Abstract
The multifunctional RNA-binding protein CUGBP1 regulates multiple aspects of nuclear and cytoplasmic messenger RNA (mRNA) processing, including splicing, stabilization, and translation of mRNAs. Previous studies have shown that CUGBP1 is overexpressed in non-small-cell lung cancer (NSCLC) tissues, but the pathological functions of CUGBP1 in tumorigenesis and development are unknown. Here, we provide the first evidence demonstrating the clinicopathological significance of CUGBP1 in NSCLC. Using immunohistochemistry, the levels of CUGBP1 expression in NSCLC tissues and adjacent non-cancerous tissues were examined and determined to be associated with differentiation. Short hairpin RNA-induced downregulation of CUGBP1 promoted apoptosis and decreased proliferation in the A549 NSCLC cell line. Moreover, Western blot analysis indicated that the depletion of CUGBP1 increased the protein levels of cyclin D1, BAD, BAX, Jun D, and E-cadherin, while the cyclin B1 level decreased. Knockdown of CUGBP1 decreased β-catenin and vimentin levels and increased E-cadherin expression, suggesting that CUGBP1 may contribute significantly to epithelial to mesenchymal transition (EMT) progression. These results demonstrate the importance of CUGBP1 in the biological and pathological functions of NSCLC and indicate its potential as a therapeutic target for NSCLC.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer is the leading cause of cancer mortality worldwide [1–3]. It can be classified into two main groups: small-cell lung cancer (SCLC), accounting for 15 % of all lung cancers, and non-small-cell lung cancer (NSCLC), accounting for 85 % [4]. Although the rates of early screening and diagnosis have improved in recent years, the prognosis of most NSCLC patients remains poor, with a 5-year survival rate of less than 15 % [5]. Effective treatment measures that significantly prolong survival and improve the quality of life in NSCLC patients are lacking. Therefore, it is imperative to discover new therapeutic methods and drug targets for the treatment of NSCLC.
CUGBP1, a member of the CUGBP and embryonic lethal abnormal vision-like factor (CEFE) family of human RNA-binding proteins, was first identified as a protein that binds to the CUG RNA repeats that are expanded in the 3′-untranslated region (UTR) of the gene encoding myotonic dystrophy protein kinase (DMPK), which is associated with DM1 (myotonic dystrophy type 1) [6–8]. It has been shown that CUGBP1 regulates many posttranscriptional processes, including alternative splicing, translation, deadenylation, and messenger RNA (mRNA) decay, which is conducted by binding to GU-rich elements (GREs) found in the 3′-UTR regions of short-lived transcripts [9–11]. Considering that CUGBP1 is involved in embryonic and cardiac development, skeletal muscle and adipose tissue differentiation, and germ cell formation, it may also play a significant role in tumorigenesis and the deterioration of certain tumors [12, 13]. Some studies have indicated that CUGBP1 plays an important role in modulating proteins during differentiation, cellular stress, or tumorigenesis and participates in the process of apoptosis in esophageal and liver cancer [12, 14]. Recently, a study revealed that CUGBP1 was overexpressed in cancer tissues, which may independently predict a poor prognosis in NSCLC [15]. However, the role of CUGBP1 in the pathogenesis of NSCLC remains unclear. To investigate the functional role of CUGBP1 in NSCLC, CUGBP1 expression was knocked down, and the resulting effects on proliferation, apoptosis, cell cycle distribution, and invasion of NSCLC cells were examined.
Materials and methods
Patients
Eighty NSCLC patients were selected from the Department of Thoracic Surgery in the Affiliated Hospital of Qingdao University, treated by curative surgical resection and never received chemotherapy or radiotherapy before. Their tumor tissues and pericancerous tissues were collected for immunohistochemical staining by the Department of Pathology. The clinical characteristics analyzed included gender, age, histology, TNM stage, N stage, and differentiation; data were recorded according to the World Health Organization (WHO) and the Union for International Cancer Control (UICC). Research approval was obtained from the Ethics Committee of the Affiliated Hospital of Qingdao University, and written informed consent was obtained from all participants.
Immunohistochemistry
The specimens were formalin-fixed and paraffin-embedded. After dewaxing and hydration, the slides were washed with phosphate-buffered saline (PBS) and endogenous peroxidase activity was blocked with 3 % hydrogen peroxide. After heating, 1 % hydrogen peroxidase was used to block endogenous peroxidase activity. The specimens were then incubated with anti-CUGBP1 (Santa Cruz Biotechnology, Shanghai, China, diluted 1:200 in PBS) for 90 min at room temperature. After washing with PBS, the samples were incubated with secondary antibodies for 60 min at room temperature. Finally, DAB staining was performed, followed by counterstaining of all sections with hematoxylin.
Cell culture and CUGBP1 depletion
The human NSCLC cell lines A549 (Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences) were grown in the designated complete medium consisting of Dulbecco’s modified Eagle medium (DMEM) supplemented with 100 units/ml penicillin, 100 units/ml streptomycin, and 10 % fetal bovine serum (FBS, Summit Biotechnology, Ft. Collins, CO). The cells were cultured as a monolayer in a humidified atmosphere containing 5 % CO2 at 37 °C in 100 % humidity. For lentivirus packaging, A549 cells were transfected with CUGBP1 short hairpin RNA (shRNA) (a GU-repeat sequence UGUUUGUUUGU) [10] or control shRNA. After 36 h of transfection, the media containing the packaged lentivirus was gathered and passed through 0.45-μm filters. A549 cells were subcultured in six-well tissue culture plates at 5 × 104 cells per well, and the prepared lentiviral particles were added after 48 h of culture at a multiplicity of infection (MOI) of 50.
Real-time qPCR analysis
Five days following infection, A549 cells were harvested for total RNA extraction, and cDNA was prepared from each sample using 10 ng of total RNA for reverse transcription. The primers were as follows: CUGBP1 (forward 5′-GTCAGTGGTGGACCTGACCT-3′, reverse 5′-AGGGGTCTACATGGCAACTG-3′). Actin was used as a control housekeeping gene (forward 5′-TGACTTCAACAGCGACACCCA-3′, reverse 5′-CACCCTGTTGCTGTAGCCAAA-3′). All primers were synthesized and purified by reverse-phase high-performance liquid chromatography (HPLC) at the central laboratory of our hospital. The PCR conditions were as follows: 1 cycle at 95 °C for 1 min, followed by 40 cycles at 95 °C for 5 s and 60 °C for 20 s. The expression of CUGBP1 was measured by normalization of the cycle threshold (Ct) with the control housekeeping gene, calculated by the formula 2−ΔΔCt. The entire process was conducted on the BioRad-Connect Real-Time PCR platform.
Western blot analysis
Whole cell lysates were prepared in buffer (100 mM Tris–HCl (pH 6.8), 10 mM EDTA, 4 % sodium dodecyl sulfate, 10 % glycerol). After separation by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), proteins were transferred to nitrocellulose membranes for immunoblotting. After blocking with 5 % non-fat milk for 1 h, the nitrocellulose membranes were incubated with specific antibodies: the primary antibodies include anti-CUGBP1 (Santa Cruz, sc-20003, dilution 1:1000) and anti-GAPDH (Santa Cruz Biotechnology, sc-32233, dilution 1:3000); the secondary antibodies are goat anti-mouse IgG-HRP (Santa Cruz Biotechnology, sc-2005, dilution 1:5000). A chemiluminescence reagent (NEN, Boston, MA) was employed to detect the signal, which was visualized by autoradiography. Densitometry analysis was performed with ImageJ software (NIH, Bethesda, MD).
Cell proliferation assay
The CCK-8 assay was performed to assess the level of cell proliferation. Cell lines with CUGBP1 shRNA or control shRNA were seeded into 96-well plates and incubated for 24, 48, and 72 h. Two hours prior to incubation completion, 10 μl of CCK-8 was added to each well. The absorbance of each well was then evaluated using a microplate reader at 450 nm. All of the experimental procedures were repeated at least three times independently.
Flow cytometry for apoptosis and cell cycle assessment
Cell apoptosis was measured using an annexin-V apoptosis analysis kit according to the manufacturer’s protocol (C1052, Beyotime Institute of Biotechnology, Jiangsu, China). Forty-eight hours after transfection with Lv-shCUGBP1 or Lv-shCon, the resulting A549 cells were centrifuged at 3000 × g for 5 min. Next, at room temperature, the transfected cells were washed with PBS, resuspended in 100 μl of buffer, and incubated with annexin-V for 15 min in the dark. After adding 400 μl of buffer to the cell suspension, fluorescence-activated cell sorting analysis was performed using a flow cytometer and CellQuest software. Similarly, after transfection of CUGBP1 shRNA or control shRNA, the A549 cells were collected by trypsinization, washed with PBS, and fixed with 70 % cold ethanol overnight. The ethanol-suspended cells were then collected, washed, and stained with propidium iodide (PI) for 30 min in the dark at room temperature. Finally, the cell cycle assessment was performed by determining the DNA content using a Cell Cycle and Apoptosis Analysis Kit (C1052, Beyotime Institute of Biotechnology, Jiangsu, China), according to the manufacturer’s protocol and analyzed by Cell Lab Quanta Beckman Coulter.
DAPI (4',6-diamidino-2-phenylindole) staining
The A549 cells transfected with CUGBP1 shRNA and control shRNA were seeded in six-well plates at a density of 2 × 105/well. The culture media was then changed to that including 10 % CCS (charcoal stripped FCS). Next, the cells were immobilized onto poly-(l-lysine)-coated slides with 4 % paraformaldehyde for 15 min, followed by permeabilization with 0.1 % Triton X-100. After washing the slides with PBS, the transfected cells were cultured with 50 ml of DAPI staining solution for 5 min. All of the above procedures were conducted at room temperature. A Nikon Eclipse Ti fluorescent microscope (Nikon TI-SR, JAPAN) was employed to observe the transfected cells at emission wavelengths of 340–380 nm.
Statistical analysis
The experimental results were evaluated by Student’s t test and expressed as the means ± SD. All statistical analyses were performed with SPSS 17.0 software. The level of statistical significance was set at P < 0.05.
Results
CUGBP1 is highly expressed in NSCLC tissues
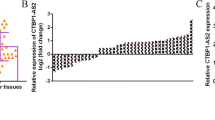
To explore the important role of CUGBP1 in NSCLC, immunohistochemistry was employed with the CUGBP1 antibody on 80 NSCLC tissues along with 80 normal tissues. As shown in Fig. 1, CUGBP1 expression was localized in the nucleus. Importantly, the level of CUGBP1 expression in the NSCLC tissue samples was significantly higher (P < 0.05) than that in the control group samples (Table 1). The relationships between the expression of CUGBP1 and clinicopathological variables were then analyzed. The expression of CUGBP1 was associated with differentiation, and maybe also with the TNM stage; no statistically significant differences were detected between CUGBP1 expression and the other variables (Table 2).
CUGBP1 expression is downregulated by transfection with CUGBP1 shRNA
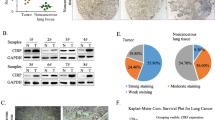
Highly specific and apparently non-toxic interfering RNA has been widely used to knock down the expression of target genes [16]. To examine the functions of CUGBP1 in NSCLC, A549 cells were collected following transfection with CUGBP1 shRNA or control shRNA. As demonstrated by real-time qPCR analysis, the transcription level of CUGBP1 was significantly decreased in A549 cells transfected with Lv-shCUGBP1 compared with cells transfected with Lv-shCon or control cells (Fig. 2a). Western blot analysis further confirmed that the level of CUGBP1 expression was downregulated significantly by transfection with CUGBP1 shRNA (Fig. 2b). These results indicated that the knockdown of CUGBP1 in A549 cells significantly decreased CUGBP1 expression at the mRNA and protein levels.
The expression levels of CUGBP1 mRNA and protein in A549 cells. a Expression analysis of CUGBP1 mRNA in A549 cells by real-time qPCR after infection with lentivirus. Data represent the means ± SD of four independent experiments. b Protein levels of CUGBP1 in A549 cells after infection with lentivirus were determined with antibodies against CUGBP1, using GAPDH as an internal control. The data represent one of four separate experiments. 1, Control; 2, Lv-shCon; 3, Lv-shCUGBP1
Suppression of CUGBP1 inhibits A549 cell proliferation
Oncogenes are typically overactivated to promote the proliferation of tumor cells and impact their invasion and migration. CUGBP1 can control the eukaryotic cell cycle by efficiently binding to the 5′-UTR region of the cyclin-dependent kinase inhibitor [12]. The proliferation of A549 cells was detected after transfection with CUGBP1 shRNA or control shRNA using the CCK-8 assay. The results demonstrate that the OD values of A549 cells transfected with Lv-shCUGBP1 (48 h, 0.274 ± 0.005; 72 h, 0.421 ± 0.004) were significantly lower than those of A549 cells transfected with Lv-shCon (48 h, 0.537 ± 0.007; 72 h, 1.114 ± 0.006) or control cells (48 h, 0.519 ± 0.010; 72 h, 1.108 ± 0.002) (Fig. 3, P < 0.001), which suggests that the depletion of CUGBP1 can inhibit A549 cell proliferation.
Suppression of CUGBP1 blocks the cell cycle in A549 cells
It is known that cell proliferation is regulated by the cell cycle, which includes a series of phases. To explore the role of CUGBP1 in regulating the cell cycle, flow cytometry was employed to determine whether the depletion of CUGBP1 affects the cell cycle in A549 cells. As the results show, the population of Lv-shCUBGP1-transfected A549 cells in the G0/G1 phase increased significantly (73.79 ± 1.31 %, P < 0.05), and the populations of cells in both the S (13.33 ± 0.56 %, P < 0.05) and G2/M (14.28 ± 1.48 %, P < 0.05) phases were decreased compared with those of the control cells (G0/G1, 60.45 ± 2.67 %; S, 19.31 ± 1.06 %; G2/M, 20.67 ± 3.12 %). However, there were no statistically significant differences between cells transfected with Lv-shCon (G0/G1, 62.62 ± 2.00 %, S, 17.83 ± 0.86 %, and G2/M, 20.29 ± 1.44 %) and control cells (Fig. 4). To further confirm that the knockdown of CUGBP1 impacts the cell cycle in A549 cells, two cell-cycle-related proteins, cyclin B1 and cyclin D1, were examined in cells transfected with Lv-shCUGBP1 or Lv-shCon and control cells. It was found that the depletion of CUGBP1 induced the downregulation of cyclin B1 and an upregulation of cyclin D1 (Fig. 5). All of the above results indicate that the suppression of CUGBP1 blocks the cell cycle.
Knockdown of CUGBP1 blocked cell cycle progression in A549 cells. a Cells were grown to 95 % confluence after transduction, and the cell cycle distribution was explored by flow cytometry analysis. b The percentage of A549 cells in the G0/G1, S, and G2/M phases. Data represent the means ± SD of three independent experiments
Depletion of CUGBP1 induces apoptosis in A549 cells
The clinicopathological function of CUGBP1 is crucial for detecting the mechanism of oncogenesis in NSCLC. In oral cancer cells, it has been found that CUGBP1 is related to mRNAs that encode proapoptotic factors and modulate the process of apoptosis [17]. To explore the effect of CUGBP1 on apoptosis, DAPI staining was performed in the A549 cell lines. The results showed that apoptosis was increased in cells transfected with Lv-shCUGBP1 compared with cells transfected with Lv-shCon or control cells (Fig. 6). Next, the expression levels of apoptosis-related proteins (such as BAD, BAX, and Jun D) were investigated by Western blot analysis. As shown in Fig. 7, the knockdown of CUGBP1 increased the level of expression of all three proteins, indicating that CUGBP1 is associated with apoptosis. We then utilized the annexin-V kit to explore the characteristics of apoptosis induced by CUGBP1 in A549 cells. It was found that, compared with control cells, there was significant variance in both early apoptosis (15.5 to 13.0 %, P < 0.05) and late apoptosis (17.7 to 10.9 %, P < 0.05) in A549 cells transfected with Lv-shCUGBP1 (Fig. 8).
Depletion of CUGBP1 inhibits the process of EMT in A549 cells
In epithelial to mesenchymal transition (EMT), when epithelial cells obtain the abilities of fibroblast-like cells, intercellular adhesion between the cells reduces and motility increases, further promoting tumor progression [18]. Thus, we initially explored the correlation between EMT in cancer and CUGBP1. We know that E-cadherin loss, high vimentin and β-catenin expression are considered to be fundamental events in EMT. The expression of EMT-related proteins in A549 cells with or without CUGBP1 knockdown was analyzed by Western blot analysis. As the results show, the level of E-cadherin was upregulated in A549 cells with knockdown of CUGBP1 compared with the negative control groups; in contrast, the levels of β-catenin and vimentin were both downregulated (Fig. 9). These results indicate that CUGBP1 potentially contributes to the development of EMT in NSCLC.
The expression of EMT regulatory molecules in A549 cells after the depletion of CUGBP1. Results of Western blot analysis of EMT regulatory molecules showing increased expression of E-cadherin with decreased vimentin and β-catenin expression after CUGBP1 knockdown in A549 cells. 1, Control; 2, Lv-shCon; 3, Lv-shCUGBP1
Discussion
According to the statistics, approximately 1.2 million new cases of NSCLC are diagnosed each year, and the overall 5-year survival rate remains at approximately 10–15 % [17]. An important reason for this poor prognosis is that the tumor has often spread beyond the primary site before diagnosis [4]. Therefore, it is extremely urgent to develop effective measures, such as predictive factors, for the diagnosis and treatment of NSCLC [19]. CUGBP1, an RNA-binding protein, is defined as a posttranscriptional regulatory network with the function of regulating cell growth, motility, and apoptosis [20]. Recent studies have indicated that CUGBP1 plays a significant role in the regulation of apoptosis, proliferation, and cell cycle in tumor cells by binding to target transcripts upon T cell activation [10, 12, 13], and the high expression of CUGBP1 prevented apoptosis by inducing the expression of p21 under stress conditions in HeLa cells [21, 22]. Although one study recently indicated the overexpression of CUGBP1 in NSCLC, the role of CUGBP1 in the pathogenesis of NSCLC remained unclear. In the current study, we first examined the level of CUGBP1 expression and its association with clinical characteristics in 80 NSCLC patients. In our study, we demonstrated that the expression of CUGBP1 in the NSCLC tissues was significantly higher than that in the adjacent non-cancerous tissues, and this overexpression is associated with TNM stage and differentiation.
Next, we explored the ability of CUGBP1 to control cell proliferation and apoptosis, and the potential correlation with EMT. First, we assessed its effects on cell proliferation in A549 cells with knockdown of CUGBP1 by the CCK-8 assay. The results demonstrated that the depletion of CUGBP1 significantly decreased cell proliferation. Considering that cell growth and proliferation are impacted by cell cycle progression, flow cytometry was employed for cell cycle analysis. It was determined that the G0/G1 phase was upregulated while the S and G2/M phases were downregulated in the CUGBP1 shRNA group compared with the control group. Moreover, the knockdown of CUGBP1 induced a downregulation of cyclin B1 and an upregulation of cyclin D1. These results suggested that CUGBP1 may regulate the process of proliferation by controlling the cell cycle. We then investigated the effect of CUGBP1 on cell apoptosis using DAPI staining, which revealed an obvious increase in apoptosis following the knockdown of CUGBP1. The annexin-V kit was used to detect the apoptotic features of A549 cells by flow cytometry, and significant differences were observed in both early and late apoptosis in A549 cells transfected with CUGBP1 shRNA compared with the control groups. Moreover, the levels of apoptosis-related proteins, such as BAD, BAX, and Jun D, were examined by Western blot analysis, and the results showed that all three proteins were upregulated in cells with CUGBP1 depletion. All of the above data indicate that CUGBP1 plays an important role in mediating cellular apoptosis. Currently, tumor invasion and metastasis are associated with EMT, suggesting that epithelial cells can gain an enhanced ability to migrate after losing polarity [23]. Western blot analysis was used to assess the levels of E-cadherin, β-catenin, and vimentin expression, which can regulate EMT in tumor cells. It was found that the knockdown of CUGBP1 induced the up-regulation of E-cadherin and the down-regulation of β-catenin and vimentin. Thus, the invasive ability was decreased prominently in A549 cells transfected with CUGBP1 shRNA. These results indicate that CUGBP1 may contribute to the EMT process.
In conclusion, this study demonstrates the importance of CUGBP1 in biological and pathological functions of NSCLC and indicates CUGBP1 as a potential therapeutic target for NSCLC. However, the mechanism of CUGBP1 in the pathogenesis of NSCLC has not been elucidated, and further research will likely be continued in the future.
References
Hecht SS. Tobacco smoke carcinogens and lung cancer. J Natl Cancer Inst. 1999;91:1194–210.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, et al. Cancer statistics. CA Cancer J Clin. 2008;58:71–96.
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29.
Kang S, Koh ES, Vinod SK, Jalaludin B. Cost analysis of lung cancer management in South Western Sydney. J Med Imaging Radiat Oncol. 2012;56:235–41.
Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108.
Koshelev M, Sarma S, Price RE, Wehrens XH, Cooper TA. Heart-specific overexpression of CUGBP1 reproduces functional and molecular abnormalities of myotonic dystrophy type 1. Hum Mol Genet. 2010;19:1066–75.
Ward AJ, Rimer M, Killian JM, Dowling JJ, Cooper TA. CUGBP1 overexpression in mouse skeletal muscle reproduces features of myotonic dystrophy type 1. Hum Mol Genet. 2010;19:3614–22.
Mulders SA, van den Broek WJ, Wheeler TM, Croes HJ, van Kuik-Romeijn P, et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc Natl Acad Sci U S A. 2009;106:13915–20.
Moraes KC, Wilusz CJ, Wilusz J. CUG-BP binds to RNA substrates and recruits PARN deadenylase. RNA. 2006;12:1084–91.
Rattenbacher B, Beisang D, Wiesner DL, Jeschke JC, von Hohenberg M, et al. Analysis of CUGBP1 targets identifies GU-repeat sequences that mediate rapid mRNA decay. Mol Cell Biol. 2010;30:3970–80.
Vlasova IA, Tahoe NM, Fan D, Larsson O, Rattenbacher B, et al. Conserved GU-rich elements mediate mRNA decay by binding to CUG-binding protein 1. Mol Cell. 2008;29:263–70.
Zheng Y, Miskimins WK. CUG-binding protein represses translation of p27Kip1 mRNA through its internal ribosomal entry site. RNA Biol. 2011;8:365–71.
Zhang L, Lee JE, Wilusz J, Wilusz CJ. The RNA-binding protein CUGBP1 regulates stability of tumor necrosis factor mRNA in muscle cells: implications for myotonic dystrophy. J Biol Chem. 2008;283:22457–63.
Salisbury E, Sakai K, Schoser B, Huichalaf C, Schneider-Gold C, et al. Ectopic expression of cyclin D3 corrects differentiation of DM1 myoblasts through activation of RNA CUG-binding protein, CUGBP1. Exp Cell Res. 2008;314:2266–78.
Jiao W, Zhao J, Wang M, Wang Y, Luo Y, et al. CUG-binding protein 1 (CUGBP1) expression and prognosis of non-small cell lung cancer. Clin Transl Oncol. 2013;15:789–95.
Gartel AL, Kandel ES. RNA interference in cancer. Biomol Eng. 2006;23:17–34.
Talwar S, Balasubramanian S, Sundaramurthy S, House R, Wilusz CJ, et al. Overexpression of RNA-binding protein CELF1 prevents apoptosis and destabilizes pro-apoptotic mRNAs in oral cancer cells. RNA Biol. 2013;10:277–86.
Zhou BP, Hung MC. Wnt, hedgehog and snail: sister pathways that control by GSK-3beta and beta-Trcp in the regulation of metastasis. Cell Cycle. 2005;4:772–6.
Ciervide R, Dhage S, Guth A, Shapiro RL, Axelrod DM, et al. Five year outcome of 145 patients with ductal carcinoma in situ (DCIS) after accelerated breast radiotherapy. Int J Radiat Oncol Biol Phys. 2012;83:e159–64.
Oji Y, Kitamura Y, Kamino E, Kitano A, Sawabata N, et al. WT1 IgG antibody for early detection of nonsmall cell lung cancer and as its prognostic factor. Int J Cancer. 2009;125:381–7.
Beisang D, Rattenbacher B, Louis IAV, Bohjanen PR. Regulation of CUG-binding protein 1 (CUGBP1) binding to target transcripts upon T cell activation. J Biol Chem. 2012;287:950–60.
Gareau C, Fournier M, Filion C, Coudert L, Martel D, et al. p21WAF1/CIP1 upregulation through the stress granule-associated protein CUGBP1 confers resistance to bortezomib-mediated apoptosis. PLoS One. 2011;6:e20254.
Greenburg G, Hay ED. Epithelia suspended in collagen gels can lose polarity and express characteristics of migrating mesenchymal cells. J Cell Biol. 1982;95:333–9.
Acknowledgments
This study was supported by the grants of Jieping Wu foundation (320.6750.13210 and 320.6753.1219).
Conflicts of interest
None
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gao, C., Yu, Z., Liu, S. et al. Overexpression of CUGBP1 is associated with the progression of non-small cell lung cancer. Tumor Biol. 36, 4583–4589 (2015). https://doi.org/10.1007/s13277-015-3103-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13277-015-3103-1