPK, a serine/threonine protein kinase and a main signal system in eukaryotes, could transduct the signal of extracellular stimuli to induce basic physiological response intracellularly. It has been identified that three MAPK signal transduction pathways were present in eukaryotic cells, namely ERK pathway, c-jun N-terminal kinase (JNK)/stress-activated protein kinase (SAPK) pathway, and p38 MAPK pathway [39]. In order to clarify which of the above pathways is related to Vav3 in MGC803 for apoptosis, we examined these key molecules associated with cell in signaling pathways. The results showed that silencing Vav3 expression inhibits activation of ERK phosphorylation, without affecting changes in other signaling pathways. We conclude that Vav3 gene might be invovled in apoptosis of GC cells through ERK pathway regulation, whereas detailed molecular mechanism in the process should be evaluted in future study.
In conclusion, this study has found that Vav3 upregulation in GC tissues and cells and siRNA-mediated Vav3 expression silencing inhibits cell growth and promotes apoptosis through upregulation of caspase-3 and Bax, and downregulation of Survivin, x-IAP, and Bcl-2. Although the exact mechanism still needs further study, these results have suggested that Vav3 may act as an oncogene in the development of GC and could be employed for targeting Vav3 as a potential therapeutic treatment of human GC.
Abbreviations
- GC:
-
Gastric cancer
- siRNA:
-
Small interfering RNA
- IHC:
-
Immunohistochemistry
- FCM:
-
Flow cytometry
- qPCR:
-
Real-time fluorescent quantitative PCR
- WB:
-
Western blotting
- PI:
-
Propidium iodide
References
Chen WQ, Zheng RS, Zhang SW, Li N, Zhao P, Li GL, et al. Report of incidence and mortality in china cancer registries, 2008. Chin J Cancer Res. 2012;24:171–80.
Wang QP, Wang Y, Wang XD, Mo XM, Gu J, Lu ZY, et al. Survivin up-regulates the expression of breast cancer resistance protein (BCRP) through attenuating the suppression of p53 on NF-κB expression in MCF-7/5-FU cells. Int J Biochem Cell Biol. 2013;45:2036–44.
Wittkopf N, Günther C, Martini E, He G, Amann K, He YW, et al. Cellular FLICE-Like inhibitory protein secures intestinal epithelial cell survival and immune homeostasis by regulating caspase-8. Gastroenterology. 2013;145:1369–79.
Sikdar S, Mukherjee A, Ghosh S, Khuda-Bukhsh AR. Condurango glycoside-rich components stimulate DNA damage-induced cell cycle arrest and ROS-mediated caspase-3 dependent apoptosis through inhibition of cell-proliferation in lung cancer, in vitro and in vivo. Environ Toxicol Pharmacol. 2013;37:300–14.
Golestani EB, Sanati MH, Houshmand M, Ataei M, Akbarian F, Shakhssalim N. Expression and prognostic significance of Bcl-2 and Bax in the progression and clinical outcome of transitional bladder cell carcinoma. Cell J. 2014;15:356–63.
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang C, et al. Dihydromyricetin reduced Bcl-2 expression via p53 in human hepatoma HepG2 cells. PLoS One. 2013;8, e76886.
Hayakawa Y, Hirata Y, Sakitani K, Nakagawa H, Nakata W, Kinoshita H, et al. Apoptosis signal-regulating kinase-1 inhibitor as a potent therapeutic drug for the treatment of gastric cancer. Cancer Sci. 2012;103:2181–5.
Korbakis D, Scorilas A. Treatment of gastric cancer cells with 5-fluorouracil/leucovorin and irinotecan induces distinct alterations in the mRNA expression of the apoptosis-related genes, including the novel gene BCL2L12. Tumor Biol. 2009;30:100–7.
Zhuo Z, Zhang L, Mu Q, Lou Y, Gong Z, Shi Y, et al. The effect of combination treatment with docosahexaenoic acid and 5-fluorouracil on the mRNA expression of apoptosis-related genes, including the novel gene BCL2L12, in gastric cancer cells. In Vitro Cell Dev Biol Anim. 2009;45:69–74.
Fernandez-Salguero PM. A remarkable new target gene for the dioxin receptor: the Vav3 proto-oncogene links AhR to adhesion and migration. Cell Adh Migr. 2010;4:172–5.
Liu Y, Wu X, Dong Z, Lu S. The molecular mechanism of Vav3 oncogene on upregulation of androgen receptor activity in prostate cancer cells. Int J Oncol. 2010;36:623–33.
Travert M, Huang Y, de Leval L, Martin-Garcia N, Delfau-Larue MH, Berger F, et al. Molecular features of hepatosplenic T-cell lymphoma unravels potential novel therapeutic targets. Blood. 2012;119:5795–806.
Lee K, Liu Y, Mo JQ, Zhang J, Dong Z, Lu S. Vav3 oncogene activates estrogen receptor and its overexpression may be involved in human breast cancer. BMC Cancer. 2008;8:158.
Lin KY, Wang LH, Hseu YC, Fang CL, Yang HL, Kumar KJ, et al. Clinical significance of increased guanine nucleotide exchange factor Vav3 expression in human gastric cancer. Mol Cancer Res. 2012;10:750–9.
Zheng KC, Aoki K, Li XQ, Lin SG, Wu BS, Zhong WL, et al. Serum pepsinogens, gastrin-17 and helicobacter pylori antibody in the residents of two cities in China with distinct mortality rates of gastric cancer. Tohoku J Exp Med. 2012;228:289–94.
Wang HM, Huang CM, Zheng CH, Li P, Xie JW, Wang JB, et al. Tumor size as a prognostic factor in patients with advanced gastric cancer in the lower third of the stomach. World J Gastroenterol. 2012;18:5470–5.
Nomura T, Yamasaki M, Hirai K, Inoue T, Sato R, Matsuura K, et al. Targeting the Vav3 oncogene enhances docetaxel-induced apoptosis through the inhibition of androgen receptor phosphorylation in LNCaP prostate cancer cells under chronic hypoxia. Mol Cancer. 2012;12:27.
Dong Z, Liu Y, Levin L, Oleksowicz L, Wang J, Lu S. Vav3 oncogene is involved in regulation of secretory phospholipase A2-IIa expression in prostate cancer. Oncol Rep. 2011;25:1511–6.
Sauzeau V, Sevilla MA, Rivas-Elena JV, de Alava E, Montero MJ, López-Novoa JM, et al. Vav3 proto-oncogene deficiency leads to sympathetic hyperactivity and cardiovascular dysfunction. Nat Med. 2006;12:841–5.
Sauzeau V, Carvajal-González JM, Riolobos AS, Sevilla MA, Menacho-Márquez M, Román AC, et al. Transcriptional factor aryl hydrocarbon receptor (Ahr) controls cardiovascular and respiratory functions by regulating the expression of the Vav3 proto-oncogene. J Biol Chem. 2011;286:2896–909.
Hannon GJ. RNA interference. Nature. 2002;418:244–51.
Hirai K, Nomura T, Yamasaki M, Inoue T, Narimatsu T, Chisato N, et al. The Vav3 oncogene enhances the malignant potential of prostate cancer cells under chronic hypoxia. Urol Oncol. 2014;32:101–9.
Citterio C, Menacho-Márquez M, García-Escudero R, Larive RM, Barreiro O, Sánchez-Madrid F, et al. The rho exchange factors vav2 and vav3 control a lung metastasis-specific transcriptional program in breast cancer cells. Sci Signal. 2012; 5: ra71.
Deng B, Zhang XF, Zhu XC, Huang H, Jia HL, Ye QH, et al. Correlation and prognostic value of osteopontin and Bcl-2 in hepatocellular carcinoma patients after curative resection. Oncol Rep. 2013;30:2795–803.
Yang XC, Wang X, Luo L, Dong DH, Yu QC, Wang XS, et al. RNA interference suppression of A100A4 reduces the growth and metastatic phenotype of human renal cancer cells via NF-kB-dependent MMP-2 and bcl-2 pathway. Eur Rev Med Pharmacol Sci. 2013;17:1669–80.
Zhang GJ, Zhang Z. Effect of Bcl-2 on apoptosis and transcription factor NF-κB activation induced by adriamycin in bladder carcinoma BIU87 cells. Asian Pac J Cancer Prev. 2013;14:2387–91.
Charoensinphon N, Qiu P, Dong P, Zheng J, Ngauv P, Cao Y, et al. 5-Demethyltangeretin inhibits human nonsmall cell lung cancer cell growth by inducing G2/M cell cycle arrest and apoptosis. Mol Nutr Food Res. 2013;57:2103–11.
Ku BM, Kim DS, Kim KH, Yoo BC, Kim SH, Gong YD, et al. Transglutaminase 2 inhibition found to induce p53 mediated apoptosis in renal cell carcinoma. FASEB J. 2013;27:3487–95.
McKenzie JA, Liu T, Jung JY, Jones BB, Ekiz HA, Welm AL, et al. Survivin promotion of melanoma metastasis requires upregulation of α5 integrin. Carcinogenesis. 2013;34:2137–44.
Li Y, Tan BB, Fan LQ, Zhao Q, Liu Y, Wang D. Expression of COX-2, survivin in regional lymph node metastases of gastric carcinoma and the correlation with prognosis. Hepato-Gastroenterology. 2010;57:1435–41.
Peng Y, Sun H, Lu J, Liu L, Cai Q, Shen R, et al. Bivalent Smac mimetics with a diazabicyclic core as highly potent antagonists of XIAP and cIAP1/2 and novel anticancer agents. J Med Chem. 2012;55:106–14.
Büneker CK, Yu R, Deedigan L, Mohr A, Zwacka RM. IFN-γ combined with targeting of XIAP leads to increased apoptosis-sensitisation of TRAIL resistant pancreatic carcinoma cells. Cancer Lett. 2012;316:168–77.
Ou JM, Ye B, Qiu MK, Dai YX, Dong Q, Shen J, et al. Knockdown of livin inhibits growth and invasion of gastric cancer cells through blockade of the MAPK pathway in vitro and in vivo. Int J Oncol. 2014;44:276–84.
Liu X, Wang A, Gao H, Yuan Z, Jiao Y. Expression and role of the inhibitor of apoptosis protein livin in chemotherapy sensitivity of ovarian carcinoma. Int J Oncol. 2012;41:1021–8.
Fritsch K, Finke J, Grüllich C. Suppression of granzyme B activity and caspase-3 activation in leukaemia cells constitutively expressing the protease inhibitor 9. Ann Hematol. 2013;92:1603–9.
Wu B, Yao H, Wang S, Xu R. DAPK1 modulates a curcumin-induced G2/M arrest and apoptosis by regulating STAT3, NF-κB, and caspase-3 activation. Biochem Biophys Res Commun. 2013;434:75–80.
Lyons LS, Rao S, Balkan W, Faysal J, Maiorino CA, Burnstein KL. Ligand-independent activation of androgen receptors by Rho GTPase signaling in prostate cancer. Mol Endocrinol. 2008;22:597–608.
Cheng YY, Yang JS, Tsai SC, Liaw CC, Chung JG, Huang LJ, et al. The newly synthesized 2-(3-hydroxy-5-methoxyphenyl)-6,7- methylenedioxyquinolin-4-one triggers cell apoptosis through induction of oxidative stress and upregulation of the p38 MAPK signaling pathway in HL-60 human leukemia cells. Oncol Rep. 2012;28:1482–90.
Zhang W, Liu HT. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002;12:9–18.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Tan, Bb., Zhang, Mm., Li, Y. et al. Inhibition of Vav3 gene can promote apoptosis of human gastric cancer cell line MGC803 by regulating ERK pathway. Tumor Biol. 37, 7823–7833 (2016). https://doi.org/10.1007/s13277-015-4505-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4505-9