Abstract
The αvβ6 integrin is a cell surface adhesion molecule that plays critical roles in the injury and inflammation processes. We hypothesized that polymorphisms in αvβ6 integrin genes (ITGAV and ITGB6) might be associated with the risk of radiation pneumonitis (RP) in lung cancer patients treated with thoracic radiation. We genotyped three potentially functional αvβ6 integrin single-nucleotide polymorphisms (rs1839123 and rs4129787 in ITGAV, and rs4665162 in ITGB6) and found that the genotypes of rs4665162 single-nucleotide polymorphisms were predictors of RP development (for grade ≥2 RP, HR = 1.505, 95 % CI 1.069–2.119, P = 0.019; for grade ≥3 RP, HR = 3.006, 95 % CI 1.431–6.313, P = 0.004). Our results showed that AG/GG genotypes of ITGB6 rs4665162 gene were associated with a higher risk of RP in patients with lung cancer receiving radiotherapy and thus may serve as a reliable predictor of RP. Further studies will be needed to confirm these findings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is one of the most common cancers and the leading cause of cancer-related deaths worldwide [1]. Radiation therapy, alone or in combination with sequential or concurrent chemotherapy, plays a critical role in the treatment of lung cancer [2]. Nevertheless, radiation pneumonitis (RP), which is known as a significant dose-limiting complication in thoracic irradiation, can occur in the inflammation process of normal lung tissues induced by irradiation, with an incidence about of 15 % for severe RP [3]. Previous studies have confirmed that multiple patient-related and therapeutic factors, such as pulmonary function, dosimetric parameters, previous treatment, smoking status, and sex, are related to the risk of RP [4]. Additionally, some genetic variants, for instance, single-nucleotide polymorphisms (SNPs) of multiple genes, can be used as biomarkers to predict RP risk, suggesting that genetic factors might have an impact upon the development of RP [5–7].
The αvβ6 integrin is a cell surface adhesion molecule composed of αv and β6 subunits that has been suggested to play critical roles during developmental and pathological processes [8–11]. αvβ6 can be induced by inflammation process and injury. For example, αvβ6 mRNA is highly expressed in response to acute injury within 5 h, and αvβ6 protein can be detected in the airway epithelial cells of patients with various inflammatory lung diseases [12–14]. Additionally, the expression of αvβ6 is significantly elevated in the radiation-induced lung injury model. Indeed, mice lacking integrin αvβ6 do not develop radiation-induced lung fibrosis (RILF), which occurs at the late stage of RP [15].
Previous researches, including genome-wide association studies, have demonstrated that the αvβ6 integrin gene (ITGAV and ITGB6 genes) polymorphisms were associated with rheumatoid arthritis [16], chronic hepatitis [17], type 2 diabetes [18], and amelogenesis imperfect [19] susceptibility. However, no evidence showed that ITGAV and ITGB6 gene polymorphisms were related to RP risk in lung cancer patients undergoing irradiation treatment. Since αvβ6 integrin may have a significant effect on the inflammation process caused by radiation, we hypothesized that SNPs of the ITGAV and ITGB6 genes were related to RP risk in patients with lung cancer and therefore could serve as biomarkers predicting RP susceptibility of lung cancer patients who received radiotherapy. Therefore, we evaluated the associations between polymorphisms in ITGAV and ITGB6 genes and the development of severe RP in lung cancer patients treated with radiotherapy.
Materials and methods
Patients
For our prospective study, data were obtained from a dataset of 301 patients with histopathologically confirmed lung cancer who were treated with radiation at Tongji Hospital, Huazhong University of Science and Technology (Wuhan, Hubei province, China) between 2009 and 2014. Among these 301 patients, 261 patients having a radiation dose at least 45 Gy, KPS > 60, and a life expectancy of at least 6 months were included for the final genotyping analysis. Patients with previous thoracic irradiation or severe cardiopulmonary diseases were excluded from our study. Samples from 169 patients were first used to genotype the two candidate SNPs by MassArray to screen for the RP susceptibility variants. The significant SNP was then genotyped by Sanger Sequencing in the remaining 92 patients. This investigation was approved by the Review Board of Tongji Hospital. Written informed consent was obtained from all patients for the use of their clinical information and for obtaining their blood and DNA.
Treatment
All patients received radiotherapy with 6-MV X-rays from a linear accelerator (Elekta Synergy, Elekta, Sweden). The median total radiation dose was 54 Gy (range, 45 to 66 Gy), with 1.5 to 2 Gy administered per radiation treatment. Intensity-modulated radiation therapy (IMRT) was administered to 62.1 % of patients (n = 162). Computed tomography simulation (CT/e, GE, Fairfield, CT, USA) was performed before the radiotherapy (RT) treatment was planned. The target volumes and critical normal organs were delineated by the three-dimensional planning system (Pinnacle Version 9.2). The baseline clinical characteristics and treatment details of the patients are shown in Table 1.
Evaluation of RP
All patients included in this investigation were examined during and 1 month after radiation therapy. Patients were followed every 3 months for the first year and every 6 months thereafter. Radiographic examination by chest X-ray or CT was performed at each follow-up visit. RP was graded by two radiation oncologists according to the Common Terminology Criteria for Adverse Events 4.0 as follows: grade 0, no change; grade 1, asymptomatic and diagnosed by radiographic findings only; grade 2, symptomatic, not interfering with daily activities; grade 3, symptomatic, interfering with daily activities or oxygen required; grade 4, assisted ventilation required; or grade 5, fatal.
Genotyping
Genomic DNA was extracted with a PureLink Genomic DNA Mini Kit (Invitrogen, K1820-01) from peripheral blood. The DNA purity and concentration were determined by spectrophotometric measurement of absorbance at 260 and 280 nm, respectively.
We searched the National Institute of Environmental Health Sciences Genome Program geneSNPs database, Ensembl, and HapMap HCB data to identify functional SNPs of the ITGAV and ITGB6 genes with a minor allele frequency more than 0.05 in the Chinese population. One SNP of the ITGAV gene locating in the 3′-untranslated region and two SNPs of the ITGB6 gene locating in the enhancer or promoter flanking region were selected. For the three candidate SNPs, genotypes were first determined using the MassARRAY system (Sequenom iPLEX assay, San Diego, USA). The sample DNA was amplified by a multiplex PCR reaction, and the PCR products were then used for a locus-specific single-base extension reaction. Finally, the resulting products were desalted and transferred to a 384-element SpectroCHIP array. The alleles were discriminated by mass spectrometry (Sequenom, San Diego, USA). After that, the RP susceptibility SNP rs4665162 was genotyped by Sanger Sequencing method in the remaining 92 patients. The primer pairs for rs4665162 were F 5′-CACAAATCCATGACAAGCTAAC-3′ and R 5′-GGGGTATGTACCACTTCCACTC-3′. The PCR products were then subjected to DNA sequencing to detect mutations.
β6 integrin expression
Immunohistochemical analysis was performed on formalin-fixed, paraffin-embedded specimens of lung tissues from 42 patients. The concentration of the sheep monoclonal antibody against integrin beta 6 (R&D systems, AF4155) was 200 mg/mL and working dilution was 1:50. The secondary antibody was donkey anti-sheep antibody (ZSGB-BIO, PV9000). Quantification was performed using Image-Pro Plus 6.0. Images were analyzed by a person blinded to groups and t test was used to assess the differences between two groups.
Statistical analysis
Patients were grouped according to their genotypes. Statistical analysis was performed using SPSS 20.0 statistical software (SPSS Inc., Chicago, IL). Cox proportional hazards analysis was performed to calculate the hazard ratio (HR) and confidence interval (CI) to evaluate the influence of genotypes on RP risk. In addition, multivariate Cox regression was performed to adjust for other covariates. The cumulative incidence of RP was estimated using the Kaplan-Meier method. All statistical tests were done with a two-sided P < 0.05 level.
Results
Patient’s characteristics and risk of RP
Table 1 lists the characteristics of the 261 patients. This study included 198 men and 63 women, with a median age of 58 years (range, 29 to 79 years). Among them, 60.9 % were current or former smokers, 90.0 % had stage III/IV disease, and 96.2 % were treated with a combination of chemotherapy and radiotherapy. The median values for radiation dose, mean lung dose (MLD), and volume of normal lung receiving 20 Gy (V20 or more radiation) were 54.0 and 13.5 Gy and 24.2 %, respectively. Thus, for this study, 54.0 and 13.0 Gy and 24.0 % were used as cutoff points for radiation dose, MLD, and V20, respectively, to define patient subgroups for further analysis. Of all the 261 patients, 37 (14.2 %) developed RP of grade 3 or higher, 107 (41.0 %) developed grade 2 RP, and 117 (44.8 %) grade 1 or lower.
Table 1 also shows the associations between patient-, tumor-, and therapy-related characteristics and RP grade ≥3 by univariate and multivariate analyses. MLD, V20, and age had statistically significant associations with RP by both univariate and multivariate analyses, while the IMRT was marginally related with RP risk by univariate analysis and significantly associated with RP risk by multivariate analysis. However, neither KPS nor smoking status was associated with RP risk in this population. The same analyses were performed for RP grade ≥2, and similar results were observed (Online Resource).
RP and αvβ6 integrin gene SNPs and genotypes
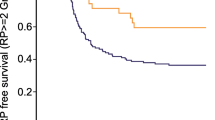
Tables 2 and 3 list the association of ITGAV (rs1839123) and ITGB6 (rs4129787, rs4665162) genotypes with the risks of grade ≥2 and grade ≥3 RP. In general, RP developed more often in patients exhibiting CT/TT in rs1839123, CT/TT in rs4129787, or AG/GG in rs4665162 genotypes, with grades ≥3 RP rates of 26.0, 21.0, and 19.6 %, respectively, than in those having CC in rs1839123, CC in rs4129787, or AA in rs4665162 genotypes, for which the incidences were 16.8, 18.7, and 8.1 %, respectively. The association was statistically significant for rs4665162 both in grade ≥2 and grade ≥3 RP (P = 0.019 and P = 0.004, respectively). Figure 1 plots the incidence of RP grade ≥2 and grade ≥3 as a function of time for rs4665162. Development of RP grade ≥2 and grade ≥3 seems to be delayed, and the incidence remained higher in the AG/GG genotype in rs4665162 (P = 0.029 and P = 0.010, respectively).
Univariate and multivariate analyses
Univariate Cox proportional hazard analyses of the data for grade ≥2 and grade ≥3 RP showed that AG/GG rs4665162 genotypes were associated with an increased risk of RP development (for grade ≥2 RP, HR = 1.446, 95 % CI 1.036 to 2.018, P = 0.030; for grade ≥3 RP, HR = 2.519, 95 % CI 1.219 to 5.204, P = 0.013, respectively; Tables 2 and 3). This effect was virtually unchanged after adjustment for age, histology, surgery, IMRT, CRT and V20, or MLD by multivariate analysis (for grade ≥2 RP, HR = 1.505, 95 % CI 1.069 to 2.119, P = 0.019; for grade ≥3 RP, HR = 3.006, 95 % CI 1.431 to 6.313, P = 0.004), suggesting that rs4665162 SNP is an independent factor for RP risk (Tables 2 and 3).
Dosimetric factors
Figure 2 shows the cumulative RP grade ≥2 and grade ≥3 probability as a function of time according to genotype and MLD. Patients with AG/GG genotypes in rs4665162 who received an MLD ≥ 13.0 Gy had a higher RP incidence than patients with an MLD ≥ 13.0 Gy with AA genotype in rs4665162 (P = 0.015, P = 0.034, respectively). We also analyzed the cumulative RP probability as a function of time according to genotypes and V20 (Fig. 3). Patients with AG/GG genotypes in rs4665162 who had V20 ≥ 24 % of their normal lung volume had a higher incidence of RP than patients with a V20 ≥ 24 % with AA genotype in rs4665162 (P = 0.017, P = 0.020, respectively). However, this difference was not significant in patients who received MLD less than 13.0 Gy (Fig. 2) or V20 less than 24 % (Fig. 3), suggesting that the effect of the dosimetric parameters on RP grade ≥2 and grade ≥3 was independent of genetic predisposition.
Genotypes of rs4665162 and β6 integrin expression
The expression of β6 integrin in lung tissue of RP patients in AG/GG genotypes and AA genotype was detected by immunohistochemical method (Fig. 4). The AG/GG genotype in rs4665162 was associated with higher β6 integrin expression in lung tissues as compared with AA genotypes (n = 40, P = 0.017).
β6 integrin immunohistochemical analysis: the low expression of β6 integrin in lung tissues of AA genotypes (×200 magnification) (a), the high expression of β6 integrin in lung tissues of AG/GG genotypes (×200 magnification) (b), the AG/GG genotypes in rs4665162 were associated with higher β6 integrin expression in lung tissues as compared with AA genotype (c)
Discussion
Our study demonstrated that patients exhibiting CT/TT in rs1839123, CT/TT in rs4129787, or AG/GG in rs4665162 genotypes of the αvβ6 integrin genes had a higher rate of RP incidence after radiation therapy for lung cancer and significantly so with AG/GG in rs4665162. The association between AG/GG genotypes in rs4665162 polymorphisms and higher RP risk was independent of MLD or V20. We identified a group of patients with the highest RP risk (AG/GG genotype in rs4665162 and V20 ≥ 24 % or MLD ≥ 13.0 Gy). Since we can get access to these information before radiotherapy starts, the gene detection could be used as a predictive biomarker to prescribe personalized irradiation treatment apart from dosimetric factors. To the best of our knowledge, this is the first study proving that AG/GG genotypes of rs4665162 predict a higher RP risk in patients with lung cancer.
Radiation therapy is one of the most commonly used approaches to inhibit primary tumor to reduce or delay the chance of local recurrence or metastasis and improve prognosis [20, 21]. Nevertheless, it may also cause severe adverse effects that can limit volumes and radiation doses, thus restricting the use of irradiation treatment. RP is a dysregulated inflammation in response to normal lung tissue injury [2]. Previous studies have demonstrated that higher values of V20 and MLD could be independent risk factors related to RP severity [7, 22], and these observations are confirmed in our current study. Additionally, we found that patients in the elderly subgroup (age ≥58) were more susceptible to grade ≥3 RP than younger patients. This may be explained by the factor that the elderly patients were prone to have chronic underlying lung diseases and inferior physical status [23]. Furthermore, patients receiving IMRT had lower risk of grade ≥3 RP, with the incidence of grade ≥3 RP significantly lower in the IMRT group than in those treated with conventional radiotherapy (11.1 versus 19.2 %). This is probably because, compared with other radiation approaches, IMRT adds fluence modulation to beam shaping, which improves dose conformity around the tumor and spares surrounding normal structures [24]. This has important implications in that IMRT might be an optimized radiation approach for patients with inherent high RP risk (AG/GG genotypes of rs4665162 and age ≥58).
The αvβ6 integrin is an epithelial restricted molecule with a variety of effects on inflammation, cell proliferation, and fibrosis. Studies have demonstrated that integrin αvβ6 can activate the latent form of transforming growth factor-β1 (TGFβ1) [25], which plays a critical role in the progress of RP [26, 27]. An animal study reported the increased expression of αvβ6 in alveolar epithelial cells after radiation injury, and mice lacking integrin αvβ6 do not develop RILF, which occurs at the later stage of the RP, because of the inactivation of TGFβ1 [15]. Additionally, multiple studies have reported the association between SNPs in the TGFβ1 gene and RP risk in patients with lung cancer [7, 28–30]. Based on the above, we performed this study to investigate whether the differences in αvβ6 integrin genotypes are related to RP severity in patients with lung cancer. We identified an association between SNP rs4665162 in ITGB6 gene and risk of RP. However, the SNP genotypes of ITGAV gene are not statistically significantly correlated with RP risk in our study. This is possibly because the αv subunit can dimerize not only with the β6 subunit but also with integrin β subunits β1, β3, β5, and β8 [31], which have not yet been proved to be associated with RP, suggesting that the β6 subunit may play a critical role in RP rather than the αv subunit.
In our current study, ITGB6 rs4665162 was significantly associated with RP development in lung cancer patients who received radiation treatment, independent of therapy-related, patient-related, and tumor-related factors. The different expression of β6 in the lung tissue of RP patients due to genotypes of rs4665162 showed that this SNP, which was located in the promoter flanking region, may play a significant role in the regulation of ITGB6 gene expression and thus regulate patient susceptibility to RP. Especially noteworthy were the remarkable variance in RP risk according to rs4665162 genotypes when patients were stratified based on V20 and MLD. This result demonstrates that genetic factors may play an even more critical role in RP susceptibility in an individual patient when either a greater volume of lung was exposed to high doses of radiation or that relatively high doses were delivered. It is necessary to point out that values of grade ≥2 RP were more statistically significant than values of grade ≥3 both in the MLD and V20 stratified analysis. This seems to imply that once the volume or dose is relatively high, the inherent radiosensitivity may potentially override dosimetric factors to play an even more important role in the progress of RP, especially of the grade ≥2 RP.
In spite of these positive observations, the current study has some limitations. Firstly, we applied the method of candidate polymorphism selection, which let us concentrate on potentially functional SNPs, so that we could not extensively cover all SNPs in the entire gene and some rare but important SNPs might not have been included or the observed association might have been a result of genetic linkages with other untyped SNPs. In addition, we were not able to investigate the mechanism by which the ITGB6 polymorphisms lead to RP in patients with lung cancer and its interaction with other factors. Finally, it is also necessary to observe the role of these SNPs playing in disease outcomes.
In summary, the current study identified genetic biomarkers to predict RP risk for lung cancer patients before initiation of radiotherapy. We identified that the AG/GG genotypes of ITGB6 rs4665162 were associated with higher RP risk in patients with lung cancer who received radiotherapy, suggesting the probability of using these gene polymorphisms as biomarkers to predict RP susceptibility. Further investigations are required to validate these findings.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. United States.
Cannon DM, Mehta MP, Adkison JB, Khuntia D, Traynor AM, Tome WA, et al. Dose-limiting toxicity after hypofractionated dose-escalated radiotherapy in non-small-cell lung cancer. J Clin Oncol. 2013;31:4343–8. United States.
Yin M, Liao Z, Liu Z, Wang L-E, O’Reilly M, Gomez D, et al. Genetic variants of the nonhomologous end joining gene LIG4 and severe radiation pneumonitis in nonsmall cell lung cancer patients treated with definitive radiotherapy. Cancer. 2012;118:528–35. United States.
Provatopoulou X, Athanasiou E, Gounaris A. Predictive markers of radiation pneumonitis. Anticancer Res. 2008;28:2421–32. Greece.
Pu X, Wang L, Chang JY, Hildebrandt MAT, Ye Y, Lu C, et al. Inflammation-related genetic variants predict toxicity following definitive radiotherapy for lung cancer. Clin Pharmacol Ther. 2014;96:609–15. United States.
Mak RH, Alexander BM, Asomaning K, Heist RS, Liu C, Su L, et al. A single-nucleotide polymorphism in the methylene tetrahydrofolate reductase (MTHFR) gene is associated with risk of radiation pneumonitis in lung cancer patients treated with thoracic radiation therapy. Cancer. 2012;118:3654–65. United States.
Yuan X, Liao Z, Liu Z, Wang L-E, Tucker SL, Mao L, et al. Single nucleotide polymorphism at rs1982073:T869C of the TGFbeta 1 gene is associated with the risk of radiation pneumonitis in patients with non-small-cell lung cancer treated with definitive radiotherapy. J Clin Oncol. 2009;27:3370–8. United States.
Munger JS, Huang X, Kawakatsu H, Griffiths MJ, Dalton SL, Wu J, et al. The integrin alpha v beta 6 binds and activates latent TGF beta 1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell. 1999;96:319–28. United States.
Sugimoto K, Kudo M, Sundaram A, Ren X, Huang K, Bernstein X, et al. The alphavbeta6 integrin modulates airway hyperresponsiveness in mice by regulating intraepithelial mast cells. J Clin Invest. 2012;122:748–58. United States.
Moore KM, Thomas GJ, Duffy SW, Warwick J, Gabe R, Chou P, et al. Therapeutic targeting of integrin alphavbeta6 in breast cancer. J Natl Cancer Inst. 2014;106:pii: dju169. United States.
Pi L, Robinson PM, Jorgensen M, Oh S-H, Brown AR, Weinreb PH, et al. Connective tissue growth factor and integrin alphavbeta6: a new pair of regulators critical for ductular reaction and biliary fibrosis in mice. Hepatology. 2015;61:678–91. United States.
Vujaskovic Z, Thrasher BA, Jackson IL, Brizel MB, Brizel DM. Radioprotective effects of amifostine on acute and chronic esophageal injury in rodents. Int J Radiat Oncol Biol Phys. 2007;69:534–40. United States.
Breuss JM, Gallo J, DeLisser HM, Klimanskaya IV, Folkesson HG, Pittet JF, et al. Expression of the beta 6 integrin subunit in development, neoplasia and tissue repair suggests a role in epithelial remodeling. J Cell Sci. 1995;108(Pt 6):2241–51. England.
Weinacker A, Ferrando R, Elliott M, Hogg J, Balmes J, Sheppard D. Distribution of integrins alpha v beta 6 and alpha 9 beta 1 and their known ligands, fibronectin and tenascin, in human airways. Am J Respir Cell Mol Biol. 1995;12:547–56. United States.
Puthawala K, Hadjiangelis N, Jacoby SC, Bayongan E, Zhao Z, Yang Z, et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am J Respir Crit Care Med. 2008;177:82–90. United States.
Shakiba E, Tavilani H, Goodarzi MT, Kiani A, Pourmotabbed T, Vaisi-Raygani A. The ITGAV-rs3911238 polymorphism is associated with disease activity in rheumatoid arthritis. Iran J Allergy Asthma Immunol. 2014;13:356–63. Iran
Lee SK, Kim M-H, Cheong JY, Cho SW, Yang S-J, Kwack K. Integrin alpha V polymorphisms and haplotypes in a Korean population are associated with susceptibility to chronic hepatitis and hepatocellular carcinoma. Liver Int. 2009;29:187–95. England.
Qi L, Cornelis MC, Kraft P, Stanya KJ, Linda Kao WH, Pankow JS, et al. Genetic variants at 2q24 are associated with susceptibility to type 2 diabetes. Hum Mol Genet. 2010;19:2706–15. England.
Poulter JA, Brookes SJ, Shore RC, Smith CEL, Abi Farraj L, Kirkham J, et al. A missense mutation in ITGB6 causes pitted hypomineralized amelogenesis imperfecta. Hum Mol Genet. 2014;23:2189–97. England.
Van Meerbeeck JP, Fennell DA, De Ruysscher DKM. Small-cell lung cancer. Lancet. 2011;378:1741–55. (London, England). England.
Goldstraw P, Ball D, Jett JR, Le Chevalier T, Lim E, Nicholson AG, et al. Non-small-cell lung cancer. Lancet. 2011;378:1727–40. (London, England). England.
Schallenkamp JM, Miller RC, Brinkmann DH, Foote T, Garces YI. Incidence of radiation pneumonitis after thoracic irradiation: dose-volume correlates. Int J Radiat Oncol Biol Phys. 2007;67:410–6. United States.
Kong F-MS, Wang S. Nondosimetric risk factors for radiation-induced lung toxicity. Semin Radiat Oncol. 2015;25:100–9. United States.
Chan C, Lang S, Rowbottom C, Guckenberger M, Faivre-Finn C. Intensity-modulated radiotherapy for lung cancer: current status and future developments. J Thorac Oncol. 2014;9:1598–608. United States.
Shi M, Zhu J, Wang R, Chen X, Mi L, Walz T, et al. Latent TGF-beta structure and activation. Nature. 2011;474:343–9. England.
Park J, Ryu S-H, Choi EK, Ahn SD, Park E, Choi K-C, et al. SKI2162, an inhibitor of the TGF-beta type I receptor (ALK5), inhibits radiation-induced fibrosis in mice. Oncotarget. 2015;6:4171–9. United States.
Li J, Mu S, Mu L, Zhang X, Pang R, Gao S. Transforming growth factor-beta-1 is a serum biomarker of radiation-induced pneumonitis in esophageal cancer patients treated with thoracic radiotherapy: preliminary results of a prospective study. Onco Targets Ther. 2015;8:1129–36. New Zealand.
Niu X, Li H, Chen Z, Liu Y, Kan M, Zhou D, et al. A study of ethnic differences in TGFbeta1 gene polymorphisms and effects on the risk of radiation pneumonitis in non-small-cell lung cancer. J Thorac Oncol. 2012;7:1668–75. United States.
Alsbeih G, Al-Harbi N, Al-Hadyan K, El-Sebaie M, Al-Rajhi N. Association between normal tissue complications after radiotherapy and polymorphic variations in TGFB1 and XRCC1 genes. Radiat Res. 2010;173:505–11. United States.
Zhang L, Yang M, Bi N, Ji W, Wu C, Tan W, et al. Association of TGF-beta1 and XPD polymorphisms with severe acute radiation-induced esophageal toxicity in locally advanced lung cancer patients treated with radiotherapy. Radiother Oncol. 2010;97:19–25. Ireland.
Henderson NC, Arnold TD, Katamura Y, Giacomini MM, Rodriguez JD, McCarty JH, et al. Targeting of alphav integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013;19:1617–24. United States.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Compliance with ethical standards
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Fund
This study was funded by National Natural Science Foundation of China (grant numbers 81272492 and 81472921).
Conflicts of interest
None.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Yi, M., Tang, Y., Liu, B. et al. Genetic variants in the ITGB6 gene is associated with the risk of radiation pneumonitis in lung cancer patients treated with thoracic radiation therapy. Tumor Biol. 37, 3469–3477 (2016). https://doi.org/10.1007/s13277-015-4171-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-4171-y