Abstract
Hypoxia-inducible factor-1α (HIF-1α) is a major effector in cell survival response to hypoxia, while the roles of HIF-1α in radiation-induced autophagy are still unclear in breast cancer cells. Human breast cancer carcinoma MCF-7 cells were stably transfected with pSUPER-shRNA against human HIF-1α or a scrambled sequence with no homology to mammalian genes, named as pSUPER-HIF-1α and pSUPER-SC, respectively. Cell Counting Kit-8 (CCK-8) assay and colony formation assay were used to detect cell viability, Western blot was used to detect protein expression, monodansylcadaverine (MDC) staining was used to analyze autophagy, and Hoechts/PI staining was used to assess apoptosis. Ionizing radiation (IR) and cobalt chloride (CoCl2) could induce HIF-1α expression and increase the microtubule-associated protein 1 light chain 3 (MAPLC3)-II/MAPLC3-I ratio, especially in radiation + CoCl2 group. After the silencing of HIF-1α, the radiosensitivity of MCF-7 cells increased and the autophagy level decreased in response to DNA damage induced by ionizing radiation, but there was no influence on IR-induced apoptosis. HIF-1α silencing also increased the expression of phospho-Akt, mTOR, and P70S6K and activated the mTOR signals significantly. Hypoxia can induce autophagy and also improve the IR-induced autophagy via the suppression of Akt/mTOR/P70S6K pathway, which consequently lead to radioresistance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Local hypoxic microenvironment of solid tumors is one of the most important characteristics. The relative genes will participate in the regulation of cell death in response to DNA damage under the hypoxic environment; among them, hypoxia-inducible factor (HIF)-1 plays central roles [1]. Nowadays, HIF-boosting drugs could be used for the treatment of infectious diseases, anemia, and so on [2]. HIF-1, an important intermediary factor to adapt hypoxia response, consists of subunits HIF-1α and HIF-1β, and its post-translational modifications can regulate HIF-1α activity [3]. HIF-1 is associated with tumor angiogenesis, energy metabolism, invasion, metastasis, and the resistance to chemotherapy and radiotherapy [4].
Although apoptosis is previously thought of as the primary mechanism of radiation-induced cell death, an alternative cell death type, autophagic cell death (programmed cell death, type II), has emerged recently as an important mechanism for radiation-induced cell death in tumors [5]. Increasing evidence proved the potential regulatory roles of p53 in the process of autophagy [6]. More importantly, some investigations have demonstrated that the co-regulation of both apoptosis and autophagy can participate in mammalian cell death [7], apoptosis, and autophagy, may be interconnected, and even simultaneously regulated by the same triggering factors [8]. Under certain circumstance, apoptosis and autophagy seem to be interconnected positively or negatively, and there might be a “molecular switch” between them. Undoubtedly, there are multiple connections between apoptotic and autophagic processes that can jointly seal the fate of tumor cells [9].
In this study, we managed to analyze the roles of HIF-1α in radiation-induced autophagic cell death in breast cancer cells, to elucidate whether HIF-1α participates in the regulation of cell death induced by ionizing radiation (IR) and its underlying mechanism.
Material and methods
Cell line, antibodies, and reagents
Human breast cancer cell line, MCF-7, were cultured in Dulbecco’s modified Eagle’s medium (DMEM) (GIBCO) supplemented with 10 % fetal bovine serum (FBS) and 1 % penicillin∕streptomycin (Invitrogen, Carlsbad, CA) in glass Petri dishes at 37 °C in a 5 %CO2 incubator.
Fetal bovine serum (FBS), Cell Counting Kit-8 (CCK-8, Dojin Laboratories, Kumamoto, Japan), 3-methyladenine (3-MA), and monodansylcadaverine (MDC) were purchased from Sigma Chemical (St. Louis, MO, USA), pSUPER retroviral vector was obtained from OligoEngine (Seattle, WA, USA). Rabbit polyclonal antibodies against microtubule-associated protein 1 light chain 3 (MAPLC3), Akt, mTOR, and P70S6K were purchased from Cell Signaling Technology (Cell Signaling, Beverly, MA, USA); mouse polyclonal antibodies against glyceraldehyde 3-phosphate dehydrogenase (GAPDH) and mouse monoclonal antibody against HIF-1α were purchased from BD Biosciences (San Jose, CA, USA), peroxidase-conjugated anti-mouse IgG and peroxidase-conjugated anti-rabbit IgG were purchased at Santa Cruz (Santa Cruz, CA, USA).
Radiation
X-ray generator (X-RAD 320ix, Precision X-ray Inc., USA) was utilized to deliver radiation at a dose rate of 0.40 Gy/min.
The establishment of HIF-1α silencing cells
ShRNAs were designed according to “www.idtdna.com.” ShRNAs were synthesized, denatured, and ligated to pSUPER vector at BglII and HindIII sites. ShRNA sequences are as follows: for HIF-1α, sense sequence: 5′-GATCTGAACTAGCCGAGGAAGAACTATGATCAAGAGTCATAGTTCTTCCTCGGCTAGTTTTTTTTA-3′; antisense sequence: 5′-AGCTTAAAAAAAACTAGCCGAGGAAGAACTATGACTCTTGATCATAGTTCTTCCTCGGCTAGTTCA-3′. The pSUPER-HIF-1α and the vector with a scrambled sequence, i.e., pSUPER, were constructed in our lab. Then they were transfected into packaging cells 293 T by calcium phosphate co-precipitation to produce pseudovirus particle [(Ampho Pack plasmid 10 μg, pSUPER pSUPER-HIF-1α 10 μg, 2 mol/LCaCl2 31 μl, ddH2O to 250 μl, and 2 × HEPES buffer salt solution (HBS) 250 μl)], the supernatant containing pseudovirus particle was collected and used to infect MCF-7. Positive stable clones were selected by growing cells with puromycin (1 μg/ml) for 1 week.
Colony formation analysis
Cells were plated into 60-mm petri dishes using standard culture medium; different doses of radiation (0, 2, 4, 6, 8 Gy) were given. Two weeks later, cells were fixed with 4 % formaldehyde and stained with 0.5 % crystal violet, and surviving colonies of >50 cells were scored under a dissection microscope (Olympus XSZ-D2, Japan). The surviving fraction for a given treatment dose was calculated as the plating efficiency of the irradiated samples relative to that of the sham-irradiated ones. Multi-target click model of GraphPad Prism 5.0 (Systat Software, USA) was used to fit cell survival curves. The dose quasithreshold (Dq) and mean lethal dose (D0) were calculated.
Cell viability assay
MCF-7 cells were seeded in 96-well plates at a density of 4000 cells/well, 48 h after different treatments; 10 μl of the CCK-8 solution was added to each well, and cells were incubated for 1 h in an incubator. The absorbance was measured at 450 nm using a microplate reader (Synergy HT, Bio-Tek, USA).
Western bolt analysis
The total proteins were extracted with RIPA lysis buffer [HEPES(50 mM), Nacl(150 mM), EDTA (1 mM), EGTA (2.5 mM), NaF (10 mM), DTT (1 mM), SV (1 mM), PMSF (1 mM), NP-40 (1 %), SDS (0.1 %)]; 2 ml aliquot was mixed with 20-μl protease inhibitor cocktail. Unless indicated, 40 μg of total protein was separated by SDS-PAGE, transferred to nitrocellulose membrane. The membrane was then blocked in 5 % dried milk or 3 % bovine serum albumin in Tris-buffered saline and Tween 20 (10 mmol/L Tris, pH 7.5;100 mmol/l NaCl; 0.1 % Tween 20), incubated with primary antibodies, and then with appropriate horseradish peroxidase-conjugated secondary antibodies. The signals were visualized by chemiluminescence system according to the manufacturer’s instruction (Santa Cruz, USA). GAPDH was used as an internal standard. The intensity of protein bands were quantified using image software (The Discovery Series), and the ratio of specific band to control was analyzed.
Apoptosis assay
The morphological observation of apoptosis was measured by Hoechts/PI staining. Cells were seeded in a 6-well flat-bottomed plate; 48 h after, different treatment cells were washed with cold phosphate buffered saline (PBS) twice followed by incubation with 5 μl Hoechts and 5 μl PI at 4 °C for 30 min. After 15 min of fixation with a solution of 4 % paraformaldehyde, cells were visualized by fluorescence microscopy. Cells with condensed chromatin, and fragmented nuclei were taken as apoptotic cells.
Autophagy assay
Cells were cultured on cover slips overnight. Twenty-four hours after the indicated irradiation treatment, cells were washed with cold phosphate buffered saline (PBS) twice. Autophagic vacuoles were labeled with 0.05 mM monodansylcadaverine (MDC) in PBS at 37 °C for 10 min. Cells were then washed three times with PBS and immediately analyzed by fluorescence microscopy using an inverted microscope equipped with a filter system (excitation filter V-2A: 380–420 nm, barrier filter: 450 nm). Images were obtained with a CCD camera (Orca I, Hamamatsu) and processed using the program Meta View, version 4.5 (Universal Images Corporation). The percentage of MDC positive cells expressing punctuate staining was analyzed under fluorescence microscope.
Statistical analysis
Statistical evaluations are presented as mean ± S.E. Data were analyzed using Student’s t test, one-way ANOVA test, or χ 2 test using SPSS v17.10 software for statistical significance. P < 0.05 was considered significant.
Results
HIF-1α induce radioresistance in MCF-7 cells
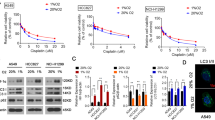
In order to elucidate the roles of hypoxia in radiation-induced cell death, CoCl2 (150 μmol/L) was used to mimic hypoxic environment. MCF-7 cells were treated with IR (8 Gy) or CoCl2, and cell viability was detected by CCK-8 assay (Fig. 1a). The result showed that after 48 h treatment, the survival rate in IR group decreased significantly. However, the viability of the group co-treated with hypoxia and IR did not change compared with that of the control group, indicating that hypoxia could antagonize the IR-induced cell death. Since hypoxia can increase HIF-1α expression, then we want to know whether HIF-α plays roles in this process. We established isogenic MCF-7 cell models in which control shRNA or HIF-1α shRNA were stably expressed. The results showed that HIF-1α silencing could successfully decrease hypoxia-induced HIF-1α expression (Fig. 1b). HIF-1α knockdown decreased the colony formation in response to IR (Fig. 1c), suggesting the increase of radiosensitivity.
HIF-1α changes the cell survival and induces radioresistance. a MCF-7 cells were exposed to normoxia, hypoxia, or IR. After 48 h, the cell viability was analyzed by CCK-8 assay. b Establishment of isogenic cell lines with HIF-1α silencing. Knockdown effects were confirmed by Western blot. Cells were exposed to the indicated doses of IR with or without hypoxia. c Both HIF-1α silencing cells and control cells were pretreated with CoCl2 24 h before radiation (0–8 Gy), then the radiosensitivity was assessed by the colony formation assay. Data were presented as mean ± SD of three independent experiments. *P < 0.05 versus control group
HIF-1α did not change the radiation-induced apoptosis in MCF-7 cells
As HIF-1α had an effect on cell death, we sought to figure out whether HIF-1α enhances radiation-induced apoptosis. Apoptosis was evaluated by Hoechts/PI staining (Fig. 2a, b). We found that the apoptosis increased when wild-type and HIF-1α silencing cells were treated with hypoxia and radiation. The important hallmarker for apoptosis, cleavage of PARP-1, was also detected, since caspase-3 and caspase-7 also cleaved PARP-1 [10]. There were no detectable change between the control group and HIF-1α knockdown group (Fig. 2c, d), indicating that HIF-1α did not change the radiation-induced apoptosis in MCF-7 cells.
HIF-1α did not change the radiation-induced apoptosis in MCF-7 cells. a Both HIF-1α silencing cells and control cells were incubated with CoCl2 (H group,150 μmol/L) for 48 h, treated with IR (8 Gy) for 24 h, or pretreated with CoCl2 (150 μmol/L) for 24 h followed by IR and stained with DAPI (1 ug/ml). Scale bar: 20 μm. b Quantitative analysis of apoptotic cells from a, data were presented as mean ± SD of three independent experiments. *P < 0.05 versus mock group. c Whole cell lysates were harvested and subjected to Western blot. d Quantitative analysis of cleaved PARP from c, GAPDH was used as an internal standard
HIF-1α enhanced the autophagy induced by IR in MCF-7 cells
Since HIF-1α participated in the regulation of cell death and it had no effect on apoptosis, how about autophagy? Autophagic cell death is also named type II programmed cell death. Then we detected whether HIF-1α can change autophagy level, which consequently affects cell death. Autofluorescent substance MDC was used as a marker for autophagy vacuoles. After 8 Gy radiation and the treatment of CoCl2, the percentage of MDC-positive cells increased, especially in radiation + CoCl2 group (Fig. 3a), suggesting that hypoxia and radiation are able to induce autophagy. MAPLC3-II is a widely used marker for autophagy; upon autophagy, the conversion from a cytosolic MAPLC3-I form to an autophagosome-bound MAPLC3-II form will appear [11, 12]. In Fig. 3b, it was illustrated radiation had no effect on HIF-1α expression, while HIF-1α expression was much higher in the combination treatment group compared with that in the single treatment group. The conversion from MAPLC3-I to MAPLC3-II indicated that both of hypoxia- and radiation-induced autophagy and autophagy increased significantly in radiation + CoCl2 group, suggesting that HIF-1α could promote the IR-induced autophagy in MCF-7 cells (Fig. 3b). 3-Methyladenine (3-MA) is an autophagic inhibitor which can control PI3KIII and prevent autophagosome formation. Forty-eight hours after the treatment of 2 mM 3-MA, cell survival was measured (Fig. 3c). 3-MA significantly sensitized MCF-7 to IR, resulting in the decrease of cell survival as compared with IR or hypoxia merely group (P < 0.05), and the survival percentage is highest in the control group as compared with the other two groups. These results support that autophagy plays a protective role in response to IR and hypoxia stress in MCF-7 cells, and the autophagy induced by HIF-1α increased MCF-7 survival. The MDC assay showed that hypoxia and radiation could induce autophagy; however, silencing of HIF-1α inhibited autophagy induced by radiation (Fig. 3d). Western blot indicated the similar results that the level of MAPLC3-II decreased in HIF-1α knockdown cells when treated with radiation, suggesting that HIF-1α can upregulate autophagy under radiation stress (Fig. 3e).
HIF-1α enhanced the autophagy induced by IR in MCF-7 cells. a MCF-7 cells were incubated with CoCl2 (H,150 μmol/L) for 48 h, treated with IR (8 Gy) for 24 h, or pretreated with CoCl2 (H,150 μmol/L) for 24 h followed by IR and stained with MDC (0.05 mM). Cells undergoing autophagy were quantitated as a percentage of MDC-positive cells. The graph shows the total number of puncta-positive cells. Scale bar: 20 μm. Data were presented as mean ± SD of three independent experiments. b In the Western blot analysis of HIF-1α and LC3 expression, GAPDH was used as an internal standard. c Three groups were divided: control, CoCl2 + IR, CoCl2 + IR + 3MA (2 mM); different doses of radiation (0, 2, 4, 6, 8 Gy) were used, and the radiosensitivity was assessed by colony formation assay. d Cells undergoing autophagy were quantitated as a percentage of MDC-positive cells. Scale bar: 20 μm. e Western blot analysis was performed to detect MAPLC3-I/II; GAPDH was used as an internal standard. Data were presented as mean ± SD of three independent experiments. *P < 0.05 versus control group
HIF-1α induce autophagy via HIF-1/Akt/mTOR pathway
It is well appreciated now that mTOR serves as the master negative regulator of autophagy [13]. In our study, Western blotting analysis showed a significant decrease in phospho-mTOR, mTOR, and P70S6K in MCF-7 cells when treated with hypoxia + IR (Fig. 4a, b). However, knockdown of HIF-1α induced the expression of phospho-Akt, mTOR, and P70S6K. These results suggested that the radiation induced the suppression of phospho-Akt, mTOR, and P70S6K could be reversed by the silencing of HIF-1α. Since Akt, mTOR, and P70S6K regulate autophagy negatively, HIF-1α could regulate IR-induced autophagy via the suppression of Akt/mTOR/P70 pathways.
HIF-1α-induced autophagy via HIF-1/Akt/mTOR pathway. a The isogenic cell lines were treated with CoCl2 (H,150 μmol/L) or IR (8 Gy). Whole cell lysates were harvested and subjected to Western blot 24 h after radiation. b Quantitative analysis of Western blot; data were presented as mean ± SD of three independent experiments. *P < 0.05 versus control group
Discussion
Because malignant proliferation as well as disorders of the blood supply of tumor cells leads to the existence of a large number of entities within the tumor hypoxic cells. When radiation is given, the peripheral portion of cancer cells with rapid growing are killed because they are sensitive to radiation, but the central portion of hypoxic cells were with higher resistance to radiation; therefore, oxygen effect is important in cancer radiotherapy. HIF-1 can respond to intracellular-reduced oxygen (O2) availably [14], and it is a heterodimer, which regulates a series of genes which participate in cell proliferation/survival, angiogenesis, and so on [15]. In our study, when MCF-7 cells were treated with CoCl2 and radiation, the cell viability increased in the CoCl2 + IR group as compared with CoCl2 alone and IR alone. It had been reported that HIF-1α showed high expression under hypoxic condition and cells displayed radiation resistance. However, the cells exhibit hypersensitivity when HIF-1α was inhibited [16]. In order to explore the role of HIF-1α in breast cancer, we knock down HIF-1α and found that HIF-1α silencing led to hypersensitivity in response to DNA damage induced by IR, which is consistent with a previous report.
Apoptosis (a type I programmed cell death) and autophagic death (type II programmed cell death) are the most important mechanisms for cell death. We found that HIF-1α could change MCF-7 viability and enhance radioresistance. Then the next question is whether HIF-1α would affect the apoptosis and autophagy. We found that both IR and hypoxia could induce apoptosis by Hoechts/PI staining and PARP expression analysis. However, HIF-1α could not influence IR-induced apoptosis in MCF-7 cells.
Autophagy is a kind of cell catabolic process which can target unfolded proteins, damaged organelles, and so on [17]. Autophagy could either induce cell apoptosis or promote autophagic cell death or inhibit cell apoptosis [18]. We demonstrated that HIF-1α did not affect the apoptosis induced by IR. Then we would like to check if the autophagy was regulated by HIF-1α. MAPLC3 is always used as a marker for autophagy, and the conversion from LC3I to LC3II is associated with autophagosome formation [11]. In this study, we detected the conversion from MAPLC3I to MAPLC3II and acidic vacuoles (MDC staining) to analyze the change of autophagy; the results illustrated that IR and hypoxia could induce autophagy in MCF-7 cells. The HIF-1α level in IR + hypoxia group was much higher than that in radiation or hypoxia treatment. IR-induced autophagy was inhibited by HIF-1α knockdown, confirming that HIF-1α did participate in the IR-induced autophagy in MCF-7 cells.
Autophagy is able to lead to different outcomes, i.e., adaptive response or inducing cell death under different conditions [19]. To make sure if the high occurrence of autophagy is associated with cell viability or sensitivity to IR, 3-MA, an inhibitor for autophagy, was used. We found that 3-MA inhibited autophagy as well as cell viability, suggesting that the autophagy might play a protective role in response to IR. To our knowledge, this is the first report highlighting the interplay between HIF-1 and autophagy induced by IR. In addition, we used two other kinds of breast cancer cells for the research: MDA-231 and SKBR3. However, only in MCF-7 cells we found that HIF-1α played important roles in autophagy, while apoptosis showed little change in MCF-7. It might be due to the lack of apoptosis genes like caspase-3 in MCF-7 cells [20].
PI3K/Akt/mTOR pathway was involved in many cellular processes, including cell growth, survival, and promoting angiogenesis [21]. TOR, specifically TORC1, can regulate autophagy by phosphorylating Atg proteins or by functioning in a signal transduction cascade [22]. It had been reported that PI3K/Akt signaling mediated cell proliferation, migration, and HIF-1α expression [23]. Gambogic acid partly inhibited the PI3K⁄Akt⁄mTOR signaling to suppress hypoxia-activated pathways [24]. Doxazosin could inhibit HIF-1α and VEGF expression and also suppress Akt/mTOR signaling in ovarian carcinoma cells [25]. In our study, the expression of mTORC1, p-Akt, and P70S6K decreased in response to IR and hypoxia in the MCF-7 cells, which was reversed by HIF-1α silencing, indicating that HIF-1α might play roles in IR-induced autophagy by the suppression of PI3K/AKT/mTOR/p70 pathway. Collectively, our findings imply the role of HIF-1α in determining the response of breast cancer cells to IR.
References
Gruber M, Simon MC. Hypoxia-inducible factors, hypoxia, and tumor angiogenesis. Curr Opin Hematol. 2006;13(3):169–74.
Bhandari T, Nizet V. Hypoxia-Inducible Factor (HIF) as a pharmacological target for prevention and treatment of infectious diseases. Infect Dis Ther. 2014;3(2):159–174.
Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70(5):1469–80.
Semenza GL. HIF-1 and human disease: one highly involved factor. Genes Dev. 2000;14(16):1983–91.
Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35(4):495–516.
Kong D, Ma S, Liang B, Yi H, Zhao Y, Xin R, et al. The different regulatory effects of p53 status on multidrug resistance are determined by autophagy in ovarian cancer cells. Biomed Pharmacother. 2012;66(4):271–8.
Cheng GH, Kong DJ, Hou X, Liang B, He MZ, Liang N, et al. The tumor suppressor, p53, contributes to radiosensitivity of lung cancer cells by regulating autophagy and apoptosis. Cancer Biother Radiopharm. 2013;28(2):153–9.
Gozuacik D, Kimchi A. Autophagy as a cell death and tumor suppressor mechanism. Oncogene. 2004;23(16):2891–906.
Liu B, Cheng Y, Zhang B, Bian HJ, Bao JK. Polygonatum cyrtonema lectin induces apoptosis and autophagy in human melanoma A375 cells through a mitochondria-mediated ROS-p38-p53 pathway. Cancer Lett. 2009;275(1):54–60.
Chaitanya GV, Steven AJ, Babu PP. PARP-1 cleavage fragments: signatures of cell-death proteases in neurodegeneration. Cell Commun Signal. 2010;8:31. doi:10.1186/1478-811X-8-31
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19(21):5720–8.
Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci. 2004;117(Pt 13):2805–12.
Codogno P, Meijer AJ. Autophagy and signaling: their role in cell survival and cell death. Cell Death Differ. 2005;12:1509–18.
Kai S, Tanaka T, Daijo H, Harada H, Kishimoto S, Suzuki K, et al. Hydrogen sulfide inhibits hypoxia- but not anoxia-induced hypoxia-inducible factor 1 activation in a von hippel-lindau- and mitochondria-dependent manner. Antioxid Redox Signal. 2012;16(3):203–16.
Zhang Z, Yan J, Chang Y, Yan SSD, Shi H. Hypoxia inducible Factor-1 as a target for neurodegenerative diseases. Curr Med Chem. 2011;18(28):4335–43.
Moon SY, Chang HW, Roh JL, Kim GC, Choi SH, Lee SW, et al. Using YC-1 to overcome the radioresistance of hypoxic cancer cells. Oral Oncol. 2009;45(10):915–9.
Lavallard VJ, Gual P. Autophagy and non-alcoholic fatty liver disease. Biomed Res Int. 2014;2014:120179.
Fimia GM, Piacentini M. Regulation of autophagy in mammals and its interplay with apoptosis. Cell Mol Life Sci. 2010;67(10):1581–8.
Chen Y, Klionsky DJ. The regulation of autophagy—unanswered questions. J Cell Sci. 2011;124(Pt 2):161–70.
Lomonaco SL, Finniss S, Xiang C, Decarvalho A, Umansky F, Kalkanis SN, et al. The induction of autophagy by gamma-radiation contributes to the radioresistance of glioma stem cells. Int J Cancer. 2009;125(3):717–22.
Morgan TM, Koreckij TD, Corey E. Targeted therapy for advanced prostate cancer: inhibition of the PI3K/Akt/mTOR pathway. Curr Cancer Drug Targets. 2009;9(2):237–49.
He C, Klionsky DJ. Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet. 2009;43:67–93.
Tahmatzopoulos A, Rowland RG, Kyprianou N. The role of alpha-blockers in the management of prostate cancer. Expert Opin Pharmacother. 2004;5(6):1279–85.
Wang F, Zhang W, Guo L, Bao W, Jin N, Liu R, et al. Gambogic acid suppresses hypoxia-induced hypoxia-inducible factor-1alpha/vascular endothelial growth factor expression via inhibiting phosphatidylinositol 3-kinase/Akt/mammalian target protein of rapamycin pathway in multiple myeloma cells. Cancer Sci. 2014;105(8):1063–70.
Park MS, Kim BR, Dong SM, Lee SH, Kim DY, Rho SB. The antihypertension drug doxazosin inhibits tumor growth and angiogenesis by decreasing VEGFR-2/Akt/mTOR signaling and VEGF and HIF-1 alpha expression. Oncotarget. 2014;5(13):4935–44.
Acknowledgments
This study was supported by NSFC grant (30770649, 30970682, 131370837), and Research Fund for the Doctoral Program of Higher Education of China (20100061110070), Program for New Century Excellent Talents in University, Fundamental Research Funds for the Jilin Universities.
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding authors
Additional information
Huiying Xu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhong, R., Xu, H., Chen, G. et al. The role of hypoxia-inducible factor-1α in radiation-induced autophagic cell death in breast cancer cells. Tumor Biol. 36, 7077–7083 (2015). https://doi.org/10.1007/s13277-015-3425-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3425-z