Abstract
Recent studies cite β2-adrenergic receptor (β2AR) antagonists as novel therapeutic agents for melanoma, as they may reduce the disease progression. The β2AR has shown to be expressed in malignant melanoma. However, it remains unclear whether the β2AR expression has a clinical and pathological significance in patients with cutaneous malignant melanoma. We herein conducted a clinicopathological study to investigate the protein expression of β2AR in malignant melanoma of the skin and its prognostic significance. One hundred thirty-three patients with surgically resected cutaneous malignant melanoma were evaluated. Tumor sections were stained by immunohistochemistry for β2AR, Ki-67, the microvessel density (MVD) determined by CD34, and p53. β2AR was highly expressed in 44.4 % (59 out of 133) of the patients. The expression of β2AR was significantly associated with the tumor thickness, ulceration, T factor, N factor, disease stage, tumor size, cell proliferation (Ki-67), and MVD (CD34). Using Spearman’s rank test, the β2AR expression was correlated with Ki-67 (r = 0.278; 95 % CI, 0.108 to 0.432; P = 0.001), CD34 (r = 0.445; 95 %CI, 0.293 to 0.575; P < 0.001), and the tumor size (r = 0.226; 95 % CI, 0.053 to 0.386; P = 0.008). Using a univariate analysis, the tumor thickness, ulceration, disease stage, β2AR, Ki-67, and CD34 had a significant relationship with the overall and progression-free survivals. A multivariable analysis confirmed that β2AR was an independent prognostic factor for predicting a poor overall survival (HR 1.730; 95 % CI 1.221–2.515) and progression-free survival (HR 1.576; 95 % CI 1.176–2.143) of malignant melanoma of the skin. β2AR can serve as a promising prognostic factor for predicting a worse outcome after surgical treatment and may play an important role in the development and aggressiveness of malignant melanoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Background
Malignant melanoma is an aggressive malignancy borne from melanocytes. Malignant melanoma of the skin is responsible for ~60 % of deaths from skin cancers, and both the incidence and prevalence of the disease have increased over the past few decades; meanwhile, the overall mortality rates have remained somewhat stable [1]. As metastatic melanoma carries a poor prognosis, there is a need for novel therapies [2]. More than 60 % of cutaneous melanomas harbor a mutation in BRAF [3], of which more than 80 % feature the specific V600E amino acid substitution mutation [4]. Single-molecule targeting approaches, such as BRAF inhibitors, in melanoma cannot cure the disease and some resistance occurs. Therefore, alternative therapies are required for melanoma that can be combined with BRAF inhibitors.
Beta2-adrenergic receptor (β2AR) is the prototypic and ubiquitous cell-surface proteins known as G protein-coupled receptors (GPCRs) or seven transmembrane receptors. β2AR is involved in production of physiological responses to adrenaline and noradrenaline [5]. Beta-blockers are commonly used for the treatment of cardiac disease [6]. Recently, the fortuitous discovery that the noncardioselective beta-blocker, propranolol, can safely induce involution of infantile haemangiomas has led to important insights regarding their effects on tumor growth [7]. The expression of β2AR has been shown in various human cancers [8–12]. Melhem-Bertrandt et al. reported that breast cancer patients taking beta-blockers have a significant reduction in tumor recurrence compared to those not prescribed beta-blockers, and beta-blocker intake is found to be closely associated with a better relapse-free survival in patients with breast cancer [13]. In preclinical studies, β-adrenergic signaling was shown to be involved in the inhibition of apoptosis, the induction of vascular endothelial growth factor (VEGF) expression, and the development of metastasis [12, 14]. Thus, Barron et al. suggested that the inhibition of β2-adrenergic signaling could suppress the tumor progression and mortality in breast cancer [14]. Regarding malignant melanoma, the clinical potential of targeted therapy for β2AR was suggested by the inhibition of β2AR using propranolol [15, 16]. Immunohistochemical studies on the β2AR expression in malignant melanoma were performed and the expression rate was reported to be high in malignant melanoma compared to melanocytic nevi [12, 17]. However, it remains unclear whether the β2AR expression has a clinical and pathological significance in patients with cutaneous malignant melanoma.
In this study, we examined the β2AR expression in resected tissue specimens to evaluate the clinicopathological and prognostic significance of β2AR in patients with cutaneous malignant melanoma.
Materials and methods
Patients
We analyzed 156 consecutive patients with malignant melanoma who underwent surgical resection at Gunma University Hospital between September 1989 and October 2011. Twenty-three patients were excluded due to unavailable patient information. In total, 133 patients were analyzed in the study. The clinical stages were defined according to the 2009 guidelines of the American Joint Committee on Cancer (AJCC). We further analyzed 30 resected lesions with melanocytic nevi as a negative control. The approach used for the evaluation and resection of these tumors has been described previously [18]. This study was approved by the institutional review board of Gunma University Hospital (ethical committee for clinical studies-Gunma University Faculty of Medicine). In the present study, the morality and recurrence were determined using medical records.
Immunohistochemical staining
The β2AR expression was examined by immunohistochemical staining with a rabbit anti-human β2AR monoclonal antibody (Abcam, Inc., Cambridge, UK; 1:100 dilution) raised against a C-terminal peptide of human β2AR. Immunohistochemical staining was performed on paraffin sections using a polymer peroxidase method (Histofine Simple Stain MAX PO (MULTI) kit; Nichirei Corporation, Tokyo, Japan). Briefly, deparaffinized, rehydrated sections were treated with 0.3 % hydrogen peroxidase in methanol for 30 min to block endogenous peroxidase activity. To expose antigens, the sections were autoclaved in 10 mmol/L sodium citrate buffer (pH 6.0) for 5 min and cooled for 30 min. After rinsing in phosphate-buffered saline (PBS), the sections were incubated with anti-β2AR antibody (1:100) overnight. Thereafter, they were incubated with the Histofine Simple Stain MAX PO (MULTI) kit (Nichirei Corporation). The peroxidase reaction was performed using 0.02 % 3,3-diaminobenzidine tetrahydrochloride and 0.01 % hydrogen peroxidase in 0.05 M Tris-HCl buffer, pH 7.6. Negative control tissue sections were prepared by omitting the primary antibody. The expression of β2AR was considered to be positive only if distinct cytoplasmic and plasma membrane staining was present. The β2AR expression scores were assessed by the extent of staining as follows: 1, ≤10 % of the tumor area was stained; 2, 11–25 % was stained; 3, 26–50 % was stained; and 4, ≥51 % was stained. The tumors in which stained tumor cells were scored as ≥3 were defined to have a high expression.
For CD34, Ki-67, and p53, immunohistochemical staining was performed according to the procedures described in previous reports [19, 20]. The following antibodies were used: mouse monoclonal antibodies against CD34 (Nichirei, Tokyo, Japan, 1:800 dilution), Ki-67 (Dako, Glostrup, Denmark, 1:40 dilution), and p53 (D07; Dako, 1:50 dilution). The number of CD34-positive vessels was counted in four selected hot spots in a 400× field (0.26 mm [2] field area). The microvessel density (MVD) was defined as the mean count of microvessels per 0.26 mm [2] field area. The median number of CD34-positive vessels was evaluated, and the tumors in which stained tumor cells made up more than each median value were defined as having a high expression. For p53, a microscopic examination for the nuclear reaction product was performed and scored, and p53 expression in greater than 10 % of the tumor cells was defined as a positive expression [18]. For Ki-67, a highly cellular area of the immunostained sections was evaluated. All melanoma cells with nuclear staining of any intensity were defined as having a high expression. Approximately 1000 nuclei were counted on each slide. The proliferative activity was assessed as the percentage of Ki-67-stained nuclei (Ki-67 labeling index) in the sample. The median value of the Ki-67 labeling index was evaluated, and the number of tumor cells with greater than the median value were defined as having a high expression. The sections were assessed using light microscopy in a blinded fashion by at least two of the authors.
Statistical analysis
Probability values of <0.05 were considered to indicate a statistically significant difference. The significance of the difference was determined by the Mann-Whitney U test. The correlation between different variables was analyzed using a nonparametric Spearman’s rank test. The Kaplan-Meier method was used to estimate the survival as a function of time, and survival differences were analyzed by the log-rank test. The overall survival (OS) was determined as the time from tumor resection to death from any cause. The progression-free survival (PFS) was defined as the time between tumor resection and the first disease progression or death. A univariate analysis was performed using the first 5-year survival rate after resection. Multivariate analyses were performed using a stepwise Cox proportional hazards model to identify independent prognostic factors. Statistical analyses were performed using the GraphPad Prism 4 (GraphPad Software, San Diego, CA, USA) and JMP 8 software programs (SAS, Institute Inc., Cary, NC, USA) for Windows.
Results
Patient’s demographics
One hundred thirty-three patients with malignant melanoma resected in Gunma University were analyzed. The clinicopathologic results stratified by the tumor location are listed in Table 1. The median age of the patients was 71 years (range, 42 to 86 years). Most tumors (n = 126, 90.6 %) were stages I to III. The day of surgery was considered to be the starting day for measuring the postoperative survival. The median follow-up duration for all patients was 1725 days. Before 1996, 7 patients had been treated with Bacille Calmette-Guerin (BCG) immunotherapy. After that, most of the patients were treated with DAV-Feron therapy (dacarbazine, ACNU, vincristine, and IFN-β). DAV-Feron therapy was done in 88 patients, while DAC-tam therapy (dacarbazine, nimustine, cisplatin, and tamoxifen) was tried in advanced 10 patients.
Immunohistochemical analysis
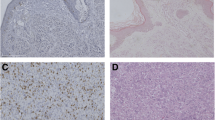
The immunohistochemical analyses were performed on 133 primary lesions with malignant melanoma and 30 resected lesions with melanocytic nevi. Figure 1 represents the immunohistochemical staining of β2AR in malignant melanoma. β2AR immunostaining was detected in melanoma cells and localized predominantly on their cytoplasmic and plasma membrane (Fig. 1a). Negative staining of β2AR in malignant melanoma and melanocytic nevi were observed (Fig. 1b, c). A high expression rate of β2AR staining was observed in 44.4 % of the patients’ sections of malignant melanoma, whereas it was expressed in 0 % of the melanocytic nevi. The positivity of β2AR expression was significantly different between malignant melanoma and melanocytic nevus lesions (P < 0.001). The cutoff points for a high CD34 expression and high Ki-67 labeling index were defined as follows. The median number of CD34-positive vessels was 4 (range, 0–90), and thus, the value of 4 was chosen as the cutoff point. The median value of the Ki-67 labeling index was 10 % (range, 0–47), and thus, the value of 10 % was chosen as the cutoff point. A positive expression of p53 was observed in 73 % (97/133) of the patients. Representative figures of each immunostaining were shown (Fig. 1d–i).
Immunohistochemical staining of β2AR in malignant melanoma. a Positive staining of the β2AR expression in the cytoplasmic and plasma membrane of malignant melanoma. b Negative staining for the β2AR expression in the malignant melanoma. c Negative staining for the β2AR expression in the melanocytic nevus. d Positive staining of the CD34 expression in the malignant melanoma. e Negative staining of the CD34 expression in the malignant melanoma. f Positive staining of the Ki-67 expression in the malignant melanoma. g Negative staining of the Ki-67 expression in the malignant melanoma. h Positive staining of the p53 expression in the malignant melanoma. i Negative staining of the p53 expression in the malignant melanoma
Table 1 shows the expression patterns of the biomarkers according to the tumor location. The rate of high expression or positivity in these biomarkers was significantly higher in melanoma than in melanocytic nevus lesions. The patient’s demographics according to the β2AR expression status are listed in Table 1. The expression of β2AR was significantly associated with the tumor thickness, ulceration, T factor, N factor, disease stage, tumor size, cell proliferation (Ki-67), and MVD (CD34).
Correlation between β2AR and different variables
Spearman’s rank test revealed that the β2AR expression significantly correlated with Ki-67 (r = 0.278, P = 0.001), CD34 (r = 0.445, P < 0.001), and tumor size (r = 0.226, P = 0.008) (Table 2).
Survival analysis according to the β2AR expression
The 5-year survival rates of the OS and PFS for all patients were 75 and 65 %, respectively. Of 133 patients, 36 died and 50 had recurrence after initial surgery. According to a univariate analysis, the tumor thickness, ulceration, disease stage, β2AR, Ki-67, and CD34 had a significant relationship with the OS and the PFS (Table 3). Only adjuvant chemotherapy was recognized as a significant prognostic marker for the PFS. A multivariable analysis confirmed that β2AR was an independent prognostic factor for predicting a poor OS and PFS after surgery in patients with cutaneous malignant melanoma. Figure 2 shows the Kaplan-Meier survival curve in patients with high and low expressions for β2AR. Significant differences in the OS and PFS were observed with respect to the intensity of the β2AR expression.
Discussion
This is the first study to elucidate the clinicopathological significance of the β2AR expression in patients with cutaneous malignant melanoma. The expression of β2AR in the tumor specimens closely correlated with cell proliferation and angiogenesis and was a significant indicator for predicting a poor outcome after surgical resection. Therefore, a high β2AR expression may play an important role in the growth of malignant melanoma.
Immunohistochemical studies on the β2AR expression in malignant melanoma using patient’s samples have been previously carried out. Yang et al. reported that 18 out of 20 melanoma biopsies were positive for β2AR [12]. Moretti et al. studied cutaneous melanocytic lesions from 40 patients using both anti-β1AR antibody and anti-β2AR antibody and found that both receptors were expressed in benign melanocytic nevi, atypical nevi, and malignant melanomas. Moreover, the expression rate was significantly higher in malignant melanomas [17]. Our data were consistent with the previous results and provide further evidence that a high expression of β2AR was an independent prognostic factor for predicting a poor prognosis of malignant melanoma.
There are several clinical studies that investigated the efficacy of beta-blockers to malignant melanoma. De Giorgi V et al. performed an epidemiological study of beta-blocker usage after the diagnosis of melanoma and melanoma-specific survival [15]. This study reported a reduction in the risk of recurrence in patients treated with beta-blockers after the diagnosis of malignant melanoma (thick melanoma: Breslow thickness > 1 mm). They also showed a significant reduction in the proportion of deaths in the beta-blocker-treated patients compared with controls. Lemeshow et al. investigated beta-blocker usage prior to the diagnosis of malignant melanoma and observed a 13 % reduction in melanoma mortality and 19 % reduction in all-cause mortality in 372 malignant melanoma patients treated with beta-blockers in the 90 days prior to the diagnosis of melanoma [16]. Contrary to the previous two studies, Livingstone et al. performed a population-based cohort study and their results did not show a significant effect of beta-blockers on the survival of malignant melanoma patients in the Netherlands [21]. Furthermore, McCourt et al. investigated patients with incident malignant melanoma diagnosed between 1998 and 2010 in the UK [22]. They reported that beta-blocker use after the diagnosis of malignant melanoma was not associated with a reduced risk of death from melanoma in this UK population-based study. Most of these studies did not define the beta-blockers used. As shown previously, there is a discrepancy regarding the effect of beta-blockers on melanoma progression. Although the results of the previous studies are controversial, our study implies the potential of β2AR inhibition in melanoma.
Several groups have described the clinicopathological studies regarding the enhanced expression of β2AR in various cancers, such as pancreatic cancer, hepatocellular carcinoma, and oral squamous cell carcinoma [23–25]. Wenjuan et al. described that single nucleotide polymorphisms in the β2AR gene could be useful biomarkers for predicting the biological behaviors and survival of pancreatic cancer throughout elevating vascularization and activating the epidermal growth factor receptor (EGFR) signaling pathway [25]. Chen et al. implied the use of the β2AR expression as a prognostic marker for predicting a poor outcome and tumor recurrence in patients with hepatocellular carcinoma after surgery [24]. On the other hand, a strong β2AR expression was shown to be a favorable prognostic factor for oral squamous cell carcinoma [23]. In our study, the strong expression of β2AR was found to have a great impact as a negative predictor. However, little is known about such discrepancy of the prognostic significance in various human neoplasms. Further study is warranted to confirm the prognostic role of the β2AR expression in cancer patients.
In the in vitro studies, it has been shown that a significant increase in the tumor growth and metastasis requires β-adrenergic signaling, which correlated with the infiltration of macrophages and pro-metastatic gene expression [26]. Recent reports demonstrated that the effects of β-adrenergic signaling on the tumor progression and metastasis are suppressed by β2 antagonists, but not β1 antagonists [27]. Several studies have revealed that the β2AR agonist, isoproterenol, promotes the growth of human cancer cells in vitro via β2AR-mediated activation of cAMP/PKA, mitogen-activated protein kinase (MAPK)/extracellular signal-regulated kinase 1/2 (ERK1/2), and PI3-kinase (PI3K)/protein kinase B (AKT) signaling pathways. Furthermore, isoproterenol has been shown to activate MAPK/ERK1/2 by a β2AR-mediated and VEGF-independent mechanism [28–30]. The stimulation of β2AR has been shown to induce cell proliferation and cell adhesion [31]. Thus, β2AR has recently received attention as a potential therapeutic target in the treatment of cancer. Recently, Wrobel and Le Gal investigated the effect of noncardioselective and cardioselective beta-blockers on melanoma progression in vitro. They revealed that the noncardioselective beta-blocker, propranolol, regulates the expression of different genes involved in tumor angiogenesis, cell death, or proliferation and inhibits melanoma progression [32]. In the present study, we did not investigate the relationship between the expression of β2AR and any signaling pathway in malignant melanoma tissues. Further study is necessary to examine the signaling pathway related to the increase of these active markers.
There are several limitations associated with our study. First, the number of malignant melanoma patients included was small, which may have biased our results. In addition, the present study showed that the expression of β2AR was closely associated with the tumor thickness, ulceration, stage, cell proliferation (Ki-67), and angiogenesis (CD34). Therefore, these factors were excluded from the multivariate analysis in order to assess the β2AR expression as an independent prognostic factor and to resolve potential confounding. Moreover, information loss occurred with the continuous variables were dichotomized.
In conclusion, a high expression of β2AR is therefore considered to be a promising pathological marker for predicting a poor prognosis in patients with malignant melanoma of the skin. The inhibition of β2AR expression may have anti-tumor efficacy, and a molecular targeting drug that selectively inhibits β2AR will aid in alternative therapeutic strategies for malignant melanoma.
References
Hawryluk EB, Tsao H. Melanoma: clinical features and genomic insights. Cold Spring Harb Perspect Med. 2014;4:a015388. doi:10.1101/cshperspect.a015388.
Marsden JR, Newton-Bishop JA, Burrows L, Cook M, Corrie PG, Cox NH, et al. Revised UK guidelines for the management of cutaneous melanoma 2010. J Plast Reconstr Aesthet Surg JPRAS. 2010;63:1401–19.
Smalley KS. Understanding melanoma signaling networks as the basis for molecular targeted therapy. J Invest Dermatol. 2010;130:28–37.
Davies H, Bignell GR, Cox C, Stephens P, Edkins S, Clegg S, et al. Mutations of the BRAF gene in human cancer. Nature. 2002;417:949–54.
Wnorowski A, Jozwiak K. Homo- and hetero-oligomerization of β2-adrenergic receptor in receptor trafficking, signaling pathways and receptor pharmacology. Cell Signal. 2014;26:2259–65.
Aronow WS. Current role of beta-blockers in the treatment of hypertension. Expert Opin Pharmacother. 2010;11:2599–607.
Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol. 2010;163:269–74.
Masur K, Niggemann B, Zanker KS, Entschladen F. Norepinephrine-induced migration of SW 480 colon carcinoma cells is inhibited by beta-blockers. Cancer Res. 2001;61:2866–9.
Palm D, Lang K, Niggemann B, Drell 4th TL, Masur K, Zaenker KS, et al. The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer J Int Cancer. 2006;118:2744–9.
Shang ZJ, Liu K, de Liang F. Expression of beta2-adrenergic receptor in oral squamous cell carcinoma. J Oral Pathol Med: Off Publ Int Assoc Oral Pathol Am Acad Oral Pathol. 2009;38:371–6.
Sood AK, Bhatty R, Kamat AA, Landen CN, Han L, Thaker PH, et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res: Off J Am Assoc Cancer Res. 2006;12:369–75.
Yang EV, Kim SJ, Donovan EL, Chen M, Gross AC, Webster Marketon JI, et al. Norepinephrine upregulates VEGF, IL-8, and IL-6 expression in human melanoma tumor cell lines: implications for stress-related enhancement of tumor progression. Brain Behav Immun. 2009;23:267–75.
Melhem-Bertrandt A, Chavez-Macgregor M, Lei X, Brown EN, Lee RT, Meric-Bernstam F, et al. Beta-blocker use is associated with improved relapse-free survival in patients with triple-negative breast cancer. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29:2645–52.
Barron TI, Connolly RM, Sharp L, Bennett K, Visvanathan K. Beta blockers and breast cancer mortality: a population- based study. J Clin Oncol: Off J Am Soc Clin Oncol. 2011;29:2635–44.
De Giorgi V, Grazzini M, Gandini S, Benemei S, Lotti T, Marchionni N, et al. Treatment with beta-blockers and reduced disease progression in patients with thick melanoma. Arch Intern Med. 2011;171:779–81.
Lemeshow S, Sørensen HT, Phillips G, Yang EV, Antonsen S, Riis AH, et al. Beta-blockers and survival among Danish patients with malignant melanoma: a population-based cohort study. Cancer Epidemiol Biomark Prev: Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2011;20:2273–9.
Moretti S, Massi D, Farini V, Baroni G, Parri M, Innocenti S, et al. Beta-adrenoceptors are upregulated in human melanoma and their activation releases pro-tumorigenic cytokines and metalloproteases in melanoma cell lines. Lab Investig: J Tech Methods Pathol. 2013;93:279–90.
Shimizu A, Kaira K, Kato M, Yasuda M, Takahashi A, Tominaga H, et al. Prognostic significance of L-type amino acid transporter 1 (LAT1) expression in cutaneous melanoma. Melanoma Res 2015; in press.
Kaira K, Endo M, Abe M, Nakagawa K, Ohde Y, Okumura T, et al. Biologic correlation of 2-[18F]-fluoro-2-deoxy-D-glucose uptake on positron emission tomography in thymic epithelial tumors. J Clin Oncol: Off J Am Soc Clin Oncol. 2010;28:3746–53.
Kaira K, Oriuchi N, Imai H, Shimizu K, Yanagitani N, Sunaga N, et al. CD98 expression is associated with poor prognosis in resected non-small-cell lung cancer with lymph node metastases. Ann Surg Oncol. 2009;16:3473–81.
Livingstone E, Hollestein LM, van Herk-Sukel MP, van de Poll-Franse L, Nijsten T, Schadendorf D, et al. beta-Blocker use and all-cause mortality of melanoma patients: results from a population-based Dutch cohort study. Eur J Cancer. 2013;49:3863–71.
McCourt C, Coleman HG, Murray LJ, Cantwell MM, Dolan O, Powe DG, et al. Beta-blocker usage after malignant melanoma diagnosis and survival: a population-based nested case-control study. Br J Dermatol. 2014;170:930–8.
Bravo-Calderon DM, Oliveira DT, Marana AN, Nonogaki S, Carvalho AL, Kowalski LP. Prognostic significance of beta-2 adrenergic receptor in oral squamous cell carcinoma. Cancer Biomark: Sect A Dis Mark. 2011;10:51–9.
Chen D, Xing W, Hong J, Wang M, Huang Y, Zhu C, et al. The beta2-adrenergic receptor is a potential prognostic biomarker for human hepatocellular carcinoma after curative resection. Ann Surg Oncol. 2012;19:3556–65.
Wenjuan Y, Yujun L, Ceng Y. Association of single nucleotide polymorphisms of beta2-adrenergic receptor gene with clinicopathological features of pancreatic carcinoma. Acta Histochem. 2013;115:198–203.
Sloan EK, Priceman SJ, Cox BF, Yu S, Pimentel MA, Tangkanangnukul V, et al. The sympathetic nervous system induces a metastatic switch in primary breast cancer. Cancer Res. 2010;70:7042–52.
Lang K, Drell 4th TL, Lindecke A, Niggemann B, Kaltschmidt C, Zaenker KS, et al. Induction of a metastatogenic tumor cell type by neurotransmitters and its pharmacological inhibition by established drugs. Int J Cancer J Int Cancer. 2004;112:231–8.
Liu X, Wu WK, Yu L, Li ZJ, Sung JJ, Zhang ST, et al. Epidermal growth factor-induced esophageal cancer cell proliferation requires transactivation of beta-adrenoceptors. J Pharmacol Exp Ther. 2008;326:69–75.
Pullar CE, Isseroff RR. The beta 2-adrenergic receptor activates pro-migratory and pro-proliferative pathways in dermal fibroblasts via divergent mechanisms. J Cell Sci. 2006;119:592–602.
Yuan A, Li Z, Li X, Yi S, Wang S, Cai Y, et al. The mitogenic effectors of isoproterenol in human hepatocellular carcinoma cells. Oncol Rep. 2010;23:151–7.
Stork PJ, Schmitt JM. Crosstalk between cAMP and MAP kinase signaling in the regulation of cell proliferation. Trends Cell Biol. 2002;12:258–66.
Wrobel LJ, Le Gal FA. Inhibition of human melanoma growth by a non-cardioselective beta-blocker. J Investig Dermatol. 2015;135:525–31.
Acknowledgements
We appreciate Ms. Yuka Matsui for her technical assistance regarding the submitted manuscript and Ms. Tomoko Okada for her help with the data collection and technical assistance. We would also like to sincerely thank Prof. Masahiko Nishiyama of Department of Molecular Pharmacology and Oncology, Gunma University Graduate School of Medicine, for his critical review of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Shimizu, A., Kaira, K., Mori, K. et al. Prognostic significance of β2-adrenergic receptor expression in malignant melanoma. Tumor Biol. 37, 5971–5978 (2016). https://doi.org/10.1007/s13277-015-4420-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4420-0